On the nonexistent Nobel Prizes for two pioneers of modern physical organic chemistry: Sir Christopher K. Ingold and Saul Winstein†
Dedicated to the memory of Sir Christopher Kelk Ingold (1893–1970) and Saul Winstein (1912–1969).
Abstract
The careers of two pioneers of modern physical organic chemistry, Sir Christopher K. Ingold and Saul Winstein, are discussed and compared. Despite the fact that Ingold received 112 nominations from 77 nominees for the Nobel Prize in Chemistry (NPch), he never received that award. Winstein, also a non-recipient of the NPch, died prematurely at the age of 57. In his last 3 years, Winstein received 22 nominations from 18 nominators, seven of whom received or would receive the NPch themselves. Analyses of the Nobel Nomination Archive along with other evidence are used to explain Ingold's experience. A detailed examination of Winstein's career along with relevant historical data suggests that Winstein was a highly probable Nobelist had he lived just a few years longer. The relationship of Ingold's and Winstein's careers and the politics of the Nobel Prize selection process including the possibility that they would have shared a Nobel Prize are presented.
1 INTRODUCTION
The Nobel Prize (NP) is the most treasured and respected honor that a chemist—or any scientist—can ever hope to receive. No other award program receives as much attention as the Nobel Prize, and the receipt of a Nobel Prize is a career-changing life event. One of the authors of this publication (JIS) has heard numerous stories of eminent chemists who have waited nervously in the early hours of certain October mornings, hoping to hear their telephones ring. Unfortunately, there are more deserving individuals than prizes to be given, a circumstance that is likely to be general for all award programs. Much has been written about “missing Nobel Prizes.”1-5
The purpose of this publication is to explore the nonexistent Nobel Prizes in Chemistry (NPch) to two pioneers of modern physical organic chemistry (POC),6-9 Sir Christopher K. Ingold (1893–1970) and Saul Winstein (1912–1969) (Figure 1). In addition to gathering and analyzing the facts around each of these chemists' nonexistent NPch, we shall also inquire into the possibility that the decision not to award the NPch to Ingold might have affected the decision not to award the NPch to Winstein. We shall also discuss the “backroom” politics that inhabit the sociology of chemistry.

We hasten to add, POC permeates throughout all chemistry, not just organic chemistry. Indeed, POC is so enmeshed within the molecular sciences that it is often not considered as its own subdiscipline by virtue of its “obliteration by incorporation.”10, 11 Honoring physical organic chemists with the Nobel Prize seems both appropriate and as it should be. But for many years, as far as the NPch is concerned, POC has been the bridesmaid to other areas of chemistry and the life sciences.5
2 ON SIR CHRISTOPHER K. INGOLD
2.1 Overview of Ingold's accomplishments
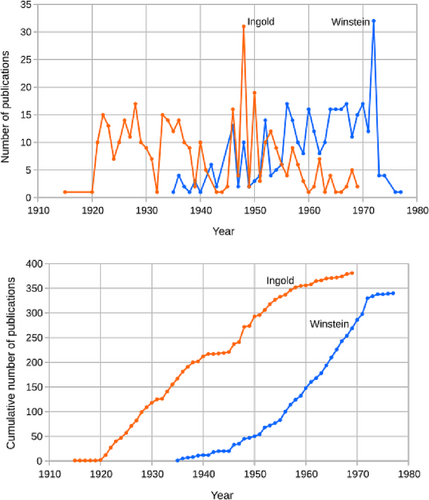
Sir Christopher K. Ingold was one of the senior founders of modern POC, though elements of POC go back to the 19th century, for example, to Nikolai Menshutkin's studies on the effect of structure (added substitutions) on ester hydrolysis and alkylation of amines.12 Ingold's scientific prowess was very early recognized, of course by the number and quality of his publications (Figure 2). Ingold's productive career embraced both pre-World War II and post-World War II periods. While he is most remembered for contributions made in the first half of his career, it would be a mistake to underestimate the importance of his publications in the second half.
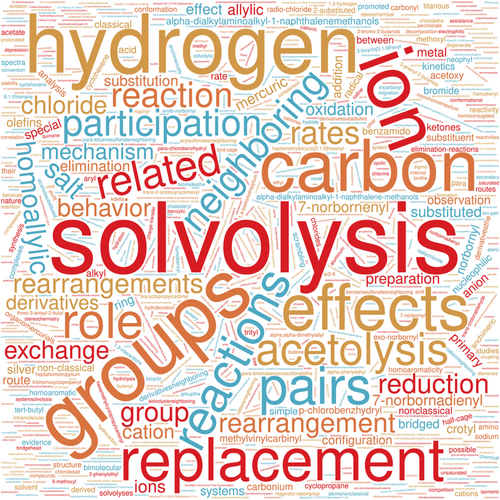
Not unexpectedly, there seems to be some shift in Ingold's research focus after 1942, as illustrated in the two-word clouds shown in Figure 3.

Ingold had a habit of publishing his work in blocks of papers. Often one paper in a block did not carry his name as an author. That was his way of increasing the stature of one of his junior collaborators, where he felt that someone had made a particularly important contribution to that paper. I published a couple of papers on my Ph.D. work, with Ingold's permission of course, but he did not put his name on them as an author. This raised my status to that of a leading author, which increased their value when the university tenure committee was looking at my work before granting me a tenured position. This practice of Ingold could very well have amounted to about 40 papers.13
Ingold provided the experimental evidence and hence the mechanisms of nucleophilic substitution reactions (SN1 and SN2), which were also studied extensively and named by him.14 He had a hand in unraveling the mechanistic differences between unimolecular and bimolecular elimination reactions, E1 versus E2 reactions, again using Ingold's terminology.14 Independent from Linus Pauling, Ingold developed the concept of “mesomerism”15 to explain the special stability of aromatic compounds and “resonance-stabilized” transition states; Pauling called it “resonance.”16 Also independently, or perhaps not so independently (according to Sir Robert Robinson17-21), Ingold devised the electronic theory of aromatic substitution.22 These discoveries are so fundamental to organic chemistry that they are among the first reactions studied by students in introductory chemistry courses.
