P53 and anti-p53 autoantibody complex versus anti-p53 antibody as a biomarker for gastrointestinal cancer
Abstract
Gastrointestinal tract cancer is among the most common types of cancer and includes colorectal cancer (CRC) and gastric cancer (GC). Early tumor detection has been shown to reduce cancer-associated mortality, emphasizing the significance of regular screening. In this regard, blood-based tumor markers have gained popularity in cancer management. The purpose of this research was to examine the potential association between serum p53 antibodies and malignant tumors. A case–control study was conducted, including 37 GC samples, 53 CRC samples, and 62 healthy control samples. Serum levels of p53 antibodies were assessed using an enzyme-linked immunosorbent assay. The results showed that the concentration of anti-p53 antibodies in GC samples was 2.35 ng/mL, significantly (p-value <.001) lower than the 9.24 ng/mL observed in the healthy control group. Similarly, the concentration of anti-p53 antibodies in CRC samples was 4.14 ng/mL, which was significantly lower than the 9.31 ng/mL found in the healthy control group (p-value <.001). These findings strongly suggest an association between the level of serum p53 antibodies and an elevated risk of cancer, implying their potential role as an early serological marker for the diagnosis of malignant tumors. However, it is important to note that measuring anti-p53 antibodies alone may not be clinically effective in distinguishing CRC and/or GC from healthy controls. Further research and a comprehensive approach would be necessary for more accurate diagnostic outcomes.
1 INTRODUCTION
Gastrointestinal malignancies, including colorectal cancer (CRC) and gastric cancer (GC), are among the most prevalent malignant tumors worldwide.1 Early detection and treatment of cancer are pivotal in reducing cancer-associated mortality, emphasizing the significance of prevention and regular screening.2
Various diagnostic procedures are employed to detect CRC, such as digital rectal examination, fecal occult blood test (FOBT), barium enema examination, colonoscopy, and sigmoidoscopy (considered the gold standard test). Nevertheless, the low specificity, compliance issues, high invasiveness, and cost of the aforementioned methods can restrict their use. Blood-based tumor markers have become increasingly prominent in cancer management due to the necessity for more accessible and repeatable screening services.3 Recent studies indicate that patients suffering from cancer produce noticeable antibodies against tumor-associated antigens (TAAs) during tumor development.4 Certain types of antibodies, such as anti-p53 antibodies, exhibit potential as prospective markers for early detection and prognosis in various malignancies, including breast, esophageal, ovarian, lung, pancreatic, and gastrointestinal cancers.5
The wild-type form of p53 protein, which is classified as a tumor suppressor marker, is believed to have a short half-life of approximately 5–20 min and is present in various cell types. The missense types of mutations that cause a constant type of the mutant p53 protein, with a half-life of 12–30 h, are the most current mutations detected in a number of human malignancies.6 The presence of a stable version of the mutant form of p53 protein or its overexpression in cancer patients can induce a particular humoral response, causing the release of anti-p53 autoantibodies.7 It is noteworthy that the healthy control groups exhibit only a wild-type p53, whereas cancer patients have both wild-type and mutant-type p53 proteins in their plasma.
A novel hypothesis suggests that the production levels of the p53-anti-p53 antibody immune complex (PIC) may be higher in individuals with cancer compared to healthy controls. As a result, the total amount of p53, including both wild-type and mutant forms, may be lower in cancer patients than in healthy individuals.8 This aspect of the study represents a new approach for monitoring patients and requires further research.
In this research, GC and CRC samples, along with a healthy control group, were evaluated for the presence of humoral antibodies against the p53 tumor suppressor protein. Enzyme-linked immunosorbent assay (ELISA) procedures were employed to assess serum anti-p53 antibodies in both CRC and GC patients.
2 MATERIALS AND METHODS
2.1 Patients
The research included 53 blood samples from patients with CRC (37 samples) and GC (16 samples), obtained from the Golestan Population-based Cancer Registry's Biobank in October 2022. Additionally, between August and December, 37 blood samples from patients with GC (21 samples) and CRC (16 samples) were collected at the Ayatollah Rouhani Hospital and Omid Clinic in Babol. Furthermore, 62 blood samples from healthy controls were included in this study, with 28 samples serving as the gastric control group and 34 samples as the colorectal control group. All serum samples were aliquoted, coded, and stored at −80°C until the assays were performed. This study was approved by the Research Ethics Committee of Golestan University of Medical Sciences (IR.GOUMS.REC.1401.134). All participants provided informed consent prior to their participation.
