Six-year experience with treatment of early donor-specific anti-HLA antibodies in pediatric lung transplantation using a human immunoglobulin-based protocol
Nicolaus Schwerk and Gregor Warnecke share senior authorship.
The results of this study were in part presented as an oral presentation at the ISHLT 39th Annual Meeting 2019, Orlando, Florida.
Abstract
Objectives
Experience with the treatment of early donor-specific anti-HLA antibodies (eDSA) after lung transplantation in children is very limited. At our institution, we have treated patients with eDSA since 2013 with successive infusions of intravenous human immunoglobulins (IVIG), combined in some cases with a single dose of Rituximab and plasmapheresis (therapeutic plasma exchange [tPE]) or immunoabsorption. The aim of this study was to present the 6-year results of IVIG-based therapy in pediatric lung recipients.
Methods
Records of pediatric (<18 years old) patients transplanted at our institution between 01/2013 and 03/2019 were reviewed. Outcomes were compared between patients with eDSA treated with IVIG (IVIG group) and without eDSA (control group). Median (interquartile range [IQR]) follow-up amounted to 28 (12-52) months.
Results
During the study period, 66 lung-transplanted pediatric patients were included, of which 27 (41%) formed the IVIG group and 38 (57%) the control group. Among the IVIG patients, 14 (52%) patients showed concomitant graft dysfunction (possible clinical antibody-mediated rejection). The median time to eDSA detection was 24 (14-63) days after transplantation. eDSA were cleared in 25 (96%) of the 26 patients which completed treatment. At 3 years, graft survival (%) was 73 vs 85 (P = .65); freedom (%) from chronic lung allograft rejection (CLAD) was 89 vs 78 (P = .82); and from infection 47 vs 31 (P = .15), in IVIG vs control patients, respectively.
Conclusions
After lung transplantation, an IVIG-based treatment for eDSA yielded high eDSA clearance. IVIG and control patients showed similar CLAD-free and graft survival.
1 INTRODUCTION
The detection of early anti-HLA donor-specific antibodies (eDSA) after lung transplantation has been associated with poorer graft survival, antibody-mediated rejection (AMR), and later development of chronic lung allograft dysfunction (CLAD).1-11 Protocols have been developed to treat eDSA and suspected AMR, but the available case series are limited to nonrandomized, usually retrospective, studies.12-19 At our Institution, since 2013, a protocol based on successive infusions of intravenous human immunoglobulins (IVIG) has shown good eDSA clearance rates and similar outcomes in patients with vs without eDSA.16, 19
Most of the studies on eDSA either included only adult patients or did not present the results separately for adult and pediatric (<18 years old) patients.1-7, 9, 10 Thus, the experience on eDSA and their treatment in pediatric lung-transplanted patients is very limited,14, 20 and usually extrapolated from the results of adult case series. However, the results obtained in adult patients might not be simply transferred to pediatric patients, due to the different transplant indications and immunologic backgrounds.
In this study, in comparison to our previous publications,15, 16, 19 we focused only on pediatric lung-transplanted patients and compared outcomes between pediatric patients who showed eDSA after transplantation and were treated, and pediatric patients who did not develop eDSA.
2 MATERIALS AND METHODS
2.1 Patients
The in-hospital and outpatient clinic records of pediatric (<18 years old) patients who underwent lung transplantation at our institution between January 2013 and March 2019 were retrospectively reviewed.
Patients who showed eDSA after transplantation and were treated with IVIG formed the eDSA+/IVIG+ group (case group). Patients who did not show eDSA after transplantation (eDSA− patients) formed the control group. Follow-up ended on 1 March 2019 and was 100% complete.
The hospital ethical review board waived the need of patient's consent to the study, since all patients and their parents had given consent to handle their personal data for research purposes at the time of listing to lung transplantation. Consent to eDSA treatment and to eDSA control after treatment end was not required, since treatment and eDSA monitoring are a routine procedure in the management of pediatric patients after lung transplantation at our institution. This study conforms to the declaration of Helsinki.
2.2 Variable definition
Early DSA (eDSA) were defined as either preformed or de novo DSA, which were detected during the first weeks after lung transplantation. eDSA clearance was defined as the absence of DSA in two consecutive Luminex-based SPA controls (LIFECODES; Immucor Transplant Diagnostics, Inc, Stamford, CT) after treatment initiation. DSA recurrence was defined as a renewed positivity of previously cleared DSA.19
Graft survival was defined as freedom from mortality or retransplantation. Pulsed-steroid therapy-free survival was defined as freedom from steroid therapy for presumed acute rejection, that occurred at follow-up after the initial hospitalization for lung transplantation. CLAD was defined as an irreversible and persistent (lasting for 3 weeks) decline in graft function (FEV1 <80% of baseline), after exclusion of other reversible reasons explaining the fall of lung function tests.21 Finally, infection occurring at follow-up was defined as a bacterial, fungal or viral infection that required hospitalization for treatment.
