Mass spectrometry-based protein analysis to unravel the tissue pathophysiology in Duchenne muscular dystrophy
Abstract
Duchenne muscular dystrophy (DMD) is a genetic muscle wasting condition with limited treatment options available and is caused by the lack of dystrophin. However, pathophysiology of different tissues is variable showing different histological and molecular signatures. Recently, a number of studies have employed gel-free proteomic approaches to unveil the molecular pathophysiology in terms of tissue-specific proteome changes in dystrophin deficiency. The authors analyzed studies in models of dystrophin deficiency and patients both from the published literature. The authors created a database containing all of the significantly differentially expressed proteins. By the integration of data from nine studies, the authors have identified 31 proteins which are commonly affected in different tissues by dystrophin deficiency. These proteins represent pathways involved in the maintenance of the actin cytoskeleton and those involved in cellular energy metabolism among others. Also represented is glyceraldehyde-3-phosphate dehydrogenase (GAPDH), often used as a loading control in protein assays, it appears to be highly variable, and should be replaced by other controls. The same intersection of data was performed using studies of the blood and urine of Duchenne muscular dystrophy patients and/or animal models and identified 33 proteins that are commonly differentially expressed. These proteins may themselves be novel therapeutic targets biomarkers that could monitor disease progression.
Abbreviations
-
- CK
-
- creatine kinase
-
- DCM
-
- dilated cardiomyopathy
-
- DGC
-
- dystrophin-associated glycoprotein complex
-
- DMD
-
- Duchenne muscular dystrophy
-
- GAPDH
-
- glyceraldehyde-3-phosphate dehydrogenase
-
- PGC-1α
-
- proliferator-activated receptor-gamma co-activator 1 alpha
1 Introduction
MS-based proteomics has been used for around two decades in a large variety of research fields including translational biomedical research of neuromuscular diseases. The technique has already had a significant impact on the identification of disease-specific protein signatures in terms of searching for changes in a proteome-wide manner upon pathophysiological changes. As the biochemical identification of disease-specific protein signatures not only improves our current understanding of the function and vulnerability of the affected tissue, but moreover allows the definition of promising starting points for the development of therapeutic intervention concepts. A comparison of findings obtained in diverse proteomic studies of one disease is a decisive step toward the definition of such starting points.
Duchenne muscular dystrophy (DMD) is a progressive muscle wasting disease caused by mutations in the DMD gene encoding the 427 kDa protein, dystrophin. It is inherited in an X-linked manner and affects between 1 in 3500 to 5000 live males born each year 1. Patients tend to be diagnosed between the ages of 3 and 5 after which they will become progressively weaker before loss of ambulation, which usually occurs in the mid to late teens. Assisted ventilation is usually required in the twenties as the respiratory muscles weaken and almost all patients will develop a left ventricular dilated cardiomyopathy. The major causes of death among patients are respiratory or cardiac failure 2. There is currently no cure for DMD and few directed therapies are available for only a small percentage of the patient population. Patients are currently given symptomatic treatments such as corticosteroids to improve muscle strength and angiotensin converting enzyme (ACE) inhibitors to manage cardiac symptoms however, many of the drugs currently prescribed have considerable side effects rendering them unsuitable for many 3, 4. Recently, a stop codon read-through drug named Translarna has been conditionally approved by the European Medicines Agency (EMA) as the first drug marketed for treatment of DMD. However, the drug is only applicable to an estimated 15% of the total patient population and is far from a cure for the condition 5.
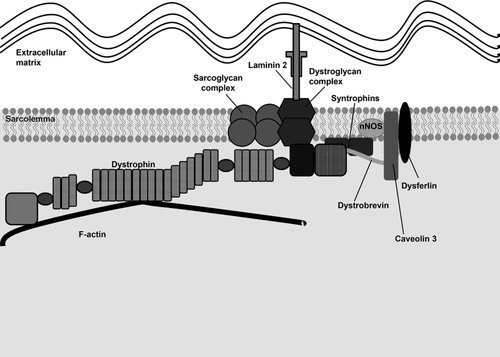
Dystrophin is a high molecular weight protein expressed highly in skeletal and cardiac muscle and the brain. Dystrophin is a protein localized to the muscle cell membrane (sarcolemma) with complex roles, many of which we are yet to fully understand. The primary function of the protein is as a structural protein linking the contractile elements of the cell to the sarcolemma via a complex of proteins known as the dystrophin-associated glycoprotein complex (DGC) (Fig. 1) 6. The dystrophin protein is comprised of four main domains: the N-terminal domain which contains an actin-binding (ABD1) site; the rod domain containing 24 spectrin-like repeats with an additional actin-binding domain (ABD2) and four hinge regions which provide flexibility and length to the protein; a cysteine-rich domain which contains binding sites for other proteins of the DGC such as dystroglycan and finally the C-terminal domain which contains the syntrophin-binding site (Fig. 1) 6-11. Mutations which result in loss of dystrophin expression also result in the lack of expression of other DGC proteins at the sarcolemma leading to membrane fragility. Beyond its structural role, dystrophin plays a part in cellular signaling and gene expression 12, 13.