2.2 Serum anti-p53 antibody assay
Anti-p53 antibodies were evaluated using a specific ELISA kit (Human p53 Antibody ELISA Kit, Catalog No. MBS700400, BioSource, San Diego, California, USA) according to the manufacturer's instructions. All samples were blindly tested with two repetitions of the same assay. The absorbance of samples was evaluated at a wavelength of 450 nm using a Biotek-Reflex800 ELISA reader device, USA.
2.3 p53 expression of colorectal and gastric cancer in tissue samples
Two groups of tissue samples from GC and CRC patients were assessed for p53 expression using immunohistochemistry (IHC). The present study included 37 GC serum samples, 53 CRC serum samples, and 62 serum samples from healthy controls, all of which were analyzed to investigate anti-p53 antibody concentrations. The results of this examination were then compared to p53 expression levels. The details of p53 expression in tissue were explained in our previous study.9
2.4 Statistical analysis
Statistical analysis was performed using SPSS software version 27, with a p-value of <.05 considered statistically significant. Graphical charts were created using GraphPad Prism 8 software. An independent sample t-test was conducted to compare the concentration of anti-p53 antibodies between GC and CRC patients and their control groups. Additionally, the anti-p53 antibody levels between GC and CRC cases were compared.
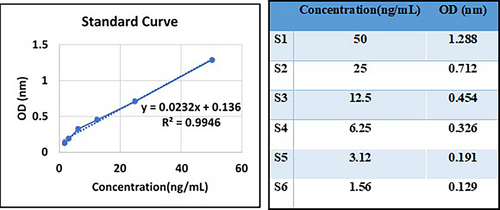
2.5 Generating the standard curve
The kit protocol was followed to acquire a standard curve for the purpose of evaluating anti-p53 antibody levels in GC and CRC samples (Figure 1).
3 RESULTS
3.1 Evaluation of serum anti-p53 antibodies in colorectal and gastric cancer samples
This research assessed serum samples from 53 patients with CRC (30 males and 23 females), 37 patients with GC (25 males and 12 females), and 62 individuals from the healthy control group (14 males and 14 females in the gastric control group, 19 males and 15 females in the colorectal control group) to detect the presence of anti-p53 antibodies. The minimum detectable dose of human anti-p53 antibody in this study was less than 0.39 ng/mL. The sensitivity of the assay, expressed as the lower limit of detection (LLD), was described as the lowest concentration of human anti-p53 antibody that could be reliably distinguished from zero. Moreover, the test demonstrated a high degree of specificity and sensitivity for detecting anti-P53 antibodies in humans, without cross-reactivity or interference from analogs. In Figure 2, the mean concentrations of anti-p53 antibodies were 2.35 and 9.24 ng/mL in GC samples and controls, respectively. In CRC samples, the average concentrations were 4.14 and 9.31 ng/mL in CRC patients and the control group, respectively.
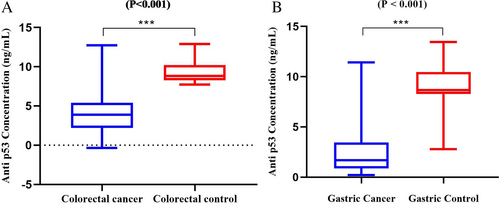
The statistical analysis revealed a significant difference in anti-p53 antibody concentrations between GC patients and the control group (p <.001), as well as between CRC patients and the control group (p <.001) (Figure 2).
3.2 Comparison of p53 expression with anti-p53 antibody concentration in colorectal and gastric cancer
In the CRC and GC groups, the p53 expression was categorized into four groups (0: no expression, 1: expression less than 10%, 2: expression between 10% and 70%, 3: expression greater than 70%). The expression ratios of p53 and anti-p53 antibodies between the GC and CRC groups are illustrated in Figure 3A,B. However, no significant difference was observed in p53 expression between GC and CRC (p = .0786). In terms of anti-p53 antibody concentration, a significant difference was not found between CRC and GC (p = .0012). Based on the results of this study, the amount of p53 expression and anti-p53 concentration in CRC is higher than GC. Overexpression of p53 can cause the production of p53 antibodies.