2.3 Patient management
At our institution, pediatric patients did not receive any induction therapy. Posttransplant immunosuppressive therapy was based on a triple therapy: tacrolimus (initial through levels between 12 and 15 µg/mL); mycophenolate mofetil (through levels between 1.2 and 3.5 µg/mL), that was later switched to everolimus (through levels of 3-5 µg/mL) in some patients; and prednisolone.
Infants, who received ABO-incompatible transplantation, underwent peritransplant sessions of therapeutic plasma exchange (tPE), repeated according to anti-A and anti-B antibody titers.
Contrarily to adult lung-transplanted patients,15, 16, 19 a surveillance protocol with transbronchial biopsies at 1, 3, and 6 months and 1 year after pediatric lung transplantation has only recently been introduced at our institution. Before 2018, only older children (>11 years old) underwent transbronchial biopsies, usually upon indication. Thus, acute rejection in most cases was a clinical diagnosis lacking histopathologic reconfirmation, and it was defined as a worsening of arterial oxygenation and/or lung function tests after the exclusion of other possible causes of graft dysfunction.
At our institution, all the blood products (packed red blood cells, platelet concentrates, and fresh frozen plasma) were leukocyte depleted.
2.4 eDSA detection protocol
All patients were screened for DSA, immediately before lung transplantation, on Day 14 after lung transplantation, before hospital discharge, and at least every 3 months during follow-up as well as upon indication. In the Luminex analysis, a threshold of 1000 median fluorescence intensity (MFI) was used to detect eDSA. In eDSA+/IVIG+ patients, Luminex-based DSA controls were additionally performed immediately before each IVIG treatment session and every 6 months after treatment end.19
In allosensitized recipients, the decision to proceed with transplantation was based on the results of the virtual crossmatch. In addition, a retrospective lymphocyte cytotoxicity test (LCT) crossmatch was performed in all patients immediately after transplantation.
Only in January 2019, we began to measure the eDSA complement-binding capacity in pediatric and adult patients, using the C3d Luminex Test (LIFECODES; Immucor Transplant Diagnostics, Inc).
2.5 eDSA treatment protocol
In 2013, an IVIG-based treatment protocol replaced the previous eDSA treatment protocol which had been based on tPE and a single dose of anti-CD 20 antibody (Rituximab).15 Although different IVIG (Pentaglobin; Biotest AG, Dreieich Germany; Kiovig; Baxter AG, Vienna, Austria; and Privigen; CSL Behring GmbH, Marburg, Germany) were employed, IgA− and IgM− enriched human immunoglobulins (Pentaglobin; IgGAM) were used in the majority of patients, due to the additional immunomodulatory and antimicrobial effects conferred by the IgA and IgM components.19, 22 Therefore, in early 2014, IgGAM became the preferred IVIG preparate for treating eDSA in pediatric lung-transplanted patients at our institution.
Treatment was performed pre-emptively in pediatric patients with serologic evidence of eDSA (possible subclinical AMR8). Patients, who showed eDSA and also graft dysfunction due to presumed acute rejection, were defined as having possible clinical AMR.8 Diagnosis of definite clinical AMR was usually not made, because, as aforementioned, transbronchial biopsies were not routinely performed, especially in younger (<11 years old) patients. Moreover, in comparison to heart and kidney transplantation, the available pathologic criteria for defining AMR in lung transplantation are still unspecific and not well-defined.8 While waiting for more specific pathologic criteria, we decided to base our treatment decision only on the presence of eDSA and graft dysfunction.
The eDSA treatment protocol was similar for adult and pediatric patients.16, 19 Treatment was started as soon as eDSA were detected. The IVIG-based treatment protocol and its modifications over the study period have been previously reported.19 Briefly, successive IVIG infusions constituted the backbone of eDSA treatment. Until April 2017, all patients received an additional single dose of Rituximab after the first IVIG infusion. Since April 2017, we have limited the use of Rituximab to patients with concomitant graft dysfunction or positive retrospective LCT crossmatch.19
Thus, as of March 2019, therapy consisted of an initial infusion of 2 g/kg of IVIG followed by additional infusions of 0.5 g/kg of IVIG every 4 weeks in patients with possible subclinical AMR. In patients with possible clinical AMR or a positive retrospective, LCT crossmatch, additional 3 or 5 tPE sessions preceded the first IVIG infusion, and a single dose (375 mg/m2) of Rituximab followed the first IVIG infusion. In all patients, treatment with IVIG was continued until eDSA clearance was achieved, or for a maximum of 6 months. Patients who did not clear eDSA by the end of treatment were carefully monitored in the outpatient clinic.