Going beyond individual reaction mechanisms, as important as that science is, Ingold made contributions to all of chemistry.15, 17-19, 23-28 We cite two examples of Ingold's range of interests and seminal contributions to POC.
First, in 1946, Ingold and his colleagues published a series of 11 consecutive publications in the Journal of the Chemical Society comprising 112 pages establishing the hexagonal structure of benzene.29-39 As mentioned above, Ingold was an author of only seven of these publications, but clearly his hand was active and central to all 11. In these studies, Ingold and his colleagues synthesized a series of deuterated benzenes and analyzed their infrared and Raman spectra, and “the structure of benzene, which had begun in 1865 by Kekulé, was finally solved in its entirety.”19
Second, much later in Ingold's career, a collaboration with R. S. Cahn and Vladimir Prelog led to the CIP R,S-rules for unambiguous nomenclature of chiral molecules.40 Ingold also proposed the term “synartetic”18, 28 for rate accelerations that later would be termed—more successfully—“anchimeric” acceleration or assistance. His 1951 book Structure and Mechanism in Organic Chemistry14 “became the bible of a new generation of organic chemists” in the 1950s.6
We add that one philosopher of chemistry wrote about what he called “The Ingold Revolution in Organic Chemistry.”41
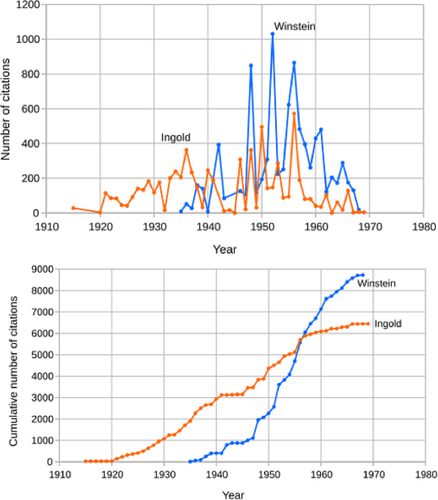
Figure 4 illustrates the citations to Ingold's publications from 1915 to 1969. Clearly, Ingold's publications had a significant impact on the research community. During these time periods, Ingold's publications were cited ~6500 times. In summary, it would be fair to say: Ingold was the premier leader in POC from the 1920s to the 1960s.
2.2 Ingold's school of Physical Organic Chemistry
Ingold's school was based mostly within the foundation Ingold laid for POC (its kinetics and mechanisms) as well as its nomenclature (that is taught to every first-year organic chemistry student). True, a number of his students became eminent scholars and researchers, for example, Peter de la Mare, Ronald S. Nyholm, Saul Patai, and C. W. Shoppee. Nonetheless, it was Ingold's own research achievements and his organization and nomenclature of the mechanisms of POC that are his legacy. And we ought not to forget the Cahn–Ingold–Prelog (CIP) system or sequence of priority rules to denote configuration.
2.3 On Ingold's nonexistent Nobel Prize
Why was it that Ingold never received a Nobel Prize? There are several possibilities that we will now consider. It could be any of these, or another, or most likely some combination.
First, perhaps he was not nominated, or not nominated frequently enough, or not nominated by sufficiently eminent scientists.
The Nobel Prize Nomination Archive incorporates a 50-year information blackout and was first made available in 1974,42 though little used until much more recently.5, 43-46 Speculations as to why Ingold was never selected for the NPch by Nobelist Derek H. R. Barton24, 47 (NP 1969) and others were often made in the absence of any idea as to the enormous volume of Ingold's nominations. We now know that during the period 1940–1970, Ingold received 112 nominations from 77 different individuals who lived in 22 different countries (Table 1). Of these 112 nominations, 13 different Nobel laureates nominated Winstein, several doing so on multiple occasions (Hans K. von Euler-Chelpin, NPch in 1929; Vladimir Prelog, NPch 1975; Tadeus Reichstein, NPphy/med 1950; Leopold Ruzicka, NPch 1939; and Sir Cyril Hinshelwood, NPch 1956). Table 1 also reveals a significant nationality-oriented bias. Table 2 reveals that among the non-recipients of the NPch, Ingold's 112 nominations from 77 different nominators far exceeded that of all other unsuccessful nominees. The closest to Ingold was Walter Rappe with 64 nominations from 34 different nominators.
| Country | Number of nominators | |||
|---|---|---|---|---|
| Robinsona | Ingolda,b | Winsteina,b | Woodwarda | |
| Nobel Prize year (birth and death years) | 1947 (1886–1975) | — (1893-1970) | — (1912–1969) | 1965 (1917–1979) |
| UK | 11 | 20 | 2 | 6 |
| France | 2 | 10 | 1 | 3 |
| Australia | 3 | 5 | 2 | |
| Israel | 5 | |||
| Italy | 5 | 2 | 1 | |
| Spain | 1 | 5 | 1 | |
| Germany | 4 | 1 | 9 | |
| Switzerland | 4 | 4 | 1 | 3 |
| USA | 4 | 3 | 8 | 23 |
| Canada | 1 | 2 | 2 | |
| New Zealand | 2 | 1 | ||
| Poland | 2 | 2 | ||
| Belgium | 1 | |||
| Egypt | 1 | |||
| Finland | 1 | 1 | 1 | |
| India | 1 | 4 | ||
| Ireland | 1 | |||
| The Netherlands | 1 | 1 | 4 | |
| Norway | 1 | |||
| Romania | 1 | 1 | ||
| Sweden | 1 | 1 | ||
| Turkey | 1 | |||
| Argentina | 1 | |||
| Austria | 1 | |||
| Chile | 1 | |||
| Croatia | 1 | |||
| Greece | ||||
| Japan | 1 | |||
| Poland | 2 | |||
| Serbia | 1 | |||
| USSR | 1 | |||
| Total nominations | 51 | 112 | 25 | 111 |
| Total nominators | 32 | 77 | 19 + 1c | 70 |
| Total number of countries | 10 | 22 | 10 | 20 |
| Years being nominated | 1928–1947(20) | 1940–1970(31) | 1963–1970(8) | 1946–1965(20) |
- a Up to and including nomination year 1970 for Robinson, Woodward, and Ingold and up to and including 1969 for Winstein.