Two additional shorter isoforms are expressed in the nervous system as well as the full-length isoform of dystrophin (Dp427). The full-length isoform is controlled by three different promoters driving expression in the brain (cortical neurons, hippocampus, and retina), Purkinje (Purkinje cells and at low levels in skeletal muscle), and skeletal muscle (skeletal muscle, cardiomyocytes, retina, and at low levels in glial cells) 14, 15. There is some evidence of a fourth promoter driving expression in the lymphocytes, however there is some conflicting evidence that this is an artefact 16, 17.
When dystrophin is lost from the sarcolemma, it becomes more susceptible to mechanically induced damage and microlesions appear in the membrane. Through these aforementioned lesions there is an efflux of intracellular components and influx of calcium ions. Chronically elevated intracellular calcium levels lead to the activation of calcium-activated proteases which break down the contractile proteins resulting in necrosis and sterile inflammation. These cycles of degeneration and regeneration exhaust the regenerative potential of the muscle and result in fibrotic and adipose replacement of muscle tissue, which presents as progressive muscle weakness in patients 18.
2 Results and discussion
2.1 DMD animal models: intersecting data from a range of species
Animal models of DMD provide a valuable insight into the disease processes; many different animal models exist for DMD representing lower invertebrate animals such as nematodes through to small mammals such as the mouse and larger species including cats, dogs, and pigs. The most commonly studied DMD model is the mdx mouse that arose due to a spontaneous mutation resulting in a premature stop codon in exon 23 of the gene 19. The mouse is genetically comparable to patients however due to more extensive regenerative capacity, is phenotypically milder than the human counterpart having a near normal lifespan. The mdx mouse is the most widely used mouse model worldwide, including 32 proteomic studies providing an improved understanding of its pathophysiology. The mdx mouse does however have progressive muscle weakness and histopathology, especially using muscle from older mice, will show variation in muscle fiber size, internalized nuclei, increased connective tissue, and necrotic fibers which are all features also observed on biopsies from DMD patients (Fig. 2) 20. To create a murine model with a more severe and patient-comparable phenotype, a double knockout (dko) mouse model was created by crossing the dystrophin-deficient mouse with a utrophin-deficient mouse. Utrophin is a protein with close homology to dystrophin and is seen to be upregulated at the sarcolemma in the mdx mouse and therefore thought to have a compensatory role 21. The dko mouse has a more severe phenotype, more similar to patients but is more distant in terms of the underlying genetic defect. Using an unbiased proteomic approach to compare this model to the mdx mouse would further our understanding of the compensatory role utrophin has in mice. To date, no proteomic studies have been carried out on this model of DMD.

Another murine model being used to study dystrophin deficiency is the mdx-4cv mouse model that has a mutation in exon 52 of the DMD gene, whereas the mdx mouse has a mutation in exon 23. The mdx-4cv displays a lower proportion of dystrophin-positive revertant fibers, more in line with levels observed in patients with DMD, whereas the mdx mouse displays a higher proportion. This has been suggested to contribute to the milder phenotype observed in the mdx mouse compared to DMD patients. However, the phenotype and muscle pathology is indistinguishable between the mdx and mdx-4cv mouse models 22, 23.
Canine models for DMD have been widely used and dystrophin deficiency has been described in around 20 different breeds of dog. The golden retriever muscular dystrophy (GRMD) dog has been the most widely used and has a frameshift point mutation within the splice acceptor site in intron 6 of the dystrophin gene 24. The clinical course in these dogs is very similar to that of patients and they have a reduced life expectancy of around 3 years however there is great heterogeneity in the phenotypes of individual dogs with some being completely asymptomatic 25, 26. Recently, some asymptomatic GRMD dogs, termed “escaper dogs,” were utilized in a study 27 whereby they identified the Notch ligand Jagged 1 as being the protein responsible for amelioration of the phenotype by employment of molecular genetic methods.
