Weaning strategies for children on home invasive mechanical ventilation
Abstract
Children who require home mechanical ventilation (HMV) with an artificial airway or invasive mechanical ventilation (HMV) have a possibility of successful weaning due to the potential of compensatory lung growth. Internationally accepted guidelines on how to wean from HMV in children is not available, we summarize the weaning strategies from the literature reviews combined with our 27-year experience in the Pediatric Home Respiratory Care program at the tertiary care center in Thailand. The readiness to wean is considered in patients with hemodynamic stability, having effective cough measured by maximal inspiratory pressure, requiring a fraction of inspired oxygen (FiO2) < 40%, positive end expiratory pressure <5 cmH2O, and acceptable arterial blood gases. The strategies of weaning is start weaning during the daytime while the child is awake and close monitoring is feasible. Disconnect time is gradually increased through naps and sleeping hours. Weaning from the conventional mechanical ventilator to Bilevel PAP or CPAP are optional. Factors affected the successful weaning are mainly the underlying diseases, complications, growth and development, caregivers, and resources. Weaning should be stopped during acute illness or increased work of breathing. The readiness for decannulation could be determined by using the speaking devices, tracheostomy capping, and measurement of end-expiratory pressure. Polysomnography and airway evaluation by bronchoscopy are recommended before decannulation. Weaning when the child is ready is crucial because living with HMV can be challenging and stressful. Failure to remove a tracheostomy when indicated can result in delayed speech, social problems as well as risk for infection.
Abbreviations
-
- CPAP
-
- continuous positive airway pressure
-
- HMV
-
- home mechanical ventilation of a tracheostomized patient with either a conventional ventilator or bilevel positive airway pressure (bilevel PAP) ventilation using a BiPAP™ or similar device
-
- PEEP
-
- positive end expiratory pressure
1 INTRODUCTION
Home mechanical ventilation (HMV) is indicated in conditions that relate to progressive respiratory failure or failure to wean from mechanical ventilation in such conditions as central nervous system (CNS) disorders, neuromuscular diseases, or chronic respiratory diseases due to either airway defects or immature lung growth.1-7
Weaning from mechanical ventilation means the process of decreasing ventilatory support so that the patients can breathe spontaneously and effectively without mechanical support. In general, challenges with weaning can be divided into airway/lung diseases, respiratory muscle weakness (pump failure), and neurological diseases.8 The primary need for home ventilation in children is a lung disease, whereas, in adults, it is an irreversible or progressive neuromuscular disease. Hence, children with lung disease historically have a higher chance of successful weaning than adults, in part due to the potential of compensatory lung growth in the younger lungs.9, 10 At birth, only approximately 15% of alveoli have formed, with the remaining subdivisions developing in the first few postnatal years.11 Most alveoli (85%) are added through multiplication after birth12 and lung size increases until about the age of 20−25 years.12 Therefore, children with respiratory failure due to lung disease during the infancy period are more likely to be weaned from mechanical ventilation with age. A systematic review of weaning from long-term invasive ventilation in children showed that the median proportion weaned was 17%, ranging from 0% to 75%.13 Considering only children with severe bronchopulmonary dysplasia (BPD), which immature alveolarization and pulmonary vasculatures are the main pathophysiology, the median age of weaning from HMV ranged from 24 to 27 months, whereas decannulation usually occurs by the age of 4 years.14, 15 On the other hand, weaning children with neurological disease depends largely on the reversibility of the underlying disease itself, which is frequently progressive, limiting weaning ability. In these cases, chest wall mechanics and respiratory muscle strength may be the determining factors in weaning (Table 1).