- b During the period 1940–1970, Ingold received 112 nominations. During the period 1963–1970, Winstein received 25 nominations.
- c Winstein received one nomination in 1970, by the American chemist Harold Urey. Presumably, that nomination was intended for 1969 but was received after the nomination deadline.
| Nobel laureates in chemistry | Non-recipients of the NPch | ||||
|---|---|---|---|---|---|
| Name | Nominations | Nominators | Name | Nominations | Nominators |
| Woodward | 111 | 70 | Ingold | 112 | 77 |
| Nernst | 76 | 52 | Reppe | 64 | 34 |
| Staudinger | 74 | 34 | Urbain | 56 | 26 |
| Pauling | 65 | 50 | Bartlett (Neil) | 46 | 37 |
| Barton | 54 | 38 | Lewis | 41 | 29 |
| Heyrovský | 51 | 41 | Clusius | 39 | 23 |
| Robinson | 51 | 32 | Tammann | 35 | 26 |
| Onsager | 45 | 38 | Kögl | 35 | 24 |
| Natta | 44 | 35 | Paneth | 35 | 17 |
| Moissan | 41 | 31 | Hedvall | 34 | 15 |
| Leloir | 36 | 18 | Le Châtelier | 31 | 23 |
| Richards | 33 | 17 | Curtius | 31 | 21 |
| Ziegler | 32 | 24 | Craig | 30 | 7 |
| Diels | 32 | 16 | Bijvoet | 28 | 21 |
| Hevesy | 31 | 30 | Meerwein | 27 | 13 |
| Eigen | 31 | 22 | Schlesinger | 26 | 13 |
| du Vigneaud | 30 | 27 | Eyring | 25 | 23 |
| Ramsay | 30 | 25 | Winstein | 25 | 20 |
| Karrer | 28 | 21 | Freudenberg | 24 | 19 |
| Willstätter | 28 | 20 | Bertrand | 24 | 15 |
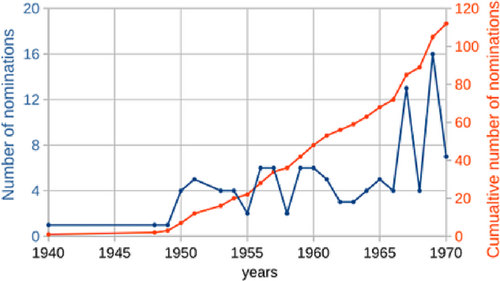
Ingold's nomination chronology continued into the year after he died; in fact, it accelerated in his later years (Figure 5). That Ingold received 16 nominations in 1969, the year before he died, was certainly a message delivered to the Academy—a message that was ignored. Ingold's overall nomination chronology was marked with an interesting and certainly not random up-down-up sequence in the mid and late 1960s. This feature is observed in the nomination chronologies of other NPch nominees and suggests some type of organized behavior.48 Clearly, possibility #1 is discredited.
Second, perhaps Ingold's achievements were judged insufficient for a Nobel Prize by the Nobel Committee for Chemistry and/or the Royal Swedish Academy of Sciences (the “Academy”). The recipient of many awards, Ingold was elected a Fellow of the Royal Society (FRS) in 1924, at the extraordinarily early age of 30. Judging scientific accomplishments—especially prior to bibliographic analyses, and even with bibliographic analysis—is a momentous task.
Admittedly, comparing the achievements of individuals at the highest level is even more difficult. But that is what the Nobel Committee for Chemistry and the Academy have been doing since 1901. The Nobel Committee for Chemistry had (and has) the luxury of enlisting the assistance from the entire membership of the Royal Swedish Academy of Sciences and, even more impressively, from the entire body of scientists worldwide—to serve as consultants to the Committee. Ingold's enormous nomination record clearly demonstrates that the well-placed chemists, all selected by the Nobel Committee to serve as nominators and for their advice and recommendations, had judged Ingold's achievements and impact to be at the highest level within their community. Clearly, possibility #2 is discredited.
Third, perhaps Ingold was always the “bridesmaid” and never the “bride” when compared to pressing nominations of other well-deserving candidates. That is, perhaps Ingold was consistently judged as being less deserving than those chosen for the NPch. But if the Nobel Committee sincerely wanted to award Ingold the Prize, they could have awarded the 1956 Nobel Prize jointly with Sir Cyril Hinshelwood and Nikolay Semenov who were laureated “for their researches into the mechanism of chemical reactions.” That is exactly what Ingold also achieved, though from a physical organic chemical perspective rather than a physical chemistry perspective. Or they could have broadened the 1961 Nobel Prize received solely by Melvin Calvin “for his research on the carbon dioxide assimilation in plants.” Calvin actually determined the mechanism of biochemical reactions; the citation could have been for “the mechanism of chemical and biochemical reactions.” In other words, the argument that there was too much competition, that there were “no available slots” for Ingold falls flat. Possibility #3 is discredited.
Fourth, perhaps not awarding the NPch to Ingold was an “error and oversight of the Nobel Prize Committee.” Much has been written on errors and oversights of the Royal Swedish Academy of Sciences and the Nobel Prize.6, 15, 17-20, 23-25, 28, 41, 47, 49-52 Errors and oversights, unfortunate as they are, do happen. But in the face of 112 nominations over a 31-year period for Ingold, simple errors and oversights do not happen to organizations as astute as the Royal Swedish Academy of Sciences and their Nobel Committee for Chemistry. Intentionality happens. Possibility #4 is discredited.
We simply do not believe any of the previous four reasons are viable. Ingold's chemistry was deserving of an NPch. Ingold received more than enough nominations from more than enough eminent chemists to justify an NPch. There were several opportunities, over the years, for the Royal Swedish Academy of Sciences to have Ingold jointly share a Nobel Prize “slot” such that no other deserving candidate would have been excluded. The Nobel Committee for Chemistry is such a deliberate body, they do not make careless errors or oversights. They are strategic and deep thinkers.
