Persistent tachypnea of infancy, neuroendocrine cell hyperplasia of infancy, and pulmonary interstitial glycogenosis: “A3-Specific conditions of undefined etiology”
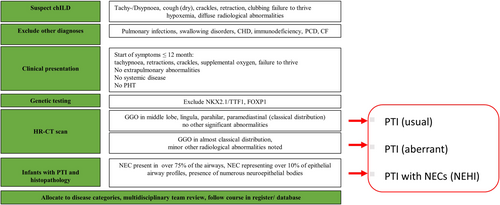
Interstitial lung diseases, also known as diffuse parenchymal lung diseases, encompass a large spectrum of conditions. Their prevalence increases with age.1 In childhood, all conditions are rare and several entities not observed in adults need to be considered. To consolidate research on these conditions among children and address their often overlooked status, the term childhood interstitial lung diseases (chILDs) was introduced.2 To learn more about these conditions and build clinical cohorts, continuous updating of their classification and disease definitions play an important role. A recent suggestion of an etiologic classification system differentiates four easy memorizable groups: conditions involving only the lung, systemic disease-related disorders (or conditions with a dominant lung parenchymal phenotype with extra-pulmonary manifestation), exposure-related disorders, and vascular disorders3 (Table 1). Within each of these groups, further differentiation is possible by the use of “specifiers” characterizing the subgroups, such as “lung developmental conditions” or “conditions occurring with immune deficiency state.” Of particular importance for pediatricians are the four “A-subgroups” that is, conditions primarily manifesting in infancy (“developmental”). Here we focus on one of them, the group A3-specific conditions of undefined etiology (Table 1). A3 conditions are among the most frequent group of chILD patients under the age of about 2 years, making up more than 34% of patients.4 This group comprises persistent tachypnea of infancy (PTI), pulmonary interstitial glycogenosis (PIG), and neuroendocrine cell hyperplasia of infancy (NEHI). PTI is further subdivided into PTI (usual) and PTI (aberrant).5, 6
| Lung-only (native parenchymal) disorders |
| Developmental (conditions manifesting in infancy) |
| A1—Diffuse developmental disorders (e.g., alveolar capillary dysplasia, acinar dysplasia, congenital alveolar dysplasia) |
| A2—Growth abnormalities with deficient alveolarisation (e.g., related to preterm birth, associated with diaphragmatic hernia, associated with oligohydramnion) |
| A3—Infant conditions of undefined etiology (e.g., PTI/NEHI, PIG) |
| A4—Related to alveolar surfactant region (e.g., CPI) |
| All ages |
| (e.g., NSIP, DIP, LIP, PAP, genetic surfactant protein deficiencies) |
| Systemic diseases-related disorders or dominant lung parenchymal phenotype with extrapulmonary manifestation |
| Immunocompetent (e.g., Lane–Hamilton syndrome, EGPA) |
| Immuno-deficient (e.g., Ataxia-teleangiectasia) or transplanted (e.g., CLAD) |
| Autoinflammatory (e.g., COPA syndrome, STING-associated vasculopathy, other interferonopathies) |
| Immunedysregulated (e.g., Hermansky–Pudlak syndrome type 2) |
| Exposure-related disorders |
| Noninfectious (e.g., hypersensitive pneumonitis, drug-induced interstitial lung disease, radiation pneumonitis) |
| Infectious (e.g., postinfectious constrictive bronchiolitis) |
| Vascular disorders |
| (e.g., DAH, pulmonary capillary hemangiomatosis, VOD) |
- Note: Adapted from Griese.3
- Abbreviations: CLAD, chronic lung allograph dysfunction; CPI, chronic pneumonitis of infancy; DAH, diffuse alveolar hemorrhage; DIP, desquamative interstitial pneumonia; EGPA, eosinophilic granulomatosis with polyangiitis; LIP, lymphocytic interstitial pneumonia; NEHI, neuroendocrine cell hyperplasia of infancy; NSIP, nonspecific interstitial pneumonia; PAP, pulmonary alveolar proteinosis; PIG, pulmonary interstitial glycogenosis; PTI, persistent tachypnea of infancy; VOD, vena occlusive disease.