2.6 Statistics
IBM SPSS 25.0 (IBM, NY) was used for data analysis. The study was a retrospective analysis of prospectively collected data.
Primary endpoints were graft survival and eDSA clearance at treatment end. Secondary endpoints were patient survival, pulsed-steroid therapy-free, CLAD-free, and infection-free survival, and the composite endpoint including mortality, CLAD, or retransplantation. Patients who died in-hospital were censored for analysis of pulsed-steroid therapy-free, CLAD-free and infection-free survival.
Categorical and continuous variables were summarized as percentages and median with interquartile range (IQR), respectively. The nonparametric Mann-Whitney test and the χ2 test or the Fisher's exact test were used for group comparisons of continuous and categorical variables, respectively.
Survival estimates were calculated by the product-limit method of Kaplan-Meier. Differences between groups were quantified using the log-rank test. In eDSA+/IVIG+ patients, outcomes were stratified according to Rituximab use, cumulative MFI values higher than 5000, presence of eDSA against more than one HLA antigen, presence of possible clinical AMR, and presence of preformed eDSA (online supporting information).
P ≤ .05 were considered significant.
3 RESULTS
3.1 Patient groups
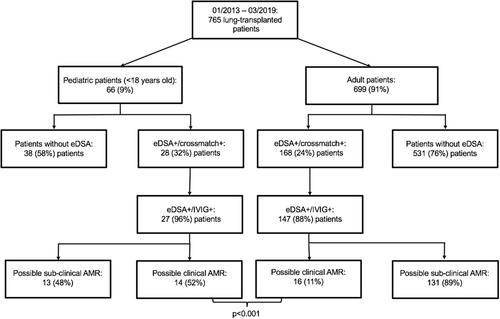
Between January 2013 and March 2019, 66 (9%) out of the 765 lung-transplanted recipients were pediatric patients (Table 1). Twenty-seven (41%) patients showed eDSA and were treated with IVIG, while 38 (57%) patients did not develop eDSA. One (2%) patient showed eDSA but was treated only with tPE and Rituximab, and thus was excluded from the study (Figure 1). The patient, a 17-year-old girl, was transplanted in March 2013 for cystic fibrosis, developed eDSA 1 week after transplantation, and was still treated with the “old” tPE-based protocol.15 She developed CLAD 3 years after transplantation and she is still alive.
| Variable | eDSA+/IVIG+ (n = 27) | eDSA− (n = 38) | P |
|---|---|---|---|
| Number of pediatric lung transplantations per year | |||
| 2013 (n = 138a) | 3 (11) | 8 (21) | |
| 2014 (n = 130a) | 4 (15) | 4 (11) | |
| 2015 (n = 119a) | 3 (11) | 6 (16) | |
| 2016 (n = 132a) | 6 (22) | 6 (16) | |
| 2017 (n = 116a) | 4 (15) | 5 (13) | |
| 2018 (n = 116a) | 7 (26) | 8 (21) | |
| 2019 (until March, n = 13a) | 0 | 1 (3) | |
| Female sex | 17 (63) | 17 (45) | .15 |
| Age, y | 12 (6-16) | 14 (10-15) | .089 |
| Age <11 y | 9 (33) | 10 (26) | .54 |
| BSA, m2 | 1.17 (0.71-1.34) | 1.18 (0.96-1.36) | .92 |
| CMV risk profile | |||
| Low | 10 (37) | 10 (26) | .36 |
| Intermediate | 10 (37) | 12 (32) | .65 |
| High | 7 (26) | 16 (42) | .18 |
| Blood group | |||
| A | 15 (56) | 21 (55) | .98 |
| B | 3 (11) | 4 (11) | 1.00 |
| AB | 0 | 2 (5) | .51 |
| 0 | 9 (33) | 10 (26) | .54 |
| Incompatible recipient-donor blood group transplantation | 2 (7) | 0 | .17 |
| Transplant indication | |||
| Children's interstitial lung disease | 5 (19) | 6 (16) | .51 |
| Cystic fibrosis | 12 (44) | 22 (58) | .28 |
| Pulmonary hypertension | 9 (33) | 7 (18) | .17 |
| Retransplant | 1 (4) | 3 (8) | .64 |
| Pulmonary arterial hypertension | 10 (37) | 7 (18) | .092 |
| LAS score | 83.7 (38.9-100) | 44.2 (36-100) | .10 |
| Preoperative mechanical ventilation | 2 (7) | 4 (11) | .51 |
| Preoperative intensive care unit | 11 (41) | 15 (40) | .92 |
| Preoperative ECMO | 8 (30) | 5 (13) | .10 |
- Abbreviations: BSA, body surface area; CMV, cytomegalovirus; COPD, chronic obstructive pulmonary disease; ECMO, extracorporeal membrane oxygenation; LAS, lung allocating score.