In addition, Table 1 demonstrates the correlation between expression of p53 and the carcinoma grade, gender, and age of patients. According to Fisher's exact test, there was no significant difference between p53 expression and gender or age in either CRC and GC. However, Fisher's exact test showed a significant association between carcinoma grade and expression of p53 in CRC (p = .005) and GC (p = .001).
| Gastric cancer (p53 expression) | Colorectal cancer (p53 expression) | |||||||
|---|---|---|---|---|---|---|---|---|
| No expression | Less than 10% | 10%–70% | More than 70% | No expression | Less than 10% | 10%–70% | More than 70% | |
| Gender | ||||||||
| Male | 7 | 9 | 2 | 7 | 6 | 9 | 5 | 10 |
| Female | 4 | 2 | 3 | 3 | 6 | 1 | 5 | 11 |
| Total | 11 | 11 | 5 | 10 | 12 | 10 | 10 | 21 |
| p-Value | .411 | .121 | ||||||
| Age | ||||||||
| 0–20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 20–40 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 40–60 | 2 | 1 | 1 | 1 | 5 | 2 | 6 | 11 |
| More than 60 | 9 | 10 | 4 | 9 | 7 | 8 | 4 | 10 |
| Total | 11 | 11 | 5 | 10 | 12 | 10 | 10 | 21 |
| p-Value | 1.000 | .273 | ||||||
| Carcinoma grade | ||||||||
| Well | 1 | 6 | 1 | 2 | 5 | 7 | 4 | 5 |
| Moderate | 0 | 1 | 0 | 2 | 2 | 1 | 2 | 14 |
| Poorly | 1 | 2 | 4 | 5 | 0 | 1 | 1 | 1 |
| Unknown | 9 | 2 | 0 | 1 | 5 | 1 | 3 | 1 |
| Total | 11 | 11 | 5 | 10 | 12 | 10 | 10 | 21 |
| p-Value | .001*** | .005** | ||||||
4 DISCUSSION
The TP53 gene, which encodes the tumor suppressor p53, mediates cell cycle arrest, apoptosis, or senescence in response to various cellular stresses, such as hypoxia, DNA damage, and tumorigenic signaling. p53 enhances these responses by regulating key molecules, such as p21 (a cyclin-dependent kinase inhibitor), Puma (a p53-upregulated modulator of apoptosis), and PAI-1 (a single-chain glycoprotein from the serpins superfamily). TP53 variations include missense mutations and nonsense or frameshift mutations.10 The occurrence of mutations in this gene is an important phenomenon in the tumorigenesis of various types of tumors. However, the precise mechanisms underlying autoantibody generation against p53 have not been fully understood. According to previous studies, missense mutations and the overexpression of the p53 protein can result in p53 protein accumulation in tumors.11, 12 Abdel-Aziz et al.13 collected serum samples from 48 CRC patients and 20 healthy individuals as a control group. Their findings revealed a significant rise in serum levels of the mutant p53 among CRC samples compared to the healthy control group (p < .001). Furthermore, Zusman et al.14 demonstrated a significant increase in the serum concentration of the p53 antigen in cancer samples (3.6 mg/mL) in comparison to patients with benign tumors (1.7 mg/mL) or non-tumorigenic disorders (0.49 mg/mL). This increase was attributed to a higher p53 protein concentration in tumorigenic cells. Our results regarding the p53 evaluation were consistent with their findings. These observations are attributed to the overexpression and p53 mutation, which leads to the production of p53 autoantibodies.
As depicted in Figure 2, the levels of anti-p53 antibodies were significantly higher in gastric and colorectal control samples (p = .87) compared to the GC and CRC groups (p = .001). In contrast to our findings, Mattioni et al.15 reported elevated concentrations of anti-p53 antibodies in 15.3% of GC samples, with this specificity being absent in healthy controls. They found that elevated serum concentrations of anti-p53 antibodies were associated with the accumulation of mutant p53 in the corresponding tumors.
Various studies have described an association between anti-p53 antibodies and the progression of different neoplasms. However, these antibodies are not specific enough to distinguish a particular type of neoplasm. Therefore, combined testing is crucial for identifying specific biomarkers for CRC rather than merely adjusting the sensitivity of detection.16
Actually, there is considerable interest in using autoantibodies as blood-based diagnostic and prognostic biomarkers, as the anti-tumor immune response may be generated before other tumor proteins are clinically detectable and before the first clinical signs of cancer or its recurrence appear.17
Sobhani and colleagues conducted a meta-analysis that included 12 clinical studies with almost 2094 patients. The analysis revealed a significant relevance between the anti-p53 antibody expression in the serum and poorer survival outcomes in cancer patients with 95% confidence interval 1.48 [1.24, 1.77], and p < .00001.18
Altogether, the positive results of the anti-p53 antibody might be considered a favorable diagnostic biomarker for distinguishing between CRC and GC.19 The elevated levels of the p53 marker in cancer samples, as reported by Abdel-Aziz et al.13 suggest the production of anti-p53 autoantibodies and the potential formation of antigen–antibody complexes, which may reduce the concentration of free anti-p53 antibodies. These observations are consistent with our results and the findings of Song et al.8
Although anti-p53 antibody concentrations were measured in the cancer cohort, IHC was utilized to evaluate p53 protein expression directly. This technique revealed an increased concentration of p53 protein in the cancer samples, supporting the presence of elevated p53 levels in these tissues.
