Advancing Health Equity in Europe: Explore, Tailor, Implement, and Evaluate (ETIE)—A Framework of Diversity and Fairness in Pharmacoepidemiologic Research
Funding: The authors received no specific funding for this work.
ABSTRACT
Pharmacoepidemiology should represent and benefit populations equitably, embracing diversity and equity, and ensuring fairness. This article describes equity and fairness in pharmacoepidemiology, depicts key diversity domains, and provides an operational framework and call for action to implement diversity and fairness in pharmacoepidemiologic research. To ensure fairness, studies should address diversity and inclusion while providing equal opportunities and benefits for everyone in the target population. To implement and evaluate fairness in pharmacoepidemiology, we defined the following diversity domains: biological sex, socially constructed gender, age, life stages (e.g., pregnancy, menopause), ethnicity, race, migration, nationality, socioeconomic status, education, health literacy, and health status and capabilities. These are determinants of health, either through biological pathways or through social norms, discrimination, and barriers to healthcare or research participation. They are interlinked, their impact is study- and context-specific, and due to their sensitive and evolving nature, they should be handled with caution. Implementing diversity domains enables researchers to assess the generalizability of findings, identify and address health inequities, account for determinants of health, and ensure the fairness of algorithms, implementations, and recommendations. To successfully implement diversity domains and ensure fair pharmacoepidemiologic research, we recommend researchers to follow the Explore, Tailor, Implement, and Evaluate (ETIE) framework: Explore the role/implication of the diversity domains in the study, tailor their definitions to the study context, implement them appropriately and evaluate the study findings in their context. Increased availability of diversity data is needed, and support from stakeholders is essential. This manuscript was endorsed by the International Society for Pharmacoepidemiology (ISPE).
Summary
- Pharmacoepidemiology should represent and benefit everyone in the population equitably.
- To implement and evaluate fairness in pharmacoepidemiology, we defined important diversity domains and provided the Explore, Tailor, Implement, and Evaluate (ETIE) framework.
- Diversity domains are determinants of health and include sex, gender, age, life stages, ethnicity, race, migration, nationality, socioeconomic status, education, health literacy, and health status and capabilities.
- Implementing the ETIE framework is expected to aid in addressing diversity and inclusion, evaluating the fairness of the data-driven analyses and study findings, and identifying health disparities and inequities.
- Every stakeholder should join forces to increase availability and implementation of diversity data, and improve health equity and fairness in pharmacoepidemiology.
1 Introduction
Pharmacoepidemiology addresses a myriad of health-related questions and should be fair and benefit all individuals equitably.
Historical underrepresentation of women [1, 2], racial and ethnic minorities [3] and other groups in clinical trials relates to unequal societal benefits. Recently, there is growing appreciation of embracing diversity and inclusion (D&I) in clinical trials [4-6]. Similarly, although real-world evidence (RWE) is considered more inclusive population-wise compared to randomized controlled trials (RCTs), restrictions due to study design may occur, and real-world data (RWD) can reflect systemic societal biases. Therefore, embracing D&I is important in both RCTs and RWE. However, how to address this in pharmacoepidemiology remains unclear, and heterogeneous approaches are expected in different societal contexts.
Investigating diversity aspects in study populations enables assessing inclusion and generalizability of the findings, but also aids identifying disadvantaged groups (e.g., under-researched, underserved), addressing health inequities and establishing fairness of studies, algorithms, implementations, or recommendations. Every pharmacoepidemiologic study, also beyond health equity research, should ensure equity, and fairness.
This article aims to describe the concepts of equity and fairness in pharmacoepidemiology, to depict key diversity domains and to provide a framework for operational guidance and a call for action to implement diversity and fairness in pharmacoepidemiologic research.
2 Framework Development Process
This article and framework were developed by an international taskforce of pharmacoepidemiologists, health equity and diversity experts, citizen/patient research partners, and patient and community representatives. It is based on scientific literature, applicable regulations, and expert opinion. Input from collaborators was collected through surveys, panel discussions, and literature and manuscript review and editing. This manuscript was endorsed by the International Society for Pharmacoepidemiology (ISPE).
3 Health Equity and Fairness in Pharmacoepidemiologic Research
Health equity is “the absence of unfair and avoidable or remediable differences in health among population groups” [7]. Unlike equality, which refers to—or targets to achieve—the same for everyone, equity acknowledges that we all face different burdens and privileges throughout life, thus, needs, and subsequent strategies to tackle them may differ across population groups. Although the terms health inequalities and health inequities are often used interchangeable, health inequities may be defined as unfair health inequalities [8]. Achieving health equity implies removing burdens to health (e.g., discrimination, low resources) [9], tackling health inequities, unfair disparities, unfair inequalities.