- Values are expressed as median (IQR, interquartile range) or N of patients (%).
- a Overall number of lung transplantations performed per year at our institution.
Pre-, intra-, and posttransplant recipient and donor characteristics were similar between eDSA+/IVIG+ and eDSA− patients (Tables 1-4). eDSA+/IVIG+ patients showed a higher median lung allocating score (LAS) score than eDSA− patients, due to their younger age and higher prevalence of idiopathic or secondary pulmonary arterial hypertension (Table 1). Three eDSA− patients died in-hospital after transplantation of disseminated aspergillosis, primary graft dysfunction, and acute hemorrhagic shock, respectively.
| Variable | eDSA+/IVIG+ (n = 27) | eDSA− (n = 38) | P |
|---|---|---|---|
| Donor characteristics | |||
| Female sex | 19 (70) | 21 (55) | 0.22 |
| Age, y | 17 (8-38) | 18 (11-43) | 0.63 |
| Age >70 y | 1 (4) | 0 | 0.41 |
| BSA, m2 | 1.52 (0.98-1.81) | 1.64 (1.33-1.75) | 0.37 |
| Ventilation time, d | 4 (3-6) | 3 (5-7) | 0.81 |
| pO2 (100%, PEEP 5 mmHg) | 445 (327-503) | 399 (346-452) | 0.27 |
| Smoking history | 3 (11) | 7 (18) | 0.50 |
| Contusion | 5 (19) | 5 (13) | 0.73 |
| Aspiration | 1 (4) | 3 (8) | 0.64 |
| Lung Preservation | |||
| Cold flush with Celsior | 27 (100) | 35 (92) | 0.26 |
| Portable EVLP | 0 | 1 (3) | 0.58 |
| Intraoperative recipient characteristics | |||
| Single lung transplantation | 0 | 1 (3) | 0.58 |
| Bilateral lung transplantation | 27 (100) | 37 (97) | 0.58 |
| Cardiopulmonary bypass | 4 (15) | 2 (5) | 0.22 |
| Intraoperative ECMO | 14 (52) | 12 (32) | 0.10 |
| Postoperative extended ECMO | 8 (30) | 7 (18) | 0.29 |
| Ischemic time, min | |||
| First lung | 401 (300-463) | 363 (274-434) | 0.18 |
| Second lung | 486 (405-572) | 482 (406-572) | 1.00 |
| Blood products, intraoperative | |||
| PRBCs, units | 2 (1-5) | 2 (1-4) | 0.76 |
| PC, units | 1 (0-2) | 0 (0-1) | 0.39 |
| FFP, units | 4 (2-6) | 3 (2-6) | 0.58 |
- Abbreviations: BSA, body surface area; ECMO, extracorporeal membrane oxygenation; EVLP, ex-vivo lung perfusion; FFP, fresh frozen plasma; PC, platelet concentrate; PRBC, packed red blood cells.
- Values are expressed as median (IQR, interquartile range) or N of patients (%).
| Variable | eDSA+/IVIG+ (n = 27) | eDSA− (n = 38) | P |
|---|---|---|---|
| Preoperative anti-HLA antibodies | |||
| Anti-HLA I | 4 (15) | 4 (11) | .71 |
| Anti-HLA II | 8 (30) | 3 (8) | .041 |
| Anti-HLA I + anti-HLA II | 1 (4) | 0 | .41 |
| Cumulative mismatches | |||
| HLA A + B | 3 (2- 4) | 3 (3-4) | .34 |
| HLA A + B + DR | 5 (4-6) | 5 (4-5) | .010 |
| Postoperative anti-HLA antibodiesa | |||
| Anti-HLA I | 11 (41) | 4 (11) | .004 |
| Anti-HLA II | 25 (93) | 7 (18) | <.001 |
| Anti-HLA I + anti-HLA II | 10 (37) | 2 (5) | .002 |
| Postoperative anti-HLA eDSA | |||
| HLA A | 5 (19) | ||
| HLA B | 4 (15) | ||
| HLA C | 0 | ||
| HLA DR | 3 (11) | ||
| HLADQ | 23 (85) | ||
| Positive retrospective crossmatch | 1 (4) | ||
| Preformed DSA | 2 (7) | ||
| MFI values before treatment | 3143 (2015-5006) | ||
| Cumulative MFI values before treatmentb | 3879 (2066-6251) | ||
| eDSA treatment | |||
| IVIG | 27 (100) | ||
| tPE/immunoabsorption | 14 (52) | ||
| Rituximab | 25 (93) |
- Abbreviations: eDSA, early detectable donor-specific antibodies; HLA, human leukocyte antigen; MFI, median fluorescence intensity.