There are three other explanations that may have relevance.
First, as Friedman has discussed,3 the NPch in the 1930s and 1940s and into the 1950s were heavily influenced by German chemists, though the latter years brought more Americans into the NPch fold. Ingold had four nominations from Germans; Robinson, not a single German nominated him (see Table 1). Woodward had nominations from nine Germans.
Second, perhaps the Nobel Committee for Chemistry was not particularly disposed to favor an award in POC. An examination of the list of NPch laureates reveals no fully physical organic laureate until perhaps Vladimir Prelog in 1975. True, other laureates contributed to POC, for example, Robinson with his electronic theory of organic chemistry; Barton with conformational analysis; even Calvin, with the mechanism of photosynthesis. But few would classify these chemists as physical organic chemists.
Robinson was lucid to the end. I remember a meeting of the Tetrahedron editorial board where we discussed the final format for [the book series] Comprehensive Organic Chemistry (COC). My views prevailed over his. (He preferred a kind of Dictionary approach.) As compensation, I suggest that Sir Robert write an introduction to COC. He agreed at once.
A week later, he rang me up to tell me how much he appreciated the invitation to write the introduction. In a clear voice he asked if he could write a long introduction, say 30 pages. I replied, “Of course, Sir Robert, anything you wish.” He said, “Good, I want to write all about the electronic theory of organic chemistry and of the secondary role played by Ingold.” Two days later, at the age of 88 years, Robinson was dead.47
Perhaps Robinson was able to indirectly (or not so indirectly) influence the Nobel Committee for Chemistry to believe that Ingold's greatest achievement—the electronic theory of organic chemistry—was actually Robinson's achievement.
Regarding [Christopher] Ingold, a good many Americans think that he has been an ass in his recent papers on ion pairs and solvolysis. In fact, I am building up to murder him.57
I lived in the Winstein heyday and the latter years of Ingold. … I can think of at least one more possible contribution to the matter, as it pertains to Ingold: The scuttlebutt among physical organic chemists in the mid-20th century was that Ingold was two-faced; in the presence of other physical organic chemists, he was gracious and perhaps even complimentary about their work, whereas in his “anonymous” referee reports he was stingingly critical and acerbic and belittled the contributions of everyone but himself. There are political aspects to all awards and prizes, and the Nobel Prize committee certainly must have known the rumors about Ingold; Robinson may well have reminded them every year.
My father never commented (in my hearing) on this matter. He was a realist, a trait I inherited from both of my parents, and he simply would not have wasted his time fretting over matters that could not be changed. There was simply too much else he wanted to do (think CIP rules in particular).58
I have observed in academic settings single individuals whose sole opinion went against the majority, but because of respect and other considerations the majority bowed to that opinion, such as on faculty appointments. Given Robinson's strong feelings about Ingold's contributions to electronic theory, it is easy to conclude that Robinson was the only reason Ingold never received the Nobel Prize.
We wonder, was Robinson so powerful? Is there any collaborative evidence to support the hypothesis of Robinson's influence on the selection of Nobel Prizes in Chemistry? Table 3 provides a partial and indirect answer. Robinson nominated nine individuals for the NPch (not counting Winstein, who died prematurely); seven of them became Nobel laureates. If Robinson was so influential in securing an NPch for his nominees, could he be so influential in the opposite?
| Physics | Chemistry | |||
|---|---|---|---|---|
| Years nominated | Year received the NP | Years nominated | Year received the NP | |
| James Chadwick | 1935 | 1935 | ||
| John Warcup Cornforth, Jr. | 1965, 1966, 1967, 1968, 1970 | 1975 | ||
| Vincent de Vigneaud | 1949 | 1955 | ||
| Sir Walter Norman Haworth | 1935 | 1937 | ||
| Sir Cyril Normal Hinshelwood | 1953 | 1956 | ||
| Dorothy Crawfoot Hodgkin | 1956 | 1956, 1961, 1963, 1964 | 1964 | |
| Richard Kuhn | 1939 [sic]a | 1938 | ||
| Herman Francis Mark | 1968, 1970 | |||
| Robert Bruce Merrifield | 1969 | 1984 | ||
| Goeoge Joseph Popjak | 1966, 1968, 1970 | |||
| John Monteath Robertson | 1963 | |||
| Sir Francis Simon | 1953 | |||
| Saul Winstein | 1968 | d. 1969 | ||
- a Presumably due to a late arrival of Robinson's nomination.
2.4 Summary
We believe that the major cause for Ingold's lack of a Nobel Prize was the spite of Robinson. And we have one other supporting explanation, and that involves Saul Winstein. We shall discuss that other explanation below.
We understand that our conclusion is speculative, just as many are explanations (or mechanisms) in “hard chemistry” speculative, when complete data are unavailable. We justify our analysis because we examined many possible explanations (in chemistry, many possible hypotheses) and were led by the available evidence.
3 ON SAUL WINSTEIN
3.1 Overview of Winstein's accomplishments
Ingold shared the distinction of being one of the “early-era” pioneers of modern POC. Saul Winstein was a “later-era pioneer” of modern POC (Figure 1). Winstein, a generation younger than Ingold, was his successor and the enhancer in transforming POC into the modern era. Ingold was most active in the 1920–1950 time period and Winstein from 1945 to 1970 time period though they did somewhat overlap in the 1950s (Figure 4).