In this commentary, we make a case for a precise diagnosis and consecutive correct labeling of these important conditions, the broad differential diagnosis necessary (including the recognition and description of extrapulmonary organs possibly involved in these patients), and provide a diagnostic algorithm to aid diagnosis.
1 HISTORY AND CASE DEFINITION OF PTI AND NEHI
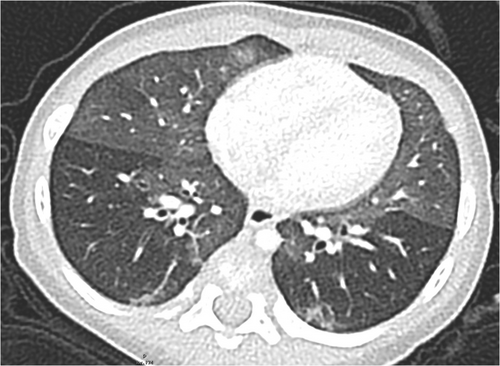
In 1997, Hull et al. described a chronic respiratory condition in eight infants characterized by tachypnoea, inspiratory crackles, and supplemental oxygen requirement. Biopsies taken from these children showed no or minimal changes (mild lymphoid infiltration of the bronchioles), no architectural disruption, no signs for fibrosis or significant inflammatory changes were seen. However, lung tissue was not analyzed for immunoreactivity. The condition was labeled “chronic idiopathic bronchiolitis of infancy.”7 The condition was clinically noted for several years as chronic bronchiolitis, with eight children investigated by percutaneous biopsy.8 Deterding et al. described later similar cases in more detail and labeled the condition “Persistent tachypnea of infancy” (PTI).9 Infants usually present in the first months of life with tachypnea, hypoxemia, and failure to thrive. Clinical examination reveals retractions, fine crackles, hyperinflation, and failure to thrive in the absence of nonpulmonary abnormalities. High-resolution computed tomography (HR-CT) scans of PTI show mainly ground-glass opacities (GGO) and only minor other abnormalities.10, 11 Depending on the distribution of GGO in the middle lobe, lingula, parahilar, and paramediastinal the disease is labeled as PTI (usual) or, if GGO are present in somewhat different distribution or minor abnormalities were present as PTI (aberrant).10, 12 Of note, the characteristic HR-CT pattern showing GGO distribution in the middle lobe, lingula, parahilar, and paramediastinal is not pathognomonic for PTI, but has been found in a variety of different respiratory conditions like FOXP1 haploinsufficiency,13 NKX2.1/TTF1 mutation,14, 15 or desquamative interstitial pneumonia.16, 17 An example is given in Figure 1. Thus, establishing the diagnosis of PTI (or NEHI) necessitates excluding imaging—mimickers by genetic analysis.
High-quality (HR-)CT scanning in infants necessitates great radiological expertise. This includes fast scanners, intelligent imaging settings to avoid noisy images, and frequently anesthesia in infants often breathing at rates above 50 times per minute, in particular, if in- and expiratory images are obtained. In this situation, lung ultrasound may be a potential option in the appropriate clinical setting. The seminal work of Urbankowska et al.18 demonstrated in a controlled prospective study that the total number of B-lines, the maximal number of B-lines in any intercostal space, the distance between B-lines, and pleural thickness were significantly increased in children with PTI compared to controls. An irregularity of the pleural line was found in all patients with PTI and in none of the healthy children. The close correlation of these findings to concurrent HR-CT scanning results pinned lung ultrasound down as a promising diagnostic tool in children with PTI.
Deterding et al.19 reviewed lung biopsies of patients with PTI and reported that the lung tissue had only few abnormalities. Immunoreactivity analyses for bombesin and serotonin, however, revealed an increased number of neuroendocrine cells (NECs). For patients with PTI and a lung biopsy showing this characteristic pattern, the authors introduced the term NEHI. Rauch et al.10 showed later that lung biopsies are only necessary in patients with contradictory clinical or radiological findings. Of note, not all subjects with typical clinical features and HR-CT abnormalities have increased NECs in their biopsies. No increased NECs have been reported previously in 12/3310 and 11/22 cases, respectively.20 In this situation the condition may be labeled as PTI usual or aberrant without increased NEC.