| Pathology | Diseases | Prognosis | Probability to wean off invasive HMV | |
| Chronic respiratory diseases | Airway | Laryngo-tracheo-bronchomalacia, TEF, vocal cord paralysis, subglottic stenosis, cystic hygroma | Improve with growth/treatment | High |
| Lungs | Severe BPD, lung hypoplasia Surfactant deficiency |
Improve with growth | High Partially wean |
|
| Respiratory muscle weakness (pump failure) | Diaphragm | Diaphragmatic paralysis | Improve with growth | High |
| Restrictive chest wall | scoliosis | Chance to improve after surgical correction | Moderate | |
| Neuromuscular disorder | Myasthenia gravis DMD, SMA |
Improve after treatment Progressive |
Moderate Low |
|
| Central nervous system | Reversible neurological condition | ADEM, GBS, encephalitis | Improve after treatment | High |
| Respiratory control | CCHS | Persistent | Partially wean | |
| Spinal cord | Poliomyelitis | Persistent | Partially wean |
- Abbreviations: ADEM, acute disseminated encephalomyelitis; BPD, bronchopulmonary dysplasia; CCHS, congenital central hypoventilation syndrome; DMD, Duchene muscular dystrophy; GBS, Guillain−Barre syndrome; SMA, spinal muscular atrophy.
Weaning children from HMV with an artificial airway (HMV) differs from the PICU setting in several ways. It is done at home where there is only minimal essential monitoring equipment. The weaning may take weeks, months, or years, and the timeline is very patient dependent because the pathophysiology of chronic respiratory failure is different from that of acute respiratory failure. Also, failure to wean may take a longer time to be recognized since the vital signs may be unchanged, but the patient may have some subtle signs of worsening, such as mood disturbance, failure to gain weight, reduction in active physical activity, acquisition of a lower respiratory tract infection, or atelectasis.
Since internationally accepted guidelines on how to wean from HMV in children are not available, and there are no strong comparison studies,16 we will summarize the weaning strategies from the literature combined with our own experience. We will start with the parameters indicating the readiness to wean, stepwise decreasing ventilatory support, when and how to implement noninvasive ventilation, and the parameters indicating the readiness to decannulate and how to decannulate. Then we will share our experience of weaning from HMV in our Thai children.
2 THE PARAMETERS INDICATING THE READINESS TO WEAN
The most important factor is the natural history of the underlying disease that led to the child requiring long term mechanical ventilation. If the nature of the disease is reversible or could be gradually improve such as BPD, laryngo/tracheomalacia, post-encephalitis, postoperative brain tumor removal, the child will have a greater chance to be weaned from mechanical ventilation. If the disease is progressive such as type 1 spinal muscular atrophy (SMA), or Duchene Muscular Dystrophy, it is rarely possible to wean the ventilator.
Some parameters used to assess readiness in a critical care setting17 can be applied to a home setting, including hemodynamic stability, lack of fever or infections, awake and alert or easily arousable, free of sedation, requiring a fraction of inspired oxygen (FiO2) ≤ 40% and positive end expiratory pressure (PEEP) ≤ 5 cmH2O, no anemia, acceptable arterial blood gases, ability to initiate inspiratory effort17 which can be measured by maximal inspiratory pressure or assessed indirectly by effective cough. Moreover, the child should have adequate weight gain and either appropriate or improvement in his developmental milestones. The spontaneous breathing pattern of the child can also be obtained from home caregivers' observations. Children tolerating some periods without ventilatory support well, such as during suctioning, changing the ventilator circuit, weighing, bathing, and brushing teeth, is helpful information in determining whether or not the child is ready to wean.