While health equity research aims to reduce health inequities, general pharmacoepidemiologic studies with other main focuses (e.g., drug utilization, safety, effectiveness, efficacy) should also consider health equity and strive for fairness.
A fair study benefits equally everyone in the target population. Otherwise, a bias occurs if it favours or underserves certain group(s). Health equity related biases are gaining importance in the field.
Regarding fairness in pharmacoepidemiology, we here discuss the following.
3.1 Equal Opportunity
Identifying and confronting health inequities is crucial to ensure equal opportunity to health care and evidence amongst everyone in the target population. Pharmacoepidemiologists can raise awareness, bridge data gaps, and provide evidence to highlight the importance of diversity, equity, and fairness in health research and practice.
3.2 Diversity and Inclusion (D&I)
Study populations should accurately represent their target population. Otherwise, the applicability of the findings can be limited in the underrepresented groups.
3.3 Equal Benefit or Model Performance
Health data (especially RWD) can reflect human and societal biases and, when not addressed, data-driven findings can perpetuate or exacerbate these inequities [10]. Biases may arise from not-at-random missing data (e.g., disparity in using resources), underrepresentation and misclassification or measurement error (e.g., unequal treatment decisions) [11]. Failing to attend to group disparities can lead to aggregation bias, erroneously using the one-size-fits-all approach [12]. There are numerous examples of data-driven findings perpetuating or enlarging biases from their data [13, 14]. This intersects with increasing discussions of fairness in statistical algorithms, machine learning (ML) and artificial intelligence (AI) [10, 15, 16]. Evaluating model performance overall is no longer sufficient and, instead, evaluating it across population groups is needed. Several fairness measurements and definitions to compare population groups have been described, and there is a growing body of bias mitigation strategies [15-18]. Choice of fairness definition should be study-specific and can include: equal benefit; equal performance; or equity in allocation of resources.
The diversity domains depicted in this article (Figure 1) should be considered when addressing these fairness aspects in pharmacoepidemiologic studies. Additionally, we provide the Explore, Tailor, Implement, and Evaluate (ETIE) framework to guide this process (Section 5).
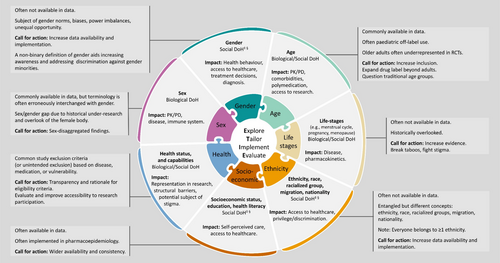
4 Diversity Domains
4.1 Sex and Gender
Sex and gender are different but entangled health determinants [1]. Biological sex (female, male and intersex), although driven by sex chromosomes, genitalia and reproductive organs, is generally assigned at birth based on visible attributes. Sex is typically categorized as female or male, though variation exists; it is estimated that up to 1.7% of the population has intersex traits, with characteristics not matching female/male categories [19], whether visible or not. Socially constructed gender is an evolving multidimensional concept. It encompasses socially constructed roles, behaviors and identities, influencing self-perception, social interactions and the distribution of power and resources [20]. Gender dimensions include gender identity (e.g., cis, trans, non-binary, etc.), gender roles/norms, gender relations, institutionalized gender [21] and gender expression [22]. Gender categories depend on the dimension [23, 24] and gender is context-dependent. We acknowledge that gender is independent of sexual orientation.
There are biological factors influenced by sex (e.g., driven by sex chromosome genes [25] and sex hormones [26]), such as the immune system [26], body composition [27, 28] and physiological mechanisms of pain [29]. Sex can also influence pharmacokinetics (e.g., due to pH of gastric fluids, body composition, or enzyme expression), pharmacodynamics [22, 30-35] and pharmacogenomics [36]. Sexual specificities are observed in disease prevalence (e.g., inflammatory and autoimmune diseases [26, 37]), disease phenotype (e.g., heart failure [38]) and drug metabolism (e.g., zolpidem clearance [39]) and response (e.g., adverse events [31, 33, 36]). Gender can also impact health-related aspects [40, 41], like self-assessed health status, health behaviour (e.g., smoking, use of healthcare services) [42, 43], access to healthcare [44], clinical communication [45], diagnosis [46] and treatment [47-50] (e.g., prescription and/or drug use [21, 51]). Gender biases in the healthcare system have been described (e.g., pain management [47]; referral to specialist [52, 53]) and can be observed due to: historical overlook of women in health research [25, 47, 54-58]; predominant health focus on male-based standards [43]; neglect of female-specific diseases (e.g., endometriosis); limited sex-disaggregated health findings; scarce structural presence of gender minorities; lack of consideration of gender dimensions; and discrimination faced by the LGBTI+ community [42] (also known as LGBTQIA+, lesbian, gay, bisexual, transgender, queer, intersex, asexual, and more).