Saul Winstein died suddenly at his home on November 23, 1969, aged 57, at the height of his career. He was perhaps the world leader in modern physical organic chemistry. With his fundamental studies of neighboring groups and reaction mechanisms he profoundly affected nearly every branch of chemistry, from industrial to biological. His results characteristically started whole new trends that can be identified with large bibliographies involving distinguished investigators the world over. He created a school of thought through his research, through his talks at national and international chemical meetings, and by his influence on former co-workers now in chemical laboratories throughout the world. Many terms and phrases that highlighted his discovery and elaboration of new phenomena or concepts have become so common that their origins may be unknown to the younger generation of investigators. Textbooks now abound in phrases such as “neighboring group participation,” “solvent participation,” “internal return,” “anchimeric assistance,” “intimate ion pair,” “ion pair return,” “bridged ions,” “nonclassical ions,” and “homoaromaticity.”62
We can add to the concepts named by Streitwieser the following: homoconjugation, solvent separated ion pairs, normal and special salt effects, common rate depression, external return, and homoallylic, homobenzylic, and homoconjugative participation.8, 62-66 And there is more: the Grunwald–Winstein equation,67, 68 which provides the linear free energy relationship between relative rate constants and the ionizing power of various solvent systems67, 69; the Winstein–Kosower70/Goering–Schewene71 diagrams, which illustrate the relationship between activation energies (kinetics) and equilibrium distributions (thermodynamics); and the Winstein–Holness equation, which provides the total reaction rate constant for a compound existing in multiple conformations, each of which reacted to give a different product.72, 73
While the term “nonclassical ions” was actually first proposed by John D. Roberts,74 it was Winstein who is most associated with establishing the validity and usefulness of this term. And this is just a partial listing of Winstein's accomplishments, achieved before his premature death at 57 years 2 months.62, 64, 65, 75-78 The word cloud extracted from the titles of Winstein's publications (Figure 6) reveals few direct overlaps in interests with Ingold (Figure 3). For Winstein, major topics include solvolysis, neighboring group(s) effects and participation, replacement reactions, acetolysis and solvolysis ions, [ion] pairs, and so forth. Indeed, there is a developing pattern that progresses from Ingold's pre- and post-1942 word clouds to Winstein's word cloud, a marker in the development of the discipline.
Returning to Figure 4, we note that citations to Winstein's publications exceeded Ingold's in number but also in its rate of acceleration. No doubt that number would have continued to increase substantially had Winstein not died in 1969. Indeed, his list of publications increased by 21 in 1971 (Figure 1), 2 years after his death. These were undoubtedly due to his students, who were publishing their results. Surely Winstein's impact on chemistry would have continued. At his death, he was at the height of his scientific productivity and influence.
After the four Titans I have described [Ingold, Robinson, Woodward, and Winstein], I was the lesser being who knew both some chemical physics and a reasonable amount of natural product chemistry. At the right moment in time, this produced a seminal article and nineteen years later in 1969 a Nobel Prize shared with Odd Hassel.24
Barton was no minor figure in organic chemistry. And he was certainly being poetically modest. Nonetheless, Barton placed Winstein in the same scientific hierarchy as Robinson and Woodward. We have one more datapoint provided by Barton. On November 12, 1969, Winstein received an undated handwritten letter from Barton. Barton was undoubtedly responding to a letter of congratulations Winstein had sent Barton on the occasion of the announcement that Barton would receive the NPch in December 1969 (Figure 7).

Ironically, Winstein was dead within a week of receiving Barton's letter, having suffered a massive heart attack while swimming in the pool at his home.
By the time of his death, Winstein had already garnered numerous awards, including the ACS Award in Pure Chemistry (1948), election to the National Academy of Sciences (1955), Richards Medal of the ACS (1962), election to the American Academy of Arts and Sciences (1966), ACS James Flack Norris Award in Physical Organic Chemistry (1967), and the Franklin Memorial Award for Outstanding Contributions to Chemistry (1968). He was awarded the US National Medal of Science posthumously in 1971 by President Richard M. Nixon.
Had he lived a few more years, he doubtlessly would have received the highest awards the world has to offer a scientist.62
3.2 The Winstein school of Physical Organic Chemistry
Saul Winstein was a well-liked and much-admired chemist's chemist.9, 54, 63, 64 It did him no harm to be on the opposite side of the nonclassical carbocation debate with H. C. Brown who was not much loved by his peer group.54, 80 It can well be said that Winstein's way of doing science spread out after him like the waves of a speeding boat, and not just by his many former students and postdoctoral associates of whom there were many. According to Donald J. Cram and William G. Young, Winstein had 72 Ph.D. students and 86 postdoctoral fellows, about 100 obtaining academic positions (Table 4).64, 75 Many of these continued Winstein's tradition as physical organic chemists or applied POC in their research in other subdisciplines of chemistry. For example, Dale Poulter at Utah investigated the isoprene biosynthetic pathway with emphasis on the mechanisms of the enzyme-catalyzed transformations. Winstein's approach to POC clearly seeped into Poulter's biosynthetic research program.81-85
| P. Ahlberg | E. Grunwald | Z. Rappoport |
| D. L. Allara | J. Grutzner | R. D. Rieke |
| R. A. Baird | H. K. Hall | R. M. Roberts |
| M. Battiste | G. S. Hammond | F. L. Scott |
| R. E. Buckles | R. F. Heck | P. Seidl |
| J. I. Brauman | E. Hedaya | S. G. Smith |
| M. Brookhart | G. Just | R. A. Sneen |
| R. F. Childs | H. D. Kaesz | T. G. Traylor |
| A. F. Diaz | E. M. Kosower | H. M. Walborsky |
| C. Eaborn | A. Ledwith | P. Warner |
| M. R. Feldman | G. C. Levy | D. Whalen |
| E. C. Friedrich | P. S. Magee | C. F. Wilcox |
| H. L. Goering | C. D. Poulter | |
| D. S. Glass | P. Radlick |
3.3 On Winstein's nonexistent Nobel Prize
According to the Nobel Prize Nomination Archive, Winstein received 24 nominations over 6 years, 22 of which came in the last 3 years of his life (Table 5). While Winstein's 24 nominations certainly did not compete with Ingold's numbers (over many more years), 24 is on par with or far greater than the number of nominations received by other laureates, for example, Sanger (NPch in 1958, 17), Libby (1960, 28), Calvin (1961, 22), Kendrew (1962, 10), Perutz (NP 1963, 13), Hodgkin (1964, 24), Mulliken (1966, 23), Eigen (1967, 31), Norrish (1967, 8), Porter (1967, 13), and Hassel (1968, 18). And with time, Winstein's number of nominations certainly would have increased substantially—just as his number of publications and citations to his publications were rising (Figures 2 and 4, respectively).