NECs are epithelial cells with paracrine functions. The cells are located in the distal airways and are involved in airway epithelial and mesenchymal cells proliferation as well as alveolar type II cell differentiation.21 NECs are physiologically present at high abundance during intrauterine growth but rapidly decline after birth.14, 22-24 Increased NECs in lung tissue have also been described in a number of respiratory diseases that need to be differentiated from PTI/NEHI. These pathological conditions include bronchopulmonary dysplasia,25, 26 sudden infant death syndrome,27, 28 pulmonary hypertension,29 and cystic fibrosis.21, 30 In adulthood, this cell type may expand and generate a heterogeneous group of malignancies including carcinoids, small-cell lung carcinoma, and large-cell neuroendocrine carcinoma.31
No correlation between the number of NECs in biopsy taken from lungs that appeared on HR-CT scans and affected areas have been found.22 A recent study reported no correlation between the clinical severity, radiological findings, and presence of an increased number NECs in lung tissue.20 Some authors argue therefore that NECs are bystanders of airway dysmaturity17, 32 without pathological importance for PTI.20 Thus, the description of the condition as NEHI may be a misnomer. Therefore, we prefer the term PTI until the relevant pathophysiology is identified.
The etiology of PTI/NEHI is currently unknown. A genetic susceptibility is suspected, but no disease-causing variant has been found yet.33 Pulmonary function tests on patients with PTI/NEHI may show irreversible airway obstruction.22, 34-37 A favorable clinical course has been published with the majority of patients being asymptomatic at school age.38 No standard therapies or guidelines are established and treatment is mostly supportive. A recent prospective observation in symptomatic children with PTI/NEHI, reported a potential benefit from the use of inhaled bronchodilators and corticosteroids on the clinical course and pulmonary function.37
2 HISTORY AND CASE DEFINITION OF PIG
PIG was first described in 200239 as the more comprehensive description of the histologically defined disease in infants referred to as “cellular interstitial pneumonitis.”40, 41 Another term initially used by pathologists was histiocytoid pneumonie.42 After birth, neonates later diagnosed as PIG, rapidly develop respiratory distress and hypoxemia requiring mechanical ventilation without signs for an infection.43, 44 HR-CT scans show a nonuniform pattern including GGO in various distributions, consolidations, mosaic attenuation, septal thickening, or emphysema.43 The diagnosis can only be made by a lung biopsy showing the expansion of the interstitium by lipofibroblasts containing periodic acid-Schiff positive diastase labile material consistent with glycogen.41, 45 PIG is unrelated to glycogen storage diseases as no abnormal glycogen deposition is to be found in any other organ.46 It is rather representing a developmental disorder of disrupted fibroblast differentiation.47 Cytoplasmic glycogen can be found in epithelial cells early during fetal lung development of primates.48 However, sometimes a diagnosis of glycogen storage disease may be made on the basis of a lung biopsy.43, 49 Thus, great care needs to be taken for the involvement of other organ systems in these infants. Increased numbers of NECs may occasionally be seen, making the categorization more complex.10, 38, 47 The clinical course of PIG is usually favorable, depending on additional abnormalities, with most patients being asymptomatic at the end of the first year.43 There is no known treatment except that there may be some role for systemic glucocorticosteroids, which have been reported to result in rapid improvement following administration.43
A majority of children with PIG cells in lung tissue have been reported with additional extrapulmonary malformation or underlying diseases and may thus be further subcategorized by these specifiers (Table 1). Mostly, patients were diagnosed with congenital heart defects,43 but also with pulmonary hypertension,50 metabolic disease,43, 49 primary ciliary dyskinesia,43 Trisomy 21,51 Noonan syndrome,52 congenital diaphragmatic hernia,53 TBX4 mutation,54 or Jacobsen syndrome.55 Although coincidental associations cannot be excluded this seems unlikely due to the multitude and spectrum of rare underlying disorders.43 PIG cells might represent impaired cell differentiation and an aberration in parenchymal lung development44 and not an entity itself.
As such, a case could be made to categorize some of these children under “Systemic diseases-related disorders,” for example, PIG as part of Noonan syndrome or glycogen storage disorders, or under “Dominant lung parenchymal phenotype with extrapulmonary manifestation,” for example, associated with congenital heart disease or hypertrophic cardiomyopathy. This again underscores the necessity for clinicians to adopt a broader perspective that extends beyond the lungs.