Other less widely used dystrophin-deficient animal models include the hypertrophic feline muscular dystrophy cat and the dystrophin-deficient pig. The hypertrophic feline muscular dystrophy cat has a deletion in the muscle promoter of dystrophin and a phenotype fairly different from patients featuring a marked muscle hypertrophy throughout life and lack of fatty infiltration of the muscle and therefore is scarcely used to model the disease 28, 29. The dystrophin-deficient pig is one of the most recently described animal models for DMD and was established by deletion of exon 52 using a targeted approach through nuclear transfer. The pig has the advantage of being comparable to humans in terms of size, physiology, and anatomy and is a well-established biomedical model. The dystrophin-deficient pig mirrors the DMD phenotype well exhibiting a marked and progressive muscle weakness, similar muscle histology, high serum creatine kinase (CK) levels, and respiratory failure 30. Recently proteomic analysis of the dystrophin-deficient pig model has allowed further understanding of the pathophysiology in this model (31; see below).
Proteomic data derived from dystrophin-deficient murine, canine, and porcine tissues and blood was included alongside data derived from patient material (Supporting Information Tables 2 and 3). Studies dealing with MS-based proteomics of dystrophin-deficient tissue and/or body fluids were selected—these will from now on be referred to as gel-free proteomic studies, whereas those employing DIGE were excluded. Those studies that published lists of the significantly differentially expressed proteins were then split into those dealing with tissue (Supporting Information Table 2) and those dealing with biomarkers from blood and urine (Supporting Information Table 3). The incorporation of different datasets in this manner may produce more robust signals if data is reproducible across a range of different models. It also allows us to list signals which may be species specific when looking at animal models of diseases.
2.2 Proteomics in investigating DMD
There is currently only one directed therapy available which is applicable only to a small percentage of patients with a particular mutation type 32. Proteomics herein represented the method of choice to gain additional insights into the molecular basis of the disease as to date, 30 proteomic studies utilizing nongel-based approaches, and 16 different tissues have been published since 2011 (Supporting Information Table 1).
2.3 Dystrophin expression in different cell types
As discussed above, there is some discussion as to whether dystrophin is indeed expressed in lymphocyte cells. We wondered if protein cataloguing based on proteomics might allow us to address this question. Thus, we compared the expression levels of dystrophin obtained from in-depth proteome studies we conducted with different cell types, including lymphocytes, primary and immortalized myoblasts, and myotubes, and quantifying on average ∼8000–10 000 proteins. Our data indicate that dystrophin is present but at low expression levels in lymphocytes, whereas expression levels in myoblasts and myotubes are in the range of 1 and 2 orders of magnitude higher, respectively (Fig. 3).

2.4 Pathophysiology of skeletal muscle
Downstream of dystrophin deficiency, it is known that there are many secondary changes in skeletal muscle but unfortunately many of these are poorly understood and undoubtedly there are more to be discovered. Recently, many groups have employed proteomic approaches to unravel these complex downstream pathways using highly sensitive LC-MS workflows discovering pathophysiological cascades and opening new doors for therapeutic intervention and bringing proteomic findings to the bedside.
Fröhlichet al. 31 performed proteomic studies on the dystrophin-deficient DMD pig and found a marked reduction in respiratory chain proteins compared to a wild-type animal. Out of the 1428 quantified proteins, 3.7% were found to be significantly altered. These findings mirror reports of reduced energy metabolism proteins in the mdx mouse and GRMD dog and confirm previous biased findings of reduced mitochondrial metabolism in dystrophin deficiency 33-37. Other mitochondrial proteins were also found to be dysregulated in the DMD pig compared to the wild-type animals such as FIS1 (mitochondrial fission 1 protein), which was upregulated in the dystrophin-deficient animals suggesting mitochondrial function may play a major role in the pathophysiology of DMD. The proteomic profile of DMD pigs also demonstrated a shift from fast to slow muscle fiber type, a phenomenon also observed in patients presumably due to susceptibility of fast fiber types to degeneration 38. The make-up of different fiber types can be identified by proteomic methods allowing us to monitor changes in the fiber-type ratios during disease and treatment 39. Analysis of skeletal muscle from pigs aged 2 days compared to aged 3 months also revealed disease stage specific proteomic changes such as an upregulation of structural proteins at the early disease stage presumably as a compensatory mechanism. Changes in structural muscle proteins were also seen in a proteomic study of mdx-4cv hindlimb muscle 31, 40. Thus proteomics has allowed the identification of potential rescue factors that could be used in the future in the development of therapeutic interventions.
A study utilizing the gastrocnemius muscle of mdx and wild-type mice found elevated levels of stress-related chaperone proteins such as GRP78 34. This is potentially a very interesting finding as GRP78 (also known as BiP) levels have already been altered as therapeutic intervention in other diseases such as cancer and photoreceptor degeneration 41-43. In this study, around 9.3% of 789 detected proteins were significantly altered. Normal function of GRP78 is needed in the maintenance of muscle homeostasis and plays a key role in calcium storage meaning it is likely a relevant target in the context of this disease 44-46. They also reported the involvement of the integrin-linked kinase (ILK) pathway in the pathology, which has previously been reported to cause skeletal muscle pathology when silenced in mice and therefore elevating levels could have a therapeutic effect 47.