| Year | Number of nominations | Nominator (* = Nobel laureate) | Country of nominator |
|---|---|---|---|
| 1963 | 1 | Y. Ogata | Japan |
| 1964 | 0 | — | — |
| 1965 | 0 | — | — |
| 1966 | 1 | W. F. Libby* | USA |
| 1967 | 10 |
F. A. Anet D. J. Cram* W. F. Libby* G. Natta* G. T. Seaborg* W. Stanley* W. Young R. Huisgen C. Nenitzescu Y. Pocker |
USA USA USA Italy USA USA USA Germany Romania USA |
| 1968 | 7 |
G. W. Kenner W. F. Libby* G. Natta* R. Robinson* M. Simonetta T. Reichstein* J. U. Koskikallio |
Great Britain USA Italy Great Britain Italy Switzerland Finland |
| 1969 | 5 |
W. F. Libby* O. A. Reutov T. Reichstein* H. Cristol P. von R. Schleyer |
USA USSR Switzerland France USA |
- a Winstein received one nomination in 1970, from Harold Urey; this nomination likely was received after the deadline for nominations for 1969. In that Winstein died on November 23, 1969, others who may have nominated him for the 1970 Nobel Prize did not do so. However, it was only in 1974 that the Nobel Foundation stipulated that a Nobel Prize cannot be awarded posthumously, unless death occurred after the announcement of the Prize and before the award ceremony.
Any effort to compare Winstein's number of nominations with that of other nominees is hampered by the fact that Winstein's premature death limited the number of years he could have been nominated. To circumvent this limitation, we compared Winstein's number of nominations in three consecutive years with several Nobel laureates. (Why might this triplet be a marker for Nobel success? Because of Winstein's premature death at 57 years old, the total number of nominations is an unfair predictor. We thus considered taking the maximum value, for each nominee, of the sum of a few consecutive years as a predictor.) As can be seen in Table 6, Winstein's 22 nominations within three consecutive years almost reaches the top 10 for Nobel laureates and is second (with Reppe) only to Ingold among all non-recipients.
| Nobel laureates | Non-recipients | ||
|---|---|---|---|
| Name | Greatest number of nominations in any 3 consecutive year period | Namec | Greatest number of nominations in any three consecutive year period |
|
Woodward (1965) |
19-7-18 = 44 (1963–1965) |
Ingold |
13-4-16 = 33 (1967–1969) |
|
Barton (1969) |
12-6-20 = 38 (1967–1969) |
Reppe |
7-9-6 = 22 (1954–1957) |
|
Staudinger (1953) |
13-12-13 = 38 (1951–1953) |
Winstein |
10-7-5 = 22 (1967–1969) |
|
Moissan (1906) |
6-22-8 = 36 (1904–1906) |
Neil Bartlettf |
9-6-6 = 21 (1965-1967) |
|
Wittigc (1979) |
14-12-9 = 35 (1967–1969) |
Hedvall |
8-4-7 = 19 (1956–1958) |
|
Nernstd (1920) |
0-8-22 = 30 (1919–1921) |
Theorell |
4-7-8 = 19 (1953–1955) |
|
Ramsay (1904) |
3-4-23 = 30 (1902–1904) |
Bijvoet |
10-0-6 = 16 (1964–1966) |
|
Pauling (1954d) |
17-5-5 = 27 (1948–1950) |
Urbain |
4-3-9 = 16 (1924–1926) |
|
Onsager (1968) |
3-6-17 = 26 (1966–1968) |
Komppa |
7-4-4 = 15 (1942–1944) |
|
Eigen (1967) |
6-8-9 = 23 (1965–1967) |
Le Châtelier |
6-4-4 = 14 (1928–1930) |
|
Natta (1963) |
7-2-14 = 23 (1961–1963) |
Lynen |
4-4-6 = 14 (1961–1963) |
|
Pauling (NPch 1954e) |
2-8-13 (1952–1954) |
||
- a For each nominee, the summation of nominations of all trios of adjacent years was computed, and the maximum for that trio of years was determined. Nominees were then ranked according to their maximum trio, and the top 11 of laureates and non-laureates were selected. In all cases presented in the table, the maximum was unique. The numbers presented as x-y-z = w correspond to the number of nominations belonging to the trio of sequential years (x, y, z), whose summation corresponds to w.
- b Data from the Nobel Prize Nomination Archive, 1901–1970.86
- c The years represented for Wittig in this table correspond to 1967, 1968, and 1969. Therefore, the data presented here may be modified by the years for which nomination data are not yet available (1970 and later years) in the Nobel Prize Nomination Archive. Wittig received the NPch in 1979.
- d It is curious that Nernst received so many nominations for the year after his receipt of the NPch.
- e Pauling also received the Nobel Prize in Peace in 1962.
- f Neal Bartlett (1932–2008) also surely received nominations after 1970 as he received 10 and 6 nominations in 1969 and 1970, respectively. In 1962, Bartlett prepared the first noble gas compound, xenon hexafluoroplatinate, for which his nominations for the NPch reflected.
We also note that the number of Winstein's nominations went from a high of 10 in 1967 to seven in 1968 and five in 1969. Analysis of the Nobel Prize Archive for chemistry has revealed many instances of a chronological trend “high, then low, then high again.”48 For example, in the same time period, from 1958 to 1965, Woodward's nomination record was 5, 7, 12, 14, 6, 19, 7, and 18 (NPch in 1965). Heyrovsky's record from 1954 to 1959 was 1, 1, 12, 4, 1, and 11 (NPch in 1959). There is every reason to believe that Winstein's future number of nominations would have increased substantially from the five he received in 1969, that is, 12, 7, 5, ???, interrupted by Winstein's death in 1969.
The main purpose of preparing Table 6 was to examine Winstein's nomination record against other nominees while controlling for his premature death and thus lessoned opportunities for receiving the NPch. Nonetheless, Table 6 provides some interesting observations. Most of the Nobel laureates received their highest number of nominations (in this sequence) in the year of their award. Nernst received 22 nominations the year following his Nobel Prize; that is odd. Georg Wittig's sequence of high nominations was a decade before his NPch. And Pauling appears twice on this list.