3 CONCLUSION
The underlying etiology and disease mechanisms for PTI/NEHI and PIG remain unknown. An increased number of NECs or the presence of PIG cells might generally indicate disrupted fetal lung development17 and there is an ongoing debate whether these conditions reflect a spectrum of specific developmental disorders.20, 47, 56, 57 Terminology and clinical characteristics for this group may be challenging for clinicians and researchers (Table 2, Figure 2). In everyday practise, we prefer the term PTI over NEHI to label the condition, as nowadays biopsies demonstrating NECs are rarely done, not all cases with PTI have increased NECs and a causal pathophysiological link between increased NECs and PTI has not been demonstrated, yet. Apart from that, currently, PIG is always a histological diagnosis. International collaboration is mandatory to better characterize early lung development and gain insight into the pathogenic mechanisms leading to disrupted lung maturation. Further research may help to develop strategies to treat and support our patients and caregivers. For clinical practice, it is imperative to avoid settling for a superficial diagnosis of PTI/NEHI or PIG and instead to search for associated and underlying conditions with the help of clinical expertise and genetic testing. Clinicians should be aware of the history of these conditions, the precise definition of the different terms introduced in the literature, and their correct usage. As these disorders are rare, for all cases the correct disease category allocation should be verified by a multidisciplinary team, and the clinical course be followed in an international database/register to learn more about the natural history, outcome, and potential treatments.4
| Persistent tachypnea of infancy | Pulmonary interstitial glycogenosis | |||
|---|---|---|---|---|
| PTI/NEHI | PIG | |||
| Clinical features | Tachypnea, hypoxemia, retractions, crackles, failure to thrive; usually no additional clinical features | Respiratory failure, PHT, often in association with underlying disease or extra-pulmonary abnormalities | ||
| Start of clinical symptoms | 1–9 months | First days of life | ||
| Genetics | Exclude conditions mimicking HR-CT pattern | |||
| HR-CTa | GGO in usual distribution, no other significant abnormalities | GGO in usual and additional distributions, some minor nonsignificant abnormalities | GGO in usual and additional distributions, some minor nonsignificant abnormalities | No uniform HR-CT pattern including GGO, consolidations, mosaic attenuation, septal thickening, emphysema |
| Histopathology | Not performed or no increased NECs | Not performed or no increased NECs | NEC found in at least 75% of total airway profiles NEC representing at least 10% of epithelial airway profiles Numerous neuroepithelial bodies |
Expansion of the interstitium by spindle-shaped cells containing PAS positive diastase labile material consistent with glycogen |
| Treatment options | Supplemental oxygen, potential benefit from the use of inhaled BD and ICS |
Supplemental oxygen, sometimes mechanical ventilation Potential benefit from the use of systemic glucocorticosteroids |
||
| Prognosis | Favorable clinical course suspected Longer follow-up time (>10 years) needed |
Depending on underlying disease or extrapulmonary abnormalities | ||
| Terminology | PTI (usual) | PTI (aberrant) | PTI (usual/aberrant) with increased NECs in biopsy (=NEHI) | PIG associated with Structural heart defect Other diseases (e.g., GSD) Other lung diseases (e.g., BO) No additional features |
| Obsolete terms | Chronic idiopathic bronchiolitis | Infantile cellular interstitial pneumonitis, histiocytoid pneumonia | ||
- Abbreviations: BD, bronchodilators; BO, bronchiolitis obliterans; CHD, congenital heart disease; GGO, ground glass opacities; GSD, glycogen storage disease; HR-CT, high-resolution computed tomography; ICS, inhaled glucocorticosteroids; NEC, neuroendocrine cells; NEHI, neuroendocrine cell hyperplasia of infancy; PAS, periodic acid-Schiff; PHT, pulmonary hypertension; PIG, pulmonary interstitial glycogenosis; PTI, persistent tachypnea of infancy.
- a GGO in usual distribution = GGO in middle lobe, lingula, parahilar, paramediastinal.
AUTHOR CONTRIBUTIONS
Matthias Griese: Supervision; writing—review and editing; project administration. Elias Seidl: Conceptualization; writing—review and editing; writing—original draft; methodology; data curation.
ACKNOWLEDGMENTS
Open access funding provided by Universitat Zurich.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data available on reasonable request from the authors.