Spontaneous breathing trials are considered the best method to reassure the readiness to wean.18 This should be done first in a short period of time while the child is awake during the daytime under closed observation. If the child exhibits nasal flaring, increased heart rate, respiratory rate (RR), or respiratory effort, or if oxygen saturation as measured by pulse oximetry drops from the baseline condition, weaning should not be continued.19 We summarize the checklist of the parameters that indicate the readiness to wean in Table 2.
| Systems | Clinical status | Parameters | |
|---|---|---|---|
| Neurology | ☑ | Awake, alert, easily arousable, free of sedation | |
| Cardiovascular | ☑ | Hemodynamic stability | Normal BP & PR for age |
| Respiratory | ☑ | Spontaneous breathing trial → no increased work of breathing | Normal RR for age requiring PEEP ≤ 5 cmH2O |
| ☑ | Adequate oxygenation | SpO2 ≥ 95% in room air or Requiring FiO2 ≤ 0.4 | |
| ☑ | Adequate ventilation | EtCO2 or PaCO2 45−50 mmHg | |
| ☑ | Effective cough | MIP (if available), CXR—no atelectasis | |
| ☑ | Ability to initiate respiratory effort; respiratory muscle strength | NIF > −30 to −60 cmH2O | |
| GI/nutrition | ☑ | Adequate weight gain | |
| Fluid & Electrolytes | ☑ | Normal electrolytes | Serum HCO3 < 27 mMol/L |
| Infection | ☑ | No frequent infection | No hospitalization within 3−6 months |
| Hematology | ☑ | No anemia, adequate oxygen content | Hb ≥12 g/dL |
- Abbreviations: BP, blood pressure; CXR, chest X-ray; EtCO2, end tidal carbon dioxide; FiO2, fractional inspiration of oxygen; GI, gastrointestinal system; Hb, hemoglobin; HCO3, serum bicarbonate; MIP, maximal inspiratory pressure; NIF, negative inspiratory force; PaCO2, partial pressure of carbon dioxide; PEEP, positive end expiratory pressure; RR, respiratory rate; SpO2, peripheral saturation of oxygen.
3 STEPWISE DECREASING VENTILATORY SUPPORT
After the child fulfills the criteria of readiness to wean as described above, the family should be informed and agree to participate in the weaning process. The family is trained at the bedside at an outpatient visit on how to decrease ventilatory support and observe signs and symptoms of weaning failure, such as nasal flaring, tachycardia, tachypnea, desaturation, and cyanosis. Very few patients were admitted to the hospital specifically for weaning.20 Various modes of mechanical ventilation were reported to be used, including pressure support and synchronized intermittent mandatory ventilation (SIMV),20 in which the pressure support level and the number of mechanical breaths is gradually reduced, respectively. We propose the algorithm of stepwise weaning from invasive HMV, as shown in Figure 1. In our practice which is similar to the common practice in the United States of America,19 we prefer to begin with complete disconnection from the ventilator for half an hour once or twice a day while the child is awake. If the child tolerates the periods of disconnection, these are gradually lengthening and increased in the frequency. This technique has some benefits over the technique of decreasing pressure support and the number of SIMV breaths, as the children will be free to do any personal activities during the period of disconnection and return to the full ventilatory support of the old settings once they feel tired. The period of disconnection then will be lengthening until the children can breathe independently all through the waking hours. We suggest to start with daytime weaning. Physiologically, the respiratory drive is less with sleep leading to a lower RR. Intercostal muscle activity is reduced during the rapid eye movement sleep results in lower tidal volume (TV). Hence the minute ventilation (RR x TV) during sleep is lower than when awake; therefore, the need for respiratory support is usually higher at night. Moreover, daytime weaning is beneficial in terms of closer monitoring if labored breathing occurs. Disconnect time is subsequently increased through naps and part of sleeping hours and finally all through the whole night sleep. Some children need only nocturnal mechanical ventilatory support and can attend school during the daytime. If the patient still needs supplemental oxygen during disconnecting time, a trach collar or speaking device will be used to administer oxygen. Gradual weaning from invasive to noninvasive ventilation could be helpful in some