It is worth pointing out that the chemical community understood the pairing of Ingold and Winstein as the premier physical organic chemists of the 20th century. In 1967, Rolf Huisgen, Costin Neniţescu, and Yeshayau Pocker each nominated both Ingold and Winstein; Pocker also nominated Louis Plack Hammett. In 1969, both Stanley J. Christol and Paul von R. Schleyer nominated Ingold and Winstein. Within this small sample, it is clear that several nominators envisioned a joint Ingold–Winstein Nobel Prize.
Of note is the fact that Robinson was one of Winstein's nominators.
Returning to Barton's letter (Figure 7), it is also interesting to note that, up to and including 1969, Barton made only five nominations for the NPch, three for R. B. Woodward, and two for Carl Djerassi (Table 7). It is noteworthy that despite his complimentary and even promising letter to Winstein (Figure 7), Barton himself had not nominated Winstein. Rather, in 1969, Barton nominated another academic also from a California university, Carl Djerassi (Stanford).
| Year | Nominee (NPch year) |
|---|---|
| 1955 | R. B. Woodward (1965) |
| 1961 | R. B. Woodward |
| 1963 | R. B. Woodward |
| 1966 | Carl Djerassi |
| 1969 | Carl Djerassi |
Barton's behavior seems to be artfully political. But perhaps Barton had been informed via the grapevine87 that Winstein's year was coming shortly and felt it was unnecessary to nominate him again. It should be noted that Barton was a major “player” within the chemistry community, in addition to his being a Nobel laureate. For example, Barton was the Chair of Pergamon Press, the publisher of Tetrahedron and Tetrahedron Letters, then the premier international organic chemistry journals.
4 THE SYNERGIES OR ANTI-SYNERGIES IN THE NOBEL PRIZE SELECTION PROCESS
We48 and Friedman3, 4 have identified a number of instances in which individuals have received the NPch, not because they were overwhelmingly nominated but because they were at the right place at the right time.
For example, 1951 was Glenn Seaborg's year. His accomplishments were such that he was clearly a Nobelist in waiting; and he had received numerous nominations by highly placed individuals including several Nobel laureates and members of the Royal Swedish Academy of sciences. Seaborg shared the 1951 Nobel Prize with his Berkeley colleague and collaborator Edwin McMillan. Prior to 1951, McMillan had received only one nomination for the Nobel Prize in 1947, and it was for the Nobel Prize in Physics.
For another example, in 1931, Friedrich Bergius shared the Nobel Prize with Carl Bosch. Bosch garnered nine nominations in 1931 matching his nine nominations in previous years (including one from Albert Einstein—Einstein's only nomination for the chemistry prize). Bergius had received only two nominations ever. According to Friedman,3 Bergius was chosen to defuse the sensitivities that remained following Bosch's failure to share the 1918 Nobel Prize with Fritz Haber for nitrogen fixation. In the minds of some, the combination of Bergius–Bosch made up for the slight of Bosch in 1918. Had Bosch joined Haber in 1918, Bergius's 1931 NPch would not have been necessary, and Bergius would not have been so awarded.
With this background, we return to Ingold and Winstein. We posit that the Royal Swedish Academy of Sciences was reluctant to choose Winstein for the NPch without simultaneously awarding the Nobel Prize in Chemistry to Ingold. But to give the NPch to Ingold would be to backtrack on 23 years of excluding him. And it would affront the strong negative influence of Robinson who, at 83 years old, would outlive Ingold by more than 4 years. Perhaps the Academy (and the Nobel Committee for Chemistry) thought they could outlast Robinson who was born 7 years before Ingold. But Robinson outwitted them.
This kind of strategic planning—taking into consideration bits and pieces of different influencers—had been part of the inner workings of the NPch for decades.3 And such a plan had a reasonable chance of succeeding, but ultimately, Winstein and Ingold died in 1969 and 1970, respectively. Robinson hung on nearly blind until he was 88, dying in 1975, despising Ingold until the bitter end.
5 CONCLUSIONS
The most obvious conclusion from this research is, as we all already know from intuitive experiences,88 that many deserving scientists never received the Nobel Prize. Whether or not we have sat on award selection committees, we also understand that there are never enough awards for all who deserved them, and that some degree of judgment and bias affects every award selection. The inability to award all those deserving is especially challenging for an award program that is open to all chemists—and now, seemingly to all life scientists5—rather than to one subdiscipline of chemistry. We also intuitively understand that the ebb and flow of science is such that there are periods of many seminal achievements and other periods less so. Timing can be everything.
One might consider that the selection process for the NPch would be sensitive to the opinions of the relevant communities. Indeed, the Royal Swedish Academy of Sciences is exposed to the review and criticism by its most relevant communities, should those communities' most deserving members fail to be chosen or if lesser deserving members are chosen or if disciplines outside of “chemistry” are considered to be “chemistry.” The authoritativeness of the Nobel Prize itself depends on the support and enthusiasm of its foundational communities. There is every reason for the Nobel Committees and the Royal Swedish Academy of Sciences to be responsive to the nominations it receives—especially since, to make a nomination, you must receive an explicit invitation from the Academy to do so.
The availability of the Nobel Prize Nomination Archive has made it possible to look more closely at the NPch award program. The Nobel Prize Nomination Archive reveals that Ingold's 112 nominations and 77 nominators over 31 years far surpasses nominations of all other chemists save one, that being R. B. Woodward. And Woodward eventually became a Nobel laureate. Ingold never did. We48 and others24, 48, 58 believe that Ingold's Nobel Prize was, at least in part, thwarted by his nemesis, Oxford's Sir Robert Robinson. Robinson was connected to Stockholm in many ways, especially after his Nobel Prize year (1947), including through his former student, the Swedish chemist and member of the Royal Swedish Academy of Sciences, Holger Erdtman. Was it such a high barrier to overcome—Robinson's strongly negative feelings about Ingold—to award the Nobel Prize to Ingold? Apparently so. The Nobel Committees and the Academy had, by Ingold's time, much history in the application of personal and professional biases in their selection processes.3, 4, 46, 48
Ingold could have shared the 1969 Nobel Prize with Barton and Hassel “for their contributions to structure and mechanism in the understanding of chemical reactions.” But had Ingold done so, what of Winstein who very rightfully could have been paired with Ingold? Barton, Hassel (the Scandinavian chemist, a heritage often favored by the Nobel selection committees), Ingold and Winstein were four, and the (artificial) limit of three laureates in any 1 year for any one Nobel Prize would have been broken. To omit Winstein and award Ingold, especially in the face of Robinson, may well have not been feasible.