Despite the role that sex and gender can play in disease and treatment, many studies fail to address them [1, 38, 59], and research on gender minorities is even scarcer. Burdens contributing to these include unawareness, inconsistent terminology, social and structural bias and data and statistical limitations.
The Sex and Gender Equity in Research (SAGER) guidelines emphasize the understanding of sex and gender differences as essential for research [60, 61], and the European Institute of Women's Health accentuates this for drug research [62]. European Good Clinical Practice guidelines suggest sex/gender subgroup analysis [63, 64]. Scientific journals require information on how sex and gender were considered in the studies [61, 65, 66], funding agencies are adopting similar requirements, [67] and research ethics committees are encouraged to do so [68, 69].
The interplay between sex and gender is complex. Researchers should question which dimensions of sex and gender are most relevant for their study and plan accordingly. Transparency and consistency in sex and gender data need improvement (e.g., indicating if sex is as assigned at birth or otherwise). When lacking gender data, using sex as a proxy or using other related variables is a possible alternative, though neither is ideal and the choice is study dependent (e.g., computational phenotyping may serve to identify trans individuals in RWD if missing self-reported gender) [70].
Studies should seek equal opportunity of inclusion of males and females (unless clearly not applicable, for example, pregnancy study) and be inclusive of sex/gender minorities. Reporting of both sex and gender is needed to address this.
The World Health Organization (WHO) states: “When data on individuals are broken down by sex, health systems are better able to identify and respond to gender inequalities in health, and allocate resources accordingly” [71]. In pharmacoepidemiology, simply adjusting for sex may not be sufficient to understand if a drug effect is independent of this factor [72]. Thus, we encourage researchers to provide sex-disaggregated findings, unless clearly not applicable. Given enough sample size, sex differences are to be evaluated using statistical interaction tests [72] and discussed in terms of clinical relevance. If sample size limits this, we encourage researchers to provide exploratory findings to support future meta-analyses. Strengths and limitations of subgroup analyses are discussed elsewhere [72, 73].
Besides sex-disaggregated data, some studies would benefit from gender-disaggregated findings, exploring relevant gender dimensions (e.g., assessing adherence; trust in healthcare system).
4.2 Age
Traditional age groups include neonates, children, adolescents, adults, and older adults.
After historical lack of paediatric drug research, the European Union Paediatric Regulation (2007) was a major milestone [74, 75], yet off-label use in paediatrics remains common. Adolescents may also be considered a neglected group, indicating a gap needing attention. RWE can highlight off-label use and investigate drug effects and comparative effectiveness in paediatric population [76].
With aging, the decline in body functions [77] increases vulnerability to diseases (e.g., infections, cataracts, osteoporosis, dementia) [77, 78] and impacts pharmacokinetics (e.g., reduced hepatic and renal clearance, reduced total body water, elevated blood–brain barrier permeability) and pharmacodynamics (e.g., altered receptor sensitivity, signalling pathways, and homeostasis), leading to altered drug sensitivity and response and increased adverse effects [79]. Aging also has psychological (e.g., loss of purpose after death of partner) and social implications (e.g., reduced income [78], social isolation [80]) affecting health. Although «drugs should be studied in all age groups» [81], older adults are often underrepresented in or excluded from clinical trials [82-84].
In pharmacoepidemiology, addressing prevention, treatment, and sustainable care in older adults is expected to significantly benefit individuals and society. Biological, psychological, and social factors associated with aging should be considered at study design.
Chronological age is a well-documented variable in data and widely accepted as determinant of health. However, age ranges are often based on legal/societal regulations, thus, we invite researchers to challenge these cut-offs. Flexible modelling (e.g., using splines) can better reduce age-related confounding than merely assuming age to have a linear association with the outcome.
Lastly, chronological age (time since birth) and biological age (cell damage accumulation) may not always correlate [85]. While it is foreseeable that we continue using chronological age as per usual, biological age could gain presence as we learn more about it (e.g., aiding high-risk treatment decisions [85]).