patients. The candidates of noninvasive positive pressure ventilation (NIPPV) such as bilevel positive airway pressure (bilevel PAP) and CPAP includes the older children in whom the underlying diseases have been improving,8 only nighttime ventilator support is requirement, having effective cough, adequate secretion clearance without the need for suctioning via tracheostomy, good airway patency, and cooperative. Polysomnography (PSG), which is recommended and available in most of the tertiary care centers, is useful as a precise tool for weaning from HMV to achieve the appropriate setting of NIPPV.8, 16 The pressure difference between inspiratory PAP (iPAP) and expiratory PAP (ePAP) determines the delivered TV, while the ePAP acts as an endotracheal tube to maintain the airway patency. We previously reported the cases of weaning from HMV to NIPPV in our patients with congenital central hypoventilation syndrome (CCHS) and SMA since 2015.4 A retrospective review in 2022 reported three patients with CCHS who had successful weaning to NIPPV.21 The high prevalence of changes in the HMV setting has been previously reported; titration with PSG is recommended.22 However, PSG may not be available in some centers.21
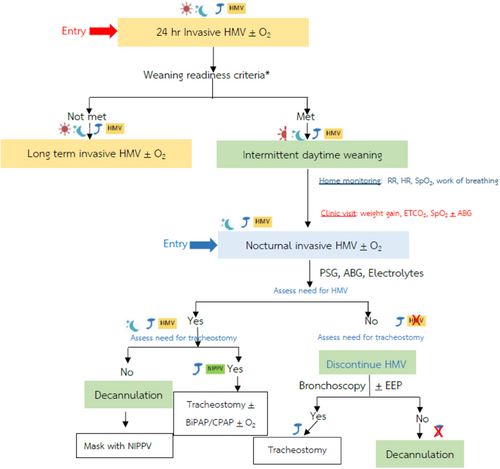
 Daytime;
Daytime; 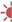 intermittent daytime;
intermittent daytime;  nighttime;
nighttime;  tracheostomy;
tracheostomy;  home mechanical ventilator;
home mechanical ventilator;  noninvasive positive pressure ventilation. ABG, arterial blood gases; EEP, end expiratory pressure; EtCO2, end tidal carbon dioxide; HR, heart rate; PSG, polysomnography; RR, respiratory rate; SpO2, peripheral saturation of oxygen.
noninvasive positive pressure ventilation. ABG, arterial blood gases; EEP, end expiratory pressure; EtCO2, end tidal carbon dioxide; HR, heart rate; PSG, polysomnography; RR, respiratory rate; SpO2, peripheral saturation of oxygen.As mentioned above, the weaning from HMV can be performed slowly at home under the supervision of the respiratory home care team, including physicians and respiratory nurses. During the weaning period, parents were requested to randomly monitor and record RR, pulse rate, oxygen saturation as well as the work of breathing during the child's various activities such as crying, sleeping, and feeding or exercise if possible. Changes from baseline, such as >20% increase in pulse rate along with signs of increased breathing effort, indicate weaning failure. For some children, supplemental oxygen is administered if hypoxemia is the only reason for weaning failure, especially if the underlying pathophysiology justifies that approach, such as BPD.
In the limited resource situation with frequent unavailable of PSG, there are some innovative devices to monitor oxygen saturation and other parameters that are more feasible to perform at home, showing trending in a graph format. The parents can share the data with the home care team on-site at the respiratory clinic or online. Also, we asked the parents to record video clips of their children to see the breathing pattern during the weaning period, especially during sleep. Regular clinical follow-up or telemedicine for further discussion and plans for the next step of weaning are recommended.
Weaning procedures should be stopped during acute illness or if there is unexpected deterioration in the underlying condition or other comorbidities. Weaning failure should include mood disturbance, failure to gain weight, reduction in active physical activity, and acquisition of lower respiratory tract infection or atelectasis. For example, our patient with surfactant deficiency used a dual mode of ventilator (daytime CPAP and nighttime ventilation) for a period of time, then stepped down to 24 h CPAP. To increase mobilization and socialization, the parents preferred an oxygen collar mask of 3 L/min instead of CPAP, as we recommended. In this case, PSG demonstrated that the child was stable without a positive pressure requirement. However, without CPAP, the child had frequent respiratory tract infections and subsequently atelectasis indicating weaning failure (Figure 2). After discussion with the rationale of using positive pressure that aligns with the pathogenesis of surfactant deficiency, with parental agreement, the child was reconnected to CPAP. The atelectasis and respiratory tract infections were diminished.
