Treatment of malignant rhabdoid tumors of the head and neck with combined chemotherapy and 125I particle implantation
Funding source
Beijing Municipal Commission of Science and Technology Capital Clinical Medicine Applied Research and Popularization Special Funding Support (No. Z151100004015159)
INTRODUCTION
The malignant rhabdoid tumor (MRT) was originally described as a highly malignant variant of Wilms’ tumor in 1978 and was designated as a distinct entity in 1981.1 This highly aggressive tumor is characterized by its rhabdoid feature and biallelic loss of SMARCB1/INI1/hSNF5.2 The prognosis is very poor with only 31% of patients surviving to 1 year,3 and there are no standardized treatment strategies available.
MRT in the head and neck region is very rare. Data from the UK showed that head and neck MRT accounted for about 15% of all extra-cranial MRT.3, 4 Because of the complexity of the anatomical factors, surgical resection is difficult to perform. To date, the information available concerning treatment strategies and outcomes for this disease is limited. To add to the current knowledge and share our treatment experience, we report two cases of pediatric head and neck MRTs treated with anthracycline and platinum-based intensive chemotherapy and brachytherapy that have remained in remission for over 3 years.
CASE REPORT
Clinical features
Case 1
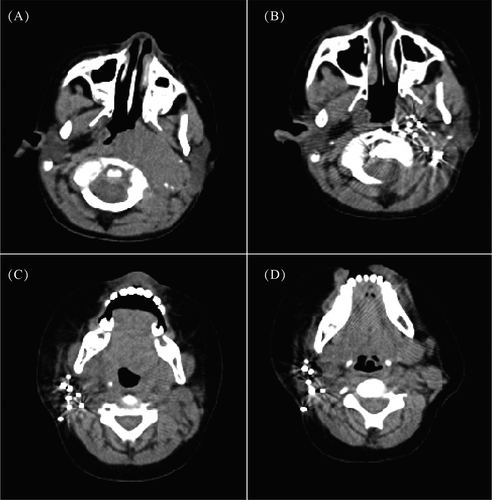
A 4 years and 7 months old boy, presented with a left neck mass first noticed 5 months prior, and the tumor size increased rapidly 2 months before admission. Physical examination revealed a hard and obscure boundary mass with a size of 8 cm × 8 cm in the left neck. The boy had hoarseness, and tongue deviation. PET/CT revealed a giant hypermetabolic lesion in the left parapharyngeal space and left nasopharynx, and the tumor crossed the midline to the right nasopharynx and right sphenoid bone (Figure 1A). A contrast-enhanced MRI scan showed a 5.8 cm × 2.7 cm × 6.2 cm soft tissue mass in the left parapharyngeal space and left retropharyngeal space. Bone marrow trephine showed no abnormal infiltration.
The patient underwent left neck tumor biopsy. Microscopic features showed small cell malignant tumors. Immunohistochemistry showed the tumor cells were positive for epithelial membrane antigen (EMA), and the staining was negative for INI-1, desmin, MyoD1, myogenin, FLI-1.The pathological diagnosis of MRT was established according to the morphological and immunohistochemical findings. According to the imaging findings, the clinical TNM staging was T2bN1M0.
Case 2
A 1 year and 2 months old boy presented with an asymptomatic right neck mass first noticed 2 months prior, and was admitted to Beijing children's hospital. The patient had no fever, fatigue, loss of body weight or any other symptoms. Physical examination revealed a 6 cm × 6 cm hard mass located in the right neck without redness and ulceration. PET/CT detected a hypermetabolic lesion in the right neck, and no other hypermetabolic lesions in other parts of the body (Figure 1C). A contrast-enhanced MRI scan showed a 3.5 cm × 3.7 cm × 4.8 cm space-occupying lesion in soft tissue of the right neck. Bone marrow trephine showed no abnormal infiltration.