Proteomic analysis has also been used to confirm changes in levels of fibrosis-related proteins in the disease in the pig model, collagen types VI and XII were found to be upregulated 31. Other than in the pig, there is an apparent lack of dysregulation of ECM proteins reported in proteomic studies of skeletal muscle from dystrophin-deficient animals, which is surprising as fibrosis is thought to be one of the major disease mechanisms in DMD.
One of the main pathomechanisms of DMD is reported to be increased cytosolic calcium levels in the muscle cells. Proteomic studies carried out on animal models of dystrophinopathies have verified this. Calcium-binding protein S100a4 was significantly upregulated in a study comparing tibialis anterior muscle from mdx to WT mice 33 which found around 16% of 3272 total proteins detected to be altered. In GRMD dogs, proteomic analysis of vastus lateralis muscles revealed increased expression of calcium-handling proteins such as calcium/calmodulin-dependant protein kinase, chloride intracellular channel, and calreticulin 35. These studies provide proteomic evidence that modulating calcium levels of skeletal muscle should remain an important therapeutic target for DMD.
In the proteomic profile obtained from skeletal muscle of GRMD dogs, Guevel et al. 35 reveal that many of the proteins with reduced abundance in the proteome profile are regulated at the transcriptional level by proliferator-activated receptor-gamma co-activator 1 alpha (PGC-1α) and that the PGC-1α protein itself was present at reduced levels. PGC-1α is known to regulate mitochondrial biogenesis and oxidative metabolism in skeletal muscle, processes shown to be affected in dystrophic muscle 36, 48, 49. PGC-1α has been found to have the potential to upregulate expression of the dystrophin homolog utrophin and several studies have focused on increasing levels of PGC-1α in mdx skeletal muscle have seen an improvement after treatment 50-52. PGC-1α seems to be a promising target in the treatment of DMD and proteomic studies will assist in the identification of downstream targets that are affected in dystrophinopathies allowing monitoring of their levels in response to treatments.
2.5 Pathophysiology of the heart
Holland et al. 53 undertook a label-free proteomic study comparing the hearts of 7-week-old and 20-month-old mdx mice. They observed age-related alterations in proteins involved in bioenergetics, iron-binding, extracellular matrix organization, the cellular stress response, and cytoskeletal organization among others. Another insight from this study was the discovery of high levels of antibodies in the hearts, particularly the aged tissue, suggesting an autoimmune contribution to the cardiac pathology. Such an autoimmune response has previously described generally in cardiomyopathy but is a novel insight into dystrophin-associated cardiomyopathy 54. High levels of autoantibodies in cardiac tissue can be modulated by immunoadsorption therapy. This therapy is carried out in patients with dilated cardiomyopathy (DCM). One such case is reported whereby a young DMD patient with end stage heart failure was treated by immunoadsorption therapy and an improvement was observed. This report is limited to only one case and therefore more evidence is needed but the observation of increased antibodies in the proteomic findings of Holland et al. 53 suggest that such treatment could be a possible intervention to improve the cardiomyopathy of DMD patients 55-57.
An interesting observation was that two proteins involved in iron binding were upregulated in mdx hearts suggesting a potential iron overload in the DMD cardiac pathology 53. This is a potentially treatable pathology as pharmaceutical interventions have been developed to treat iron overload in other disorders 58, 59. Upregulation of GSH transferase was also noted in mdx hearts, this protein is of particular interest due to its roles in modulating calcium levels and is also involved in oxidative stress response, both of which are known to be pathogenic processes at play in the DMD heart.
As in dystrophic skeletal muscle changes in structural cytoskeletal and extracellular matrix proteins were observed in the heart, these changes were distinct from those seen to occur naturally in the ageing process in wild-type animals and therefore specific to the progression of the disease 53. Changes in metabolic and mitochondrial proteins were also observed in the heart as in the skeletal muscle once more suggestive of impaired oxidative phosphorylation in affected dystrophic muscle. Such changes have previously been described from the results of an earlier gel-based proteomic study of the mdx heart suggesting that addressing these disturbances might help to treat the pathology of the skeletal and cardiac muscle 60. These studies provide further evidence for the long established theory that mitochondrial dysfunction is an important pathogenic contributor in DMD.