4 FACTORS AFFECTED THE SUCCESSFUL WEANING
Most of the patients in whom liberation was possible were weaned from ventilation within the first 5 years after initiation.8 The attributing factors are the underlying diseases, growth and development, effective cough, modes of ventilation, complications such as granulation tissue, infections, swallowing function, caregivers, and resources. Usually, BPD was the most likely group of patients with the favorable outcomes of 57% weaning in our study. A retrospective cohort study of children with BPD requiring IHMV in New Zealand during 2005−2021 revealed that half of the patients were weaned off HMV by 36 months and 90% by 52 months once home. This was longer in the patients with higher discharge age corrected for tracheostomy (DACT; chronological age at discharge minus age at tracheostomy).23
The chance of weaning from ventilation in patients with neurological disorders is far less than the patients with chronic lung disease of infancy. A 20-year review (1991−2011) from the Canadian tertiary care center reported that of 318 children on long-term HMV, most of the patients had neurological diseases. Nine percent (6 of 66) of children who were invasively ventilated via a tracheostomy had successful weaning.24 A retrospective study in Rome (2000−2017) demonstrated 432 children of long-term ventilation, 117 (27%) were invasively ventilated. Over time, 6 of 117 (5%) improved and discontinued HMV.25 A 10-year review from Ireland (2009−2018) reported that 46 children, who were commenced on long-term HMV, are being weaned and decannulated more successful in the latter period (2014−2018).26 The data from Korea gathered from 2016 to 2018 reported that 5 of 43 children (12%) were weaned from HMV. The majority of those cases had neurological disorders (CNS disorders 68% and neuromuscular diseases 13%).27 In Taiwan, 5 of 19 (26%) patients with enterovirus (EV71) encephalitis have successful weaning from ventilatory assistance from 24 h a day to nocturnal (n = 2) or episodic ventilation (n = 3).28 In our study, 12 of 47 (25%) with neurological diseases were successful weaned from invasive HMV.
5 DECANNULATION
When the etiology of tracheostomy and HMV improves, and the child has successfully weaned from HMV, decannulation is considered. The patency of the airway is usually determined by tracheostomy capping and bronchoscopy. The plan for HMV weaning time frame and decannulation should be communicated with the otolaryngologist in a timely manner. There are no clear and well-studied protocols not just for weaning but also for decannulation.
During the routine visit to the Pediatric Respiratory Clinic, speaking devices or tracheostomy caps have been using to evaluate the patency of the upper airway, thus indicating the readiness for decannulation. A previous study revealed that children who did not tolerate the use of the speaking valve or tracheostomy capping had an increased likelihood of needing laryngotracheal reconstruction compared to those who tolerated both.20
Measurement of end-expiratory pressure (EEP) at the tracheostomy tube by using the pressure gauge (Figure 3) when the one-way speaking valve is in place is another alternative and noninvasive way to assess the patency of the upper airway proximal to the tracheostomy tube.29 At our institute, we routinely measure the EEP with every clinic visit; if the EEP is higher than 6 cmH2O, it is less likely to have successful decannulation. This leads to an ENT consultation. The measurement of EEP is just an option for screening purpose. The definite diagnosis of upper airway obstruction is primarily based on a standard bronchoscopy (Figure 1).