- Values are expressed as median (IQR) or N of patients (%).
- a All patients who developed anti-HLA antibodies after lung transplantation were considered, independently of DSA positivity.
- b Sum of the single MFI, in case a patient showed eDSA against more than one antigen.
| Variable | eDSA+/IVIG+ (n = 27) | eDSA− (n = 38) | P |
|---|---|---|---|
| PGD score grade 2 or 3 | |||
| 24 h | 6 (22) | 7 (19) | .75 |
| 48 h | 7 (26) | 8 (22) | .69 |
| 72 h | 9 (33) | 6 (16) | .11 |
| Blood products, overall | |||
| PRBCs, units | 6 (2-15) | 4 (2-7) | .19 |
| PC, units | 1 (0-4) | 1 (0-2) | .23 |
| FFP, units | 5 (2-9) | 4 (2-6) | .12 |
| Rethoracotomy for bleeding | 2 (7) | 2 (5) | .55 |
| New dialysis | 1 (4) | 1 (3) | .66 |
| Postoperative pulsed-steroid therapy | 16 (59) | 8 (21) | .002 |
| Secondary ECMO | 2 (7) | 1 (3) | .56 |
| Tracheostomy | 6 (22) | 4 (11) | .29 |
| Mechanical ventilation time, d | 1 (1-3) | 1 (1-2) | .49 |
| ICU stay, d | 8 (2-19) | 2 (1-7) | .061 |
| Hospital stay, d | 40 (27-49) | 27 (21-39) | .015 |
| In-hospital mortality | 0 | 3 (8) | .13 |
| Immunosuppressive therapy at discharge after transplantationa | |||
| Cyclosporine | 0 | 0 | |
| Tacrolimus | 27 (100) | 38 (100) | 1.0 |
| Mycofenolate mofetil | 27 (100) | 38 (100) | 1.0 |
| Immunosuppressive therapy at last outpatient controlb | |||
| Cyclosporine | 0 | 0 | |
| Tacrolimus | 27 (100) | 35 (100) | 1.00 |
| Mycofenolate mofetil | 26 (96) | 32 (91) | .63 |
| Everolimus | 0 | 3 (9) | .25 |
- Abbreviations: ECMO, extracorporeal membrane oxygenation; FFP, fresh frozen plasma; ICU, intensive care unit; PC, platelet concentrate; PGD, primary graft dysfunction; PRBC, packed red blood cells.
- Values are expressed as median (IQR, interquartile range) or N of patients (%).
- a In-hospital deaths (n = 3) are excluded.
- b Switch was due to leukopenia under mycophenolate mofetil (n = 1) and to posttransplant lymphoproliferative disorder (PTLD, n = 2).
3.2 eDSA
In pediatric patients, eDSA were detected at a median of 24 (14-63) days after transplantation, significantly later than in our adult patients, where eDSA were detected at a median of 14 (11-19) days after transplantation (P = .016).
eDSA were preformed in 2 (7%) cases and de novo in the remaining 25 (93%) cases (Table 3). Both patients with preformed eDSA developed also de novo DSA against other HLA antigens after transplantation. Thirteen (48%) patients showed eDSA in the absence of graft dysfunction (possible subclinical AMR), while 14 (52%) showed eDSA and graft dysfunction (possible clinical AMR). In these 14 patients, eDSA were detected simultaneously with the diagnosis of graft dysfunction. As compared to our adult recipients, pediatric patients showed a lower incidence of preformed eDSA (P = .052) and a higher incidence of possible clinical AMR (P < .001) (Figure 1).
3.3 Treatment of eDSA and AMR
Among the 27 eDSA+/IVIG+ patients, 23 (85%) patients received IgGAM (Pentaglobin), and the remaining 4 (5%) patients other IVIG. Treatment with IVIG was combined with tPE or immunoabsorption in 14 (52%) patients and with Rituximab in 25 (93%) patients.
All 14 patients with possible clinical AMR had received pulsed-steroid therapy before the eDSA positivity had been communicated by the HLA lab. Thereafter, specific eDSA treatment was begun immediately.