Murphy et al. 61 utilized the mdx-4cv model of DMD, hearts were analyzed from 20-month-old animals by LC-MS/MS and similar to Holland et al. 53, they describe impaired mitochondrial metabolism, differences in calcium proteins, upregulation of extracellular matrix proteins, and a heightened cellular stress response. The number of significantly altered proteins they describe represents around 28% of 349 total proteins detected. They also highlight a protein with increased expression that plays a role in oxidative stress—glutathione peroxidase. Laminin decrease is also highlighted as a key pathophysiological feature in the heart—similar studies in skeletal muscles have seen no major changes in this protein of the basal lamina, as also highlighted by Holland et al. Mutations in laminin α2-chain are causative of a congenital muscular dystrophy in man and in mouse which can be associated with left ventricular dysfunction as is seen in cases of DMD. This illustrates the role that proteomics can play in molecularly linking the muscular dystrophies 62-64.
Perilipin-4 is a lipid-binding protein that has been identified in three different studies of dystrophin-deficient muscle, including the aged heart of mdx-4cv mice 61 (Supporting Information Table 2). Interestingly, a label-free proteomic study was carried out on patients with DCM (nondystrophic) who were undergoing immunoadsorption therapy to remove excess autoantibodies from the cardiomyopathic tissue. This is a routinely used therapy in patients with DCM as autoantibodies present in the cardiac tissue are known to be causative of the pathology. Some patients respond well to this therapy whereas others are nonresponders. Bhardwaj et al. 65 compared the proteomic profiles of cardiac tissue from both groups and one of the most significant findings was a less pronounced downregulation of perilipin-4 in responders to therapy. This finding could be used to predict whether DMD patients would be likely to respond to a similar therapy in treatment of their cardiomyopathy.
2.6 Pathophysiology of the diaphragm
The diaphragm is the most severely affected muscle in the mdx mouse and is the tissue which best models the human condition 66. For this reason, it is vital that there is a deep understanding of the pathophysiology of this muscle which can be achieved by studies of its proteomic signature.
Matsumura et al. 67 carried out a particularly informative study whereby the spared extraocular muscles and severely affected diaphragm of the mdx mouse were compared using a proteomic approach. Up to 7.2% of 857 total proteins identified were significantly altered. The study confirmed altered proteins seen in previous proteomic studies using gel-based approaches such as DIGE, but also described novel altered proteins such as galectin-1, annexin A5, serpin H1, and periostin. As expected, a comparison with the profiles of spared extraocular muscles revealed altered expression of ECM proteins in the diaphragm since there is no increased fibrosis in extraocular muscles. Altered expression of calcium homeostasis related proteins was also observed (calsequestrin 1, SERCA1, and SERCA2). This finding suggests improved calcium handling in the extraocular muscles and subsequent protection from calcium-induced proteolysis. The data provide evidence to Khurana et al.'s 68 claims that improved calcium handling in DMD extraocular muscles is protective of muscle degeneration. Targeting calcium dysregulation in DMD as a therapeutic approach has been under investigation for many years but results in patients have been disappointing 69. Better understanding of the exact nature of the calcium dysregulation through proteomic studies may help us design better targeted treatments with better outcomes. The main altered pathways were muscle regeneration, calcium handling, inflammation, and fibrosis, a similar pattern as seen in skeletal muscle proteomics. The particular vulnerability of the diaphragm to dystrophin deficiency in the mdx mouse is yet to be elucidated however further proteomic techniques being developed such as N-terminomics 70, 71 and the study of posttranslational modifications (i.e., phosphorylation) 72 are likely to help improve our understanding in the future.
A later proteomic study of the dystrophin-deficient diaphragm utilized the mdx-4cv mouse model and found one of the most increased proteins compared to wild-type animals was periostin, also described by Matsumura et al. 67 above, a matricellular protein with roles in collagen regulation and connective tissue properties. Expression of this protein is known to be induced following injury when it associates with various proteins such as growth factors, cytokines, and cell surface receptors promoting fibrosis. Fibrosis is a well-known pathological mechanism in the progression of DMD and contractile dysfunction of muscle, including the diaphragm, which ultimately leads to respiratory failure 73. Similar to proteomic profiles of skeletal muscle, increases in collagens were noted alongside other associated ECM proteins. Differential expression of cytoskeletal proteins was also confirmed, as seen in skeletal muscle and cardiac muscle suggesting a compensatory remodeling of the cytoskeleton in response to dystrophin deficiency.