The airway evaluations by flexible bronchoscopy performed by either the pediatric pulmonologist or otolaryngologist is crucial. Endoscopic evaluation of airway anatomy before decannulation is recommended by the American Academy of Otolaryngology-Head and Neck Surgery guidelines to confirm adequate airway patency and to exclude or manage the granulation tissue. Reconstructive laryngeal surgery before decannulation may be required. Henningfeld et al. reported that before decannulation, 65% of children required an airway surgery such as adenotonsillectomy, granuloma removal, or laryngotracheal reconstruction.20
In a retrospective data from 2001 to 2011, 426 children in the pediatric intensive care unit underwent tracheostomies, demonstrated that 163 patients (38%) were decannulated during the study period. The better prognosis in terms of the likelihood of decannulation was found in patients without congenital neurological disorders.5 Liptzin et al. proposed a protocol for decannulation such that the patients stay in the hospital for two nights, pre- and post-decannulation. During the nights, cardiorespiratory were continuously monitored along with end tidal carbon dioxide (ETCO2), followed by blood gas analysis and serum electrolytes in the morning.30
Decannulation attempts may have to be performed several times in some patients. Cristea et al. reported that approximately 80% of decannulation attempts were successful. The authors also recommended that the safe weaning protocol could be done under PSG.31 A systematic review of 24 studies, including 1395 children, to identify the role of PSG in decannulation demonstrated that apnea-hypopnea index ≤10, obstructive index ≤5, and absence of hypoventilation defined as ETCO2 > 50 mmHg less than 25% of total sleep time are good predictors of successful decannulation.32 A systematic review of decannulation protocols in 2021 revealed the large variability of pediatric decannulation protocols. Tracheostomy tube modifications include capping, downsizing, and fenestrations vary among the centers worldwide. PSG is recommended in most of the protocols. Pulse oximetry monitoring is frequently used, whereas blood gas analysis and capnography are less likely.33
Failure to remove a tracheostomy, when indicated, can result in delayed speech, social problems, as well as risk for infection. Death is another complication of delayed tracheal decannulation, secondary to airway obstruction from a plugged or partially displaced tube. Low socioeconomic status was found as a contributing factor to delayed decannulation in children with tracheostomies in one study.34
6 OUR EXPERIENCE ON HMV (TABLE 3)
| Number of patients and diseases | |||
|---|---|---|---|
| Total patients in the respiratory home care program | 324 | ||
| Invasive HMV (tracheostomy + mechanical ventilator) | 87 | 20 Conventional MV | |
| 42 Bilevel PAP | |||
| 25 CPAP | |||
| Discontinue HMV | 27 | ||
| Successful weaning | Decannulation | 14 | 4 Diaphragmatic paralysis |
| 3 Laryngomalacia | |||
| 2 Congenital heart diseases | |||
| 1 Bronchopulmonary dysplasia (BPD) | |||
| 1 Myasthenia gravis | |||
| 1 Acute disseminated encephalomyelitis (ADEM) | |||
| 1 Guillain−Barre syndrome (GBS) | |||
| 1 Encephalitis | |||
| Partial weaning | Decannulation to mask with NIPPV | 3 | 2 Congenital central hypoventilation syndrome (CCHS) |
| 1 Poliomyelitis (died at 21.5Y) | |||
| Wean to nocturnal invasive HMV | 3 | 1 ABCA-3 surfactant deficiency | |
| 1 Severe BPD with multiple complications | |||
| 1 Spinal cord injury | |||
- Abbreviations: HMV, home mechanical ventilation; NIPPV, noninvasive positive pressure ventilation.
Established in 1995, our Pediatric Home Care program at the Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand, provided home respiratory support for 324 children over a 27-year period. Eighty-seven patients underwent tracheostomy and required HMV; of these, 20 (23%) used mechanical ventilators while 42 (48%) and 25 (29%) were supported by Bilevel PAP and CPAP via tracheostomy tube, respectively. At our institute, Bilevel PAP or similar device and CPAP are being use instead of purpose designed mechanical ventilators in some selective cases because it is a less expensive option.35-37 The most frequent (54%) underlying diseases causing chronic respiratory failure requiring HMV in our patients were neurological disorders (35 cases have CNS diseases and 12 cases suffers from neuromuscular disorders) such as cerebral palsy, encephalitis, poliomyelitis, and SMA, followed by the airway defects (22 cases, 25%) such as tracheobronchomalacia, bronchial stenosis, and subglottic stenosis. Seven patients have BPD, four have cardiovascular anomalies, and four patients were diagnosed with CCHS. The rest of the patients have multiple anomalies mostly associated with some genetic disorders. The mortality rate was 31% (27 of 87) throughout the study period.