As for Winstein, the Academy and the Committee may have anticipated—sadly and incorrectly—that Ingold's contributions were too distant and that Winstein's time had yet to come. It is also quite possible that awarding the Nobel Prize to Winstein and not to Ingold might have been too awkward even for the Royal Swedish Academy of Sciences. In the eyes of the Nobel Committee for Chemistry and the Academy, that consideration might have delayed Winstein's Nobel Prize.
Since the number of scientists in the 1960s was much less than these days, human relationships were far more important also in affecting the selection of a recipient of a prize.
Another factor that we have not discussed in detail but have implied is the undervaluing of POC by the Royal Swedish Academy of Sciences and the Nobel Committee for Chemistry. That being said, some achievements in POC received Nobel recognition: Barton and Odd Hassel's conformational analysis, Hinshelwood and Semenov for reaction mechanisms, Prelog for stereochemistry, Roald Hoffmann for the Woodward–Hoffmann rules, and Donald J. Cram, Jean-Marie Lehn, and Charles J. Pederson for designing complexing agents.
The bottom line is this. Notwithstanding that nominations for the NPch are the unique privilege of those invited by the Nobel Committee for Chemistry and those automatically invited by the bylaws of the Nobel Foundation, having received 112 nominations from 77 different individuals who lived in 22 different countries during a 31-year period was not sufficient to assure an invitation to Stockholm for Ingold. Still, Ingold's legacy continues to this day, at least in terms of his chemistry and his nomenclature for organic reactions. Yes, living a long life does add years of opportunities to become a Nobel laureate. Winstein's life ended too soon for the Nobel Prize but even more sadly, for the progress and legacy of chemistry. All too often, in the minds of their relevant communities, the memories and historical worthiness of the greatest scientists is tied to their receipt, or not, of the Nobel Prize.
6 CODA
Another form of legacy is the accomplishments of one's children. Sir Christopher Ingold and Dr. Edith Hilda née Usherwood Ingold had three children, Keith, Dilys, and Sylvia. Keith Usherwood Ingold (Figure 8) is a physical chemist and expert in organic free radicals. A longtime member of the staff of the Canadian National Research Council, he is a Fellow of the Royal Society, a Fellow of the Royal Society of Canada, and a recipient of many awards, including the Sir Derek Barton Gold Medal of the Royal Society (2016).89, 90

Saul and Sylvia Winstein had two children, Bruce and Carolee. Bruce Darrell Winstein (1943–2011) (Figure 9) was an experimental physicist and cosmologist and a member of the National Academy of Sciences (1994) and the American Academy of Arts and Sciences (2007). He taught at the University of Chicago from 1972 until his death.91 Carolee J. Winstein (Figure 10) is an expert in neurology, physical therapy, motor behavior, and rehabilitation at the University of Southern California where she is a professor of neurology.92 She has published over 170 peer-reviewed articles in these fields. She is an elected Fellow of the American Society of Neurorehabilitation, a Fellow of the National Academy of Kinesiology, and a Fellow of the American Heart Association, among many other awards. She is married to Kip Thorne, an American theoretical physicist at Caltech and recipient of the 2017 Nobel Prize in Physics.


7 CODA TO A CODA
Let us now stand back from the detail and attempt a broad assessment of the significance of Hughes's scientific work, performed in the period 1928-1963. It can certainly be said that this work has changed the aspect of organic chemistry, by progressively replacing empiricism by rationality and understanding, to a degree which is now manifest in the terminology and teaching of the subject, and in the research activity all along its advancing frontier. This revolution of approach has been completed within the span of Hughes's scientific life, essentially because his particular combination of scientific and human insight enabled him, with the colleagues whose loyalty he commanded, not only to provide the required scientific concepts, but also to achieve their general acceptance, even though this task in communications involved a campaign to break through a sustained opposition. But, as the opposition ceased, and the new outlook became adopted increasingly, and particularly rapidly abroad, Hughes did feel, with a deep satisfaction, that the main purpose of his scientific life was being, and would be, fulfilled.93
8 CODA TO A CODA TO A CODA
Progress in one of the most active fields of chemical science during the present century has resulted from attempts to elucidate the detailed mechanism of organic reactions in terms of modern physical concepts. Throughout this development Ingold's contributions are especially distinguished. Possessing detailed knowledge and understanding of both the physical and organic branches of the science he has been in a position to effect the synthesis of the two modes of approach without which a successful attack on the difficult, yet fundamental, problems involved could not be achieved. … Although his theoretical contributions have attracted more attention, the originality of his experimental technique is equally noteworthy and his happy selection of crucial tests amounts to genius.100
I am greatly obliged to colleagues in the Council for their assistance in the preparation of notes on the Medallists.100
ACKNOWLEDGEMENTS
We thank Kendall N. Houk, who held the Saul Winstein Chair in Organic Chemistry at UCLA for many years, for providing access to the letter from Derek H. R. Barton to Saul Winstein (Figure 7). We also thank Carolee Winstein and several others cited in the text for the use of photographs. We thank Celia Arnaud, Keith U. Ingold, Kenneth T. Leffek, the late John D. Roberts, Stephen J. Weininger, and several highly knowledgeable and experienced reviewers for valuable discussions. One of us (JIS) thanks the staff of the Boatwright Memorial Library for continuing technical assistance.
Open Research
DATA AVAILABILITY STATEMENT
Data from the open access Nobel Foundation https://www.nobelprize.org/nomination/archive/search.php.