2.7 Pathophysiology of the brain
As well as the effects on skeletal and cardiac muscle, dystrophin-deficiency also manifests in the brain. Among DMD patients, there is a higher incidence of cognitive defects, autism spectrum disorders, and behavioral problems. However, unlike the manifestations of the disease in the skeletal and cardiac muscle the symptoms of the brain in DMD are not of a progressive nature. It has been demonstrated that the dystrophin-deficient mdx mouse displays alterations in learning, behavior, fear response, and memory 74, 75. Glial fibrillary acidic protein was upregulated in the mdx-4cv brain in an unbiased proteomic investigation, which is of considerable interest as it is a well-established indicator of brain damage further evidencing brain involvement in DMD 76, 77.
A label-free proteomic study comparing mdx-4cv and wild-type brains was conducted by Murphy et al. 77. Overall 1.78% of 2572 proteins detected in the mdx-4cv brain were differentially expressed when compared to wild-type. Among those increased were serum glycoprotein hemopexin that binds free haem and serrotransferrin, which is responsible for iron transportation. Together, this suggests that there may be altered iron metabolism in the dystrophin-deficient brain, a process which is also suggested to be altered in the dystrophin-deficient heart and can be augmented with therapeutic intervention 53. Proteomic data allowed the identification of iron metabolism as a potentially treatable pathobiochemical hallmark. Antitrypsin was also among the proteins found to be increased in the mdx-4cv brain, owing to its protease inhibitor and anti-inflammatory functions an increased expression in the brain could be suggestive of a protective effort. Previous studies have demonstrated that pharmaceutical agents can be used to augment levels of antitrypsin in other diseases therefore this approach may also have benefits in DMD patients 78. Annexin A5 and vimentin were also upregulated in the brain, both proteins which have been identified in proteomic studies of dystrophin-deficient diaphragm and skeletal muscle, respectively 34, 67.
2.8 Key pathophysiological factors
Prompted by the question of which proteins play common key roles in the different tissues affected by DMD pathology, we extracted all proteins which have been identified with altered abundance in at least three independent proteomic studies investigating dystrophin-deficient animals or patients (studies using general gel-based analyses were not taken into consideration). This meta-analysis revealed that 31 proteins (derived from nine different studies) are predominantly affected by dystrophin-mutations among different tissues, patients and/or animal models (see Supporting Information Table 2 and Fig. 4). Notably, 11 of those proteins are involved in the actin cytoskeleton (contractile proteins), nine are involved in metabolic processes, four in protein folding, and two in Ca2+ homeostasis. Whereas this finding supports the assumption that—in accordance with the function of dystrophin—disturbed cytoskeleton is a major pathophysiological hallmark of the disorder, also protein folding seems to be strongly perturbed among different DMD vulnerable tissues. Figure 5 demonstrates the interactions of these proteins at the gene level. These observations represent an important aspect in the definition of a starting point for the development of further drugs as well as in the current therapeutic management of DMD patients. For example, BiP level can be well addressed therapeutically: prolonged UPR activation is known to initiate apoptosis and has already been linked to muscle fiber death in mdx 79 thus elevated BiP level in the different proteomic studies most likely corresponds to apoptotic events and an antagonization of UPR activation—by decreasing the level of BiP as a major UPR modulator 46—might reflect a promising therapeutic intervention concept in DMD. In this context, it is important to note that BiP antagonists such as OSU-03012, verrucosidin, and HKH40A have already been successfully tested 80-82.


Importantly, HSP90 chaperone is upregulated in hearts and diaphragm of different DMD mouse models (see Supporting Information Table 2). This represents a promising target protein of therapeutic intervention in DMD: it has been demonstrated that specific HSP90 inhibitors prevented autophagic cell death in myoblasts and myotubes of dystrophic muscles 83.
Periostin was also found to be upregulated in heart, diaphragm, and skeletal muscle of different dystrophin mouse models. As discussed above, this protein induces cell attachment and spreading and also plays a role in cell adhesion and enhances modulation of the fibronectin matrix of connective tissue. As periostin has recently been described as a significant contributor to myocardial fibrosis 84, modulation of elevated periostin level might reflect a meaningful strategy to antagonize fibrosis, as well-known pathological finding in DMD.
Additionally, the downregulation of muscle-specific glycogen phosphorylase, represents an interesting observation: autosomal recessive mutations of the corresponding gene are causative for McArdle disease, a metabolic disorder characterized by onset of exercise intolerance and muscle cramps in childhood or adolescence. Moreover, dysfunctional muscle and liver glycogen metabolism is a well-known pathological hallmark of DMD-vulnerable tissues 85. Thus modulation of altered abundances of proteins involved in glycogen metabolism such as glycogen phosphorylase obviously represents a promising intervention concept to antagonize breakdown of diverse types of muscle fibers affected by DMD pathology.