7 OUTCOMES OF SUCCESSFUL WEANING (FIGURE 4 AND TABLE 3)
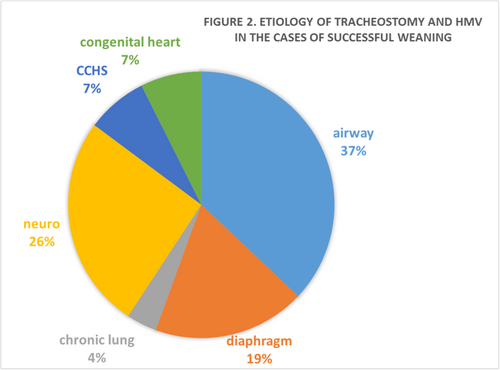
From our experience, 27 patients (31%) have successful weaning from HMV and 63% of these patients were further decannulated. The indications of tracheostomy and HMV among the cases of successful weaning are as shown in Figure 1. Of 27 patients, 10 cases (37%) had airway malacia, which could successfully wean off HMV and decannulated by the median age of 5 years old, similar to a case of severe BPD. Two of our patients with CCHS were successfully decannulated and weaned off HMV to nighttime Bilevel PAP via face mask4 when they reached 6 and 9 years old, respectively, while another two cases of CCHS still need invasive HMV. One of the patient with poliomyelitis, who had been on long-term invasive HMV then being decannulated and partially weaned to Bilevel PAP, died at the age of 21.5 years old.
In a resource-poor country like Thailand, lacking of financial support, unavailability of home care nurses and respiratory therapists, and limited number of pediatric pulmonologists and sleep physicians, we had to adapt our practice. Multidisciplinary teams are the key of success in HMV weaning which includes the caregivers, pediatric pulmonologist, sleep physician, otolaryngologist, respiratory nurses, ambulatory nurse, nutritionist, physiotherapist, occupational therapist, and speech therapist.
8 SPECIAL CONSIDERATION
Disadvantages of HMV include the limitation of patients' development and social participation,38 sleep disturbance of caregivers due to frequent alarms which are set for the safety concerns. While living at home is more “normal” than in a healthcare facility, living with HMV can be challenging and frustrating and due to major caregiver demands and uncertainty. The systematic review found that some of the caregivers felt forced to accept HMV, which may have led to some depression.39-46 It has been found that living at home could enhance the recovery in some diseases. Therefore weaning once the child is ready is important. There are some clinical practice guidelines on initiation of pediatric chronic home invasive ventilation,47 but standard guidelines for weaning are lacking. Healthcare disparities exist, especially in the low income and limited resource countries. We hope our experiences could be practically applied with minimized essential resources based on patients' safety and economical aspect.
AUTHOR CONTRIBUTIONS
Harutai Kamalaporn: conceptualization—equal, data curation—lead, project administration—lead, writing original draft—equal, writing review/editing—equal. Aroonwan Preutthipan: conceptualization—lead, resources—lead, data curation—supporting, supervision—lead, writing original draft—equal, writing review/editing—supporting. Allan L. Coates: conceptualization—equal, supervision—supporting, writing review/editing—equal.
ACKNOWLEDGMENTS
The authors would like to thank all the patients, their families, our faculty staff, respiratory nurses, and pediatric pulmonology fellows for their dedication and support throughout the long journey of our Pediatric Home Respiratory Care Program at Ramathibodi Hospital, Mahidol University, Bangkok, Thailand.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy or ethical restrictions.