The patient underwent a right neck mass biopsy. Pathological results showed an extra-renal MRT. Immunohistochemistry showed that the tumor cells were positive for vimentin, EMA, SYN, and CD99. Staining was negative for INI-1, myoglobin, and desmin. The pathological diagnosis of MRT was establis hed according to the morphological and immunohis tochemical findings. According to the imaging findings, the clinical TNM staging was T2aN0M0.
Treatment strategies
Chemotherapy
After biopsy, the two patients were treated with intensive chemotherapy. The Children's Oncology Group (COG) protocol for patients with renal MRT was adopted. The chemotherapy protocol was as follows: vincristine, cyclophosphamide, and doxorubicin (VDCPM1) at weeks 1, 7, 13, 19, and 25, and cyclophosphamide, carboplatin, and etoposide (CPM5+CE) at weeks 4, 10, 16, 22, and 28.
Local control
125I particle implantations were done in case 1 and 2 after five and six cycles of chemotherapy, respectively. According to their tumor volume, they were implanted with 80 and 96 radioactive particles respectively.
Toxicity
Both patients had grade IV neutropenia, and I-II degree anemia and thrombocytopenia after chemotherapy. No lethal infections occurred. Both patients suffered from grade I-II skin reactions after seed implantation, manifested as erythema or mild edema of the local skin, which can be improved within 1-2 months.
Follow-up
Tumor responses were evaluated at 2 months from the beginning of chemotherapy and 6 months after seed implantation, and every 3 months thereafter. The response evaluation showed very good partial regression (VGPR) after the treatment completed. Follow-up time were 70 months and 40 months respectively, and the two patients remained stable with the evaluation showing VGPR.
DISCUSSION
MRTs are rapidly progressive tumors, with most deaths occurring within 12 months of presentation. The overall survival (OS) rate for patients with MRTs enrolled in NWTS1-5 was only 23.2%. Extra-renal, extra-cranial MRT was even rarer. Radiotherapy has been shown to affect outcome, and a young age at diagnosis is strongly associated with an adverse outcome. In addition, high-stage (stage-III/IV) disease is correlated with an adverse outcome.5, 6
Regarding chemoradiotherapy, anthracycline and actinomycin have been important chemotherapeutic agents for the treatment of MRT.7 The results of the EpSSG NRSTS 2005 showed that chemotherapy regimens that include vincristine, doxorubicin, and cyclophosphamide (VDCy), alternating with cyclophosphamide, carboplatin, and etoposide (CyCE), combined with surgery and radiotherapy, can bring about a possible improvement in outcome for patients with extra-cranial MRTs. The 3-year event free survival (EFS) in the above-mentioned cohort was 32.3%, and OS was 38.4%. For metastatic disease, however, the 2-year EFS was only 8.7%, and OS was only 13%.8 Wagner et al9 also showed that alternating courses of the combination of VDCy, and the combination of ifosfamide, carboplatin, and etoposide(ICbE), have been suggested to be effective in metastatic MRTs. MRT patients may also benefit from autologous peripheral blood stem cell transplantation.10
For patients with solid tumors, local control is critical to a favorable outcome. Because of anatomic constraints, head and neck sarcomas are dif ficult to resect completely. Different from conventional radiotherapy, image-guided brachytherapy delivers a sufficiently high dose to the tumor target with a very sharp fall-off outside the implanted volume, so it can greatly reduce the side effects of radiation therapy, and our previous data showed that 125I particle implantation is effective for head and neck sarcomas.11
In conclusion, our data showed that young children with localized head and neck MRTs may enjoy a lengthy remission after treatment with combined intensive chemotherapy and 125I particle implantation. EFS time for the two patients were 70 m and 40 m respectively, and compared favorably with results reported previously. Systemic chemotherapy with an anthracycline and platinum-based protocol seems effective for patients with MRTs, and 125I particle implantation seems effective for local control. No extended resection and external radiotherapy treatment can avoid the risk of functional and cosmetic morbidity. Therefore, the combined intensive chemotherapy and 125I particle implantation may be a promising option for children with head and neck MRTs.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.