Interestingly, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), playing a role in glycolysis is also predominantly affected by deficiency of functional dystrophin among different tissues and animal models (see Supporting Information Table 2). This might be related to the additional role of this protein in modulation of actin cytoskeleton 86-88. However, GAPDH is often used as a loading control for immunoblot studies focusing on the pathophysiology of DMD. Consequently, results of our meta-analysis clearly indicate that GAPDH is not a proper control for normalization of biochemical findings achieved via immunoblot analyses (and most likely also transcript analyses) in the study of molecular DMD etiology. Proteomic analysis of protein expression across different cell types demonstrated that proteins such as COP9 Signalosome Subunit 5 (COPS5) which demonstrate relatively stable protein expression across different cell types may represent a more appropriate loading control (Fig. 3).
Perilipin-4, previously discussed in the context of the dystrophin-deficient heart, is represented in three independent proteomic studies of three different dystrophin-deficient tissues in different animal models. Perilipin-4 is member of a group of proteins, perilipins 1–5, which coat lipid droplets and play an important role in regulating lipid metabolism 89. Perilipin-4 is known to be expressed in brain, adipose cells, skeletal muscle, and heart tissue and is thought to be involved specifically with the early formation of lipid droplets 90. Pourteymour et al. 91 demonstrated that Perilipin-4 localizes to the muscle sarcolemma in humans and seems to be more highly expressed in type I oxidative slow-twitch muscle fibers.
2.9 Using body fluid based proteomics to discover novel biomarkers for DMD
Biomarkers are an invaluable tool as indicators for diagnosis and progression of disease; they can be obtained from body fluids such as urine and serum using minimally invasive techniques. There are well-established biomarkers used in the diagnosis of DMD. These are muscle enzymes such as CK which are found in the blood serum due to leakage from damaged muscles but levels vary due to a number of factors including race, age, exercise, and pharmacological compounds (Supporting Information Table 3). These biomarkers are not specific enough to be used alone and an invasive muscle biopsy is then required to confirm the diagnosis. In addition, there are few established biomarkers to assess the progression of the disease and these could be very useful when deciding on care and interventions for patients. Furthermore, there is a great need for a biomarker that could be used in clinical trials of DMD to assist in determination of efficacy 92. In recent years, proteomics has been used in efforts to tackle these issues by attempting identification of biomarkers in the serum and urine of patients which would allow a less invasive diagnosis and improved monitoring of disease progression 93.
Urine is seen as a valuable source of biomarkers as it can be obtained noninvasively in large quantities and has a relatively stable protein composition. In a study using urine from five DMD patients and five age-matched control patients, Rouillon et al. 94 used LC-MS/MS to identify proteins that were differentially expressed and found 2.9% of 1100 total proteins detected to be significantly altered. Titin was identified as the protein with the greatest fold-change between DMD and control patients. It is a structural protein and the largest in the human proteome, and mutations can be causative of muscle diseases such as limb girdle muscular dystrophy type 2J (LGMD2J). Titin fragments are found in the urine of patients with different muscular dystrophies, including DMD, when enzymes such as calpain and matrix metalloproteinases, which are found at increased levels, cleave titin into smaller fragments. In this study, N- and C-terminal titin fragments were detected alongside five other internal fragments. The level of the N-terminal titin sequence was found to have no linear correlation to CK levels in patient's urine and therefore presumably represents a different biological process. Similar titin fragments were detected in the urine of other muscular dystrophy patients (tibial muscular dystrophy (TMD), limb-girdle muscular dystrophy (LGMD) types 1D, 2D, and 2J) as well as the mdx mouse and GRMD dog models of DMD, suggesting that this could be a promising urine biomarker for muscular dystrophies as well as other diseases involving muscle degeneration. Titin was found to be differentially expressed in urine and serum of DMD patients as well as serum of mdx mice (Supporting Information Table 3). Based on the promising findings of this proteomics study, Maruyama et al. 95 and Robertson et al. (manuscript under review) have since developed ELISA-based assays capable of sensitive detection and quantitation of this N-terminal titin fragment in the urine of mdx mice, dystrophin-deficient rats, and DMD patients.
Other studies aimed at identifying novel biomarkers for DMD have focused on the blood as a source of protein. Fibronectin was identified as a potential serum biomarker after a proteomic study identified elevated levels in DMD patients compared to controls. It was also observed that plasma levels of the protein increased over time in DMD patients suggesting it has potential as a disease progression biomarker indicating levels of fibrosis 96.