The overall number of IVIG infusions amounted to 99. Twenty-three (85%) patients required a median of 3 (1-4) additional 0.5 g/kg IVIG infusions. Treatment time amounted to a median of 2.4 (1.4-5.0) months.
eDSA were cleared successfully in 25 out of 26 (96%) patients who had completed treatment. Among these 25 patients, the same eDSA recurred in 3 (12%) patients. No new DSA were detected (Figure 2). Clearance did not differ in these 26 patients after stratification according to presence of eDSA against class I or II HLA antigens (P = .77), presence of preformed or de novo eDSA (P = .077), presence of eDSA against more than one HLA antigen (P = .38), to cumulative MFI values higher than 5000 (P = .65), to presence of possible clinical AMR (P = .51), and to Rituximab use (P = .92, Table S1).
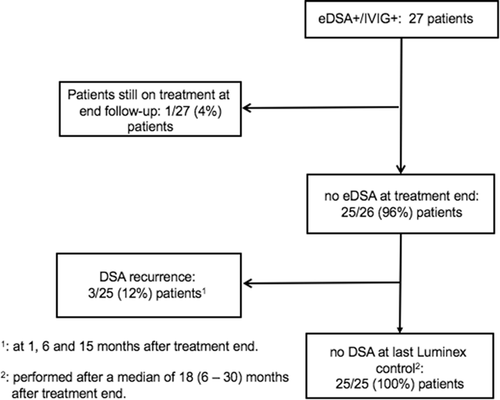
Among the 27 eDSA+/IVIG+ patients, one (4%) patient showed an allergic reaction during treatment with Kiovig. After treatment end, eight (30%) eDSA+/IVIG+ vs seven (21%) eDSA− patients showed hypogammaglobulinemia requiring IVIG substitution (P = .41). No eDSA+/IVIG+ vs 4 (12%) eDSA− patients developed a posttransplant lymphoproliferative disorder (PTLD, P = .089). At 1-year follow-up, the glomerular filtration rate (ml/min), calculated according to the equation of Cockroft-Gault, was 72 (56-92) vs 81 (61–93) in eDSA+/IVIG+ and eDSA− patients (P = .46).
3.4 Outcomes
Median follow-up amounted to 28 (12-52) months, and its length did not differ between groups (P = .68). Outcomes were similar in eDSA+/IVIG+ vs eDSA− patients (Table 5 and Figure 3A-D).
| Variable | eDSA + /IVIG + (n = 27) | eDSA-(n = 38) | P |
|---|---|---|---|
| Patient survival (%) | |||
| 1, y | 96 ± 4 | 92 ± 4 | |
| 3, y | 80 ± 9 | 92 ± 4 | |
| 5, y | 80 ± 9 | 92 ± 4 | .45 |
| Graft survival (%) | |||
| 1, y | 96 ± 4 | 89 ± 5 | |
| 3, y | 73 ± 10 | 85 ± 6 | |
| 5, y | 73 ± 10 | 85 ± 6 | .65 |
| Causes of death after hospital dischargea | |||
| CLAD | 1 (4) | 0 | .43 |
| Infection | 0 | 0 | |
| Malignancy | 0 | 0 | |
| Cardiac | 0 | 0 | |
| Other | 3 (11) | 0 | .077 |
| Biopsy-confirmed rejectionb (ISHLT Grade)a | |||
| A1 | 6 (38) | 2 (11) | .080 |
| A2 | 2 (12) | 1 (6) | .45 |
| A3 | 1 (6) | 1 (6) | 1.0 |
| Pulsed-steroid therapy-free survival (%)a | |||
| 6, mo | 85 ± 7 | 63 ± 9 | |
| 1, y | 71 ± 9 | 48 ± 9 | |
| 5, y | 41 ± 12 | 22 ± 9 | .074 |
| CLAD-free survival (%)a | |||
| 1, y | 100 | 100 | |
| 3, y | 89 ± 7 | 78 ± 9 | |
| 5, y | 67 ± 20 | 78 ± 9 | .82 |
| Retransplant-free survival (%)a | |||
| 1, y | 100 | 94 ± 4 | |
| 3, y | 92 ± 8 | 90 ± 5 | |
| 5, y | 92 ± 8 | 90 ± 5 | .48 |
| Mortality-, CLAD-, retransplant-free survival | |||
| 1, y | 96 ± 4 | 87 ± 6 | |
| 3, y | 75 ± 10 | 76 ± 8 | |
| 5, y | 56 ± 18 | 76 ± 8 | .95 |
| Infection-free survival (%)a | |||
| 1, y | 69 ± 9 | 47 ± 9 | |
| 3, y | 47 ± 11 | 31 ± 10 | |
| 5, y | 47 ± 11 | 26 ± 9 | .15 |
- Abbreviations: CLAD, chronic lung allograft dysfunction; ISHLT, International Society for Heart and Lung Transplantation.