As a marker for early detection of CRC, Ushigome and colleagues found that the levels of four antibodies—p53, RalA, Galectin1, and HSP70—did not increase in relation to disease stage development.20 Wang et al.21 analyzed various well-known antibodies and their amounts for the diagnosis of CRC. They found an odds ratio of 10.86 for the anti-p53 antibody across various categories of CRC, with moderate heterogeneity (I2 = 40.3%).
According to a study by Song et al.8 the formation of antigen–antibody complexes (specifically, the p53-anti-p53 antibody complex) in the cancer group may contribute to decreased levels of free anti-p53 antibodies. They presented new findings on this preliminary evaluation by using the PIC complex and p53 concentration to distinguish between Stages I and IV of lung cancer.
Consistent with the findings of Song et al.8 our results showed that the formation of the p53-anti-p53 antibody complex led to a decrease in the concentration of free anti-p53 antibodies. Consequently, the detection of anti-p53 antibodies in the cancer group denotes a lower ratio compared to the control group. In cancer patients, the presence of mutated or overexpressed p53 protein leads to its release into the serum, which triggers the production of anti-p53 antibodies and the formation of antigen–antibody complexes. This complex formation causes a reduction in the concentration of detectable antibodies in the serum of cancer samples.
In this research, a comparison of p53 and anti-p53 antibody concentrations revealed that these markers were higher in CRC than in GC. The coexistence of elevated levels of both p53 and anti-p53 antibodies results in the formation of PIC complexes. Consequently, the evaluation of free anti-p53 antibodies shows reduced levels in cancer groups. Consistent with previously reviewed studies, the concentration of autoantibodies demonstrated a significant correlation between cancer and control groups. This finding suggests that anti-p53 antibodies, particularly the p53-anti-p53 antibody complex, warrant further investigation as potential biomarkers for cancer screening. In this study, the level of anti-p53 antibody production in cancer patients is lower than in healthy individuals, which, according to the studies by others, may be due to the formation of PIC complexes that reduce the level of free antibodies in the serum of cancer patients.
5 CONCLUSION
The p53 protein is a vital tumor suppressor that regulates a different cellular response to protect against oncogenesis. It plays a pivotal role by inducing apoptosis in response to DNA damage. When functioning correctly, p53 acts as a transcription factor that deactivates downstream target genes and promotes cell growth arrest, programmed cell death, or DNA repair. Due to the elevated release of p53 and anti-p53 antibodies in the serum of cancer patients, there is a potential for the formation of PIC complexes, which can subsequently lead to a decrease in the concentration of free anti-p53 antibodies in the serum. Due to the inconsistent results reported across various studies, relying exclusively on anti-p53 antibody measurements as a biomarker may not be clinically beneficial for distinguishing CRC or GC from healthy controls. It is recommended that both anti-p53 antibodies and PIC complexes be measured for a more reliable biomarker assessment. Further prospective research is needed to determine the optimal combination of different biomarkers for the diagnosis of CRC and GC.
AUTHOR CONTRIBUTIONS
AM: Conceived and planned the research, provided research supervision, analyzed the data, wrote the present paper, read and confirmed the final version of the paper, critically revised the manuscript, and managed research sponsorships and key manuscript changes. VHS, AES: Collected samples, conducted experimental studies, analyzed data, read and confirmed the final version of the paper, and performed revisions. AT: Read and confirmed the final version of the paper. HRN: Read and confirmed the final version of the paper. FS: Read and confirmed the final version of the paper. GhK: Conducted pathological experimental studies. YY: Conceived and designed the experiments, provided research supervision, analyzed data, wrote the present paper, read and confirmed the final version of the paper, and performed revisions. All authors contributed to the paper and approved the final submitted version.
ACKNOWLEDGMENTS
The financial support for this study was provided by the Microbiology Department of Medical Sciences, Golestan University of Medical Sciences, Gorgan, Iran.
FUNDING INFORMATION
Partial funding for this research was provided by Golestan University of Medical Sciences (GOUMS) under Funding Code: 112792.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The study was approved by the Ethics Committee of the Golestan University of Medical Sciences (Ethics Code: IR.GOUMS.REC.1401.134) and was conducted in accordance with the principles of the Declaration of Helsinki.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