A large-scale serum biomarker proteomic study quantified proteins from almost 100 DMD patients and over 50 age-matched healthy controls which identified 44 serum biomarkers associated with DMD. A total of 3.9% of 1125 total proteins detected in this study were found to be significantly altered. Leakage proteins associated with muscle damage, such as CK, were significantly higher in younger patients but levels decreased with age, presumably as muscle mass declined. Proteins associated with connective tissue remodeling were found to be decreased in DMD patients such as cadherin-5, jagged-1, and ADAM metallopeptidase domain 9 (ADAM9). Jagged-1 was recently identified as a disease gene modifier in the GRMD dog model whereby its overexpression was cited as being responsible for almost complete amelioration of the phenotype 27. Proteins involved in inflammation including phospholipase A2 and C-X-C motif chemokine 10 (CXCL10) were increased in DMD serum in agreement with the involvement of inflammation in the pathology of the disease. The study identified neurotrophic factor persephin as being increased and a member of the signaling pathway through which it acts, proto-oncogene tyrosine kinase receptor RET, being decreased in patients. These findings suggest ongoing innervation/denervation in patients and therefore these proteins could potentially be used as biomarkers for stabilizing innervation in DMD 97.
A targeted proteomic study was carried out on the serum of DMD patients and healthy controls alongside other muscle disease groups to look at levels of the negative skeletal muscle mass regulator, myostatin. It was found that levels of myostatin were significantly lower in DMD patients than controls and most other muscle disease groups. Myostatin levels in DMD patients also showed a significant positive correlation with respiratory muscle function (measured by forced vital capacity), serum CK levels, and functional muscle testing (measured by the North Star Ambulatory Assessment) 98. The data suggest that myostatin could prove to be a potential biomarker for disease progression in DMD and might be potentially useful in monitoring outcomes in clinical trials.
A targeted affinity proteomic approach was also employed by Ayoglu et al. 99 in the identification of biomarkers from serum and plasma of patients with DMD, the milder form of the disease, Becker muscular dystrophy and female carriers of the disease. Here, highly multiplexed antibody suspension bead arrays were used to profiles for 315 unique proteins using 345 samples of serum/plasma, captured proteins were then analyzed. The study identified myosin light chain 3 (MYL3) as being significantly increased in both serum and plasma of DMD patients in comparison with controls. This is in agreement with Matsumura et al. 67 who found increased levels in the affected diaphragm of mdx mice compared to the spared extraocular muscles. MYL3 has been reported to be among proteins secreted by mdx myotubes due to impaired secretion mechanisms 100. Carbonic anhydrase 3 (CA3) was significantly higher in DMD compared to both controls and Becker muscular dystrophy. This protein was more recently reported to be increased in DMD serum in a large-scale serum biomarker study in DMD 97.
3 Conclusion
It is clear that the absence of dystrophin has profound effects on the muscle proteome which is distinct between species, muscle, and individual. Comparison of proteomic studies using different techniques, species and muscles must be interpreted with much caution, taking into consideration methods and quality control, applied cutoffs, and quality of sample material, especially if tissue and body fluids are analyzed. However, here some tissue-specific differences are quite clear.
The meta-analysis of previously published proteomic studies utilizing dystrophin-deficient tissue has provided a valuable insight into proteins which are broadly involved in the pathophysiology of Duchenne providing us with a novel insight into the disease and novel therapeutic targets to explore such as GRP78, HSP90, and periostin (Supporting Information Table 2; [31,34,35,53,61,67,73,106,109]). Intersection of different datasets may provide us with more robust signal, particularly when reproducible across different animal models. In addition, the frequency at which GAPDH is differentially expressed in proteomic studies indicates that its use as a loading control is clearly not appropriate. On a more general note, the skeletal muscle seems to show most changes in the bioenergetics and mitochondrial-related changes whereas the diaphragm shows more pronounced changes in proteins related to fibrosis. In the heart, increased levels of antibodies—which have not been reported elsewhere—is a striking finding and is distinct from other tissues. These observations allow furthering of our understanding of the pathomechanisms of the disease and how loss of the same protein can result in such distinct phenotypes in different tissues.
Proteomic studies have been used to unveil novel and reliable biomarkers not only to monitor disease progression but also to be used as outcome measures in clinical trials. Proteomic studies have so far been successful in nominating several promising candidates however much characterization is needed before any are implemented in a clinical setting. These studies have also begun to identify biomarkers that could be used to monitor specific elements of the disease such as cardiac function (Supporting Information Table 3; [94,95,97,103]).
Acknowledgments
The authors would like to acknowledge financial support from Aktion benni & Co and Deutsche Gesellschaft für Muskelkranke (DGM). S. J. Carr PhD studentship is combined funding from the Medical Research Council (MRC) and the Barbour Foundation, UK.
The authors have declared no conflict of interest.