- Values are expressed as mean ± SD (%) or N of patients (%).
- a Patients who died before hospital discharge (n = 3) were censored.
- b Available for only 16 (59%) eDSA+/IVIG+ and 18 (47%) eDSA− patients.
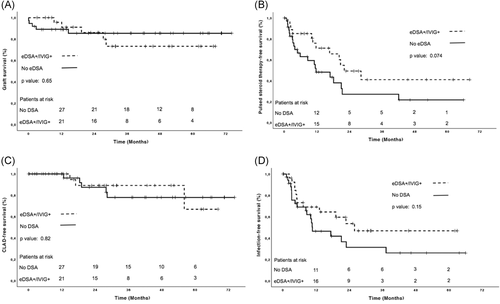
In particular, four eDSA+/IVIG+ patients died during follow-up (Table 5). The first patient died of early CLAD 1 year after retransplantation, without showing new or recurrent DSA. The second patient died of sepsis 1 year after transplantation. The third patient showed an episode of definite clinical AMR with graft dysfunction, recurrence of previously cleared DSA, and transbronchial biopsy clearly suggestive for AMR. He was treated with tPE and alemtuzumab, but the graft function did not recover and the patient died. The fourth patient developed a fulminant graft dysfunction following several successfully treated infectious episodes. She did not show new or recurrent DSA, and the biopsy was not suggestive for AMR. Although she was treated with pulsed-steroid therapy, tPE and IVIG, the graft function did not recover, and the patient died 8 months after transplantation.
Three eDSA+/IVIG+ vs five eDSA− patients developed CLAD. Among the three eDSA+/IVIG+ patients, one patient, who did not initially clear DSA, developed restrictive allograft syndrome, was again treated with tPE, IVIG, and Rituximab, but the graft function did not stabilize and she required retransplantation. Two patients developed bronchiolitis obliterans syndrome (BOS): in one patient the graft function stabilized, but in the other it did not, and the patient, as aforementioned, died.
Twelve (44%) eDSA+/IVIG+ and 20 (54%) eDSA− patients showed at least one episode of infection requiring hospitalization at follow-up, without any difference in the median number of hospital admissions between groups (2 vs 2 admissions, P = .63, respectively). Infection involved the lungs in 12 (100%) eDSA+/IVIG+ and 19 (95%) eDSA− patients (P = .62) and was viral or bacterial in 9 (75%) vs 16 (80%) and 7 (58%) vs 10 (50%) eDSA+/IVIG+ and eDSA− patients (P = .53 and P = .46) respectively.
Outcomes did not substantially differ in eDSA+/IVIG+ patients after stratification according to Rituximab use (Table S2), cumulative MFI values higher than 5000 (Table S3), presence of eDSA against more than one HLA antigen (Table S4), presence of possible clinical AMR (Table S5), and presence of preformed eDSA (Table S6).
Median forced respiratory volume in 1 second (FEV1) values (% predicted) did not differ between eDSA+/IVIG+ vs eDSA− patients at discharge (57 vs 52, P = .91), at 1-year follow-up (71 vs 78, P = .84), and at last outpatient assessment (66 vs 77, P = .39), performed at 30 (12-48) months after transplantation. FEV1 values did not differ in eDSA+/IVIG+ patients after stratification according to Rituximab use, cumulative MFI values higher than 5000, presence of eDSA against more than one HLA antigen, presence of possible clinical AMR, and presence of preformed eDSA (Table S7).
4 DISCUSSION
In comparison to our previous published studies,15, 16, 19 this study focused only on pediatric lung-transplanted patients and showed that, in comparison to adult lung-transplanted patients, more than one-third of pediatric patients developed eDSA and, among these patients, more than half showed concomitant graft dysfunction without evidence of any other reason (possible clinical AMR). Nonetheless, after treatment with an IVIG-based protocol, patients with eDSA and possible AMR had outcomes similar to patients without eDSA.
Although our study is based on a retrospective nonrandomized case series, the results are relevant, because no previous study focused specifically on DSA and AMR in pediatric lung transplantation, partly due to the worldwide limited experience with pediatric lung transplantation, with only six centers performing more than five pediatric transplants per year.23 Thus, in most of the available studies, results were not reported separately for adult and pediatric patient.1-10 Evidence from pediatric kidney and heart transplantation showed that DSA is a risk factor for mortality and acute or chronic rejection.24-26 Dipchand et al24 have recently shown in a multicenter study including 237 pediatric heart-transplant patients that one-third of patients developed de novo DSA, usually within 6 weeks after transplantation, and that DSA were a risk factor for acute cellular rejection in the first year posttransplantation. Similarly, Irving et al25 showed that 40% of pediatric heart-transplant patients developed de novo DSA and that patients with persistent vs. transient DSA showed an increased incidence of CAV, rejection and graft loss. In a case series of 103 pediatric kidney transplant recipients, Engen et al26 showed that 32% of patients developed de novo DSA after transplantation and that patients with DSA and concomitant graft dysfunction fared worse than patients with the only evidence of DSA.
This evidence supports our strategy of treating DSA as soon as they are detected, independent of graft dysfunction. The conundrum, if the DSA detected in our patients represent a memory of previous alloantigen exposure or truly de novo recipient vs. donor sensitization remains unanswered, yet has no consequences for our treatment decision, since both preformed and de novo DSA are risk factors for adverse outcomes.11 Moreover, investigation of the DSA complement-binding capacity may further help to refine our treatment strategy in the future.27
Furthermore, we think that in pediatric patients, every effort should be undertaken to preserve graft function as long as possible. In comparison to adult lung-transplant patients, pediatric patients show a worse 5-year CLAD-free survival according to the last ISHLT registry report (50.1% vs 45.8%, P = .028).23 Moreover, our study showed that more pediatric (52%) than adult (11%) patients showed graft dysfunction concomitantly with eDSA development (Figure 1).
In addition, our study showed that outcome-free survival was similar between patients who developed early possible clinical AMR and those who showed the only eDSA in the absence of clinical rejection. Thus, the treatment of eDSA and AMR in an early phase could reverse graft damage. In contrast, the two patients in our study who showed late possible or definite clinical AMR did not recover graft function, and died or required retransplantation. Similarly, other studies confirmed the detrimental and therapy-refractory prognosis of late AMR.13, 17
The pleiotropic mechanisms by which IVIG, tPE, and Rituximab inhibit DSA production have been reported elsewhere.28-32 Although other human immunoglobulin preparations are available, we chose IVIG and especially IgGAM, which contain IgG (76%), IgM (12%) and IgA (12%), because they have multiple beneficial immunomodulatory effects.28, 29 DSA treatment was well tolerated by pediatric patients and the increased immunosuppression related to treatment did not translate into increased risk of infections (Figure 3D). This result might be due in part to the IgM component of IgGAM, which confers protection against infections through pathogen opsonization.22 In addition, we adopted some precautions to decrease the treatment side effects, such as the administration of antiallergic drugs before tPE and Rituximab and the stepwise increase of the IVIG infusion rate.
Finally, our study showed that eDSA+/IVIG+ patients did not develop PTLD, which remains a significant cause of morbidity and mortality after pediatric solid organ transplantation.33 The protective effect of eDSA treatment against PTLD might have been related to Rituximab, since studies have recently demonstrated the prophylactic protective effect of low-dose Rituximab therapy against Epstein-Barr virus-induced PTLD.34, 35
5 STUDY LIMITATIONS
The retrospective design and the number of included patients may have confounded the results of this study. However, pediatric patients are rarely included in randomized trials. Moreover, a randomized multicenter trial on the treatment of eDSA and AMR might not be feasible in pediatric patients due to the limited number of transplant centers, which perform pediatric lung transplantation.
While a control group made up of eDSA+/no-treatment patients would have been more robust than a control group made of patients without eDSA to demonstrate a treatment effect, leaving pediatric patients without treatment appeared unethical, especially since DSA are already a well-proven risk factor for worse graft survival. However, a spontaneous eDSA clearance might have been also possible without treatment.
The short median follow-up time and the few patients at risk after 3 years of follow-up (Figure 3) might have confounded the outcome-free survival analysis. Moreover, DSA were detected earlier in our study than elsewhere.3, 4 However, this finding was not due to the low MFI cut-off for DSA positivity, but to the different frequency of DSA screening, follow-up time, and incidence of BOS.3, 4
The definition of infection at follow-up might be arbitrary, but we aimed at including only the most important infectious episodes, that is, those leading to hospital admission, and not just all the infectious ones.
Finally, in eDSA+/IVIG+ patients, we did not analyze outcomes according to the used IVIG preparates, due to patient number imbalance between groups (n = 4 vs n = 23 with IgGAM).
6 CONCLUSIONS
Following pediatric lung transplantation, an IVIG-based treatment for eDSA yielded high eDSA clearance. eDSA+/IVIG− and control patients showed similar graft and CLAD-free and graft survival.
CONFLICT OF INTERESTS
Fabio Ius and Gregor Warnecke report personal and congress fees paid from Biotest AG. The remaining authors report no conflict of interests concerning this manuscript.