4.3 Life Stages
Life stages characterized by shifts in sex hormonal levels, physiological changes and social factors include menarche, menstrual cycle (fluctuations of estradiol and progesterone [86]), perimenopause, menopause (no menstrual cycle for at least 12 months; loss of ovarian follicles, reduction of oestrogen [87]), pregnancy (high estradiol and progesterone), antepartum, postpartum (drop in estradiol and progesterone), breastfeeding, and andropause (male hypogonadism, low testosterone [88]).
Sex hormones influence the immune system [89, 90], the intestinal barrier function and gut microbiome [91], inflammatory biomarkers [92] and pharmacokinetics [32]. From a social perspective, life stages can involve changes in societal roles and norms, and may be stigmatized. Though largely overlooked, there are examples of clinical disparities across life stages. For example, rheumatoid arthritis (RA) disease changes are observed across pregnancy, post-partum [93] and menopause [94]; and disease variability linked to the menses has been reported in persons with RA [95-97] and persons with inflammatory bowel disease [98-101]. Pregnancy involves physiological changes that can affect pharmacokinetics [102], and drug exposure in the offspring is a concern during pregnancy and lactation. After menopause, cardiovascular and osteoporosis risk increases [87] and there are changes in body composition [103]. And low testosterone in males was associated with sarcopenia, low bone density, mood-cognitive disorders, and anaemia [88].
These life stages could be understood as determinants of health, yet their impact on disease and treatment remains largely unknown.
RWE in pregnancy and lactation is essential to understand medications in these life stages, as pregnant and breast-feeding women are commonly systematically excluded from RCTs due to ethical concerns. There is growing debate on the need to reevaluate this longstanding exclusion. From the regulatory perspective, steps to improve drug safety in the pregnancy and breastfeeding population are ongoing, with interdisciplinary approaches sought to optimize this [104]; and acknowledgement that under certain circumstances, inclusion of pregnant individuals in clinical trials may be scientifically and ethically appropriate [105, 106].
Other life stages are often overlooked. Menopause data is limited to a date, and perimenopause and menstrual cycle are largely undocumented, or restricted to pathological conditions. This gap is likely due to social misconceptions and taboos. While emerging technologies capturing self-reported data (e.g., period tracker apps) offer an opportunity to address this issue, traditional databases should also enlarge these data.
Understanding how hormonal changes affect disease progression and drug response is expected to improve disease management and personalized care. Recognizing disease flares due to hormone fluctuations (e.g., menstrual cycle) can empower patients, aiding self-management of symptoms. Also, a deeper understanding of the social and health implications of life stages could shape policies to mitigate their undesired impact.
4.4 Ethnicity, Race, Migration, Nationality
Ethnicity is a multidimensional dynamic concept [107], referring to groups of people with shared attributes such as language [108, 109], culture [110], social norms, roles, or behaviours. Ethnic groups are fluid, better seen as clusters instead of fixed categories, and people can belong to several clusters. While ethnic pride may vary between groups, every person falls under one or several ethnicities. Majority groups are not exempt to be ethnic cluster(s), since ethnicity is not “others”, but “all of us”.
In English-speaking contexts, race and ethnicity are often used together due to their interconnection, with race referring to groups with similar physical appearance. In some European contexts, the term “ethnicity” is preferred over “race,” due historical and linguistic connotations. However, avoiding “race” in Europe could hinder awareness/recognition of existing racism [111]. Alternatively, “racialized” is used for social grouping based on perceived characteristics, often resulting in unequal treatment (e.g., privilege, discrimination). Racialization is entirely a social concept, based on socially established differences of ethno-racial features [111] and can disconnect from self-defined race and ethnicity.
Sociocultural norms, ethnicity, race, and racialized groups can affect access to healthcare and be subjects of discrimination. For example, European ethnic minorities suffer health inequities [43]; and the Roma community experiences healthcare discrimination [112]. Discrimination in healthcare can result in late diagnosis, different treatment, worse prognosis, and lower inclusion in studies, amongst other consequences.
Despite the European Commission's encouragement to collect racial and ethnic data, most member states do not collect data on ethnicity, race or racial origin, and sometimes this has legal restrictions [113]. European surveys (not necessarily health related) resort to different variables to characterize racial and ethnic origin, such as: racial origin, ethnic origin, colour, descent, citizenship, place of birth, place of birth of the parents, nationality, religion [113]. These interlinked variables do not have interchangeable meanings/equivalence.
Self-reported information is the gold-standard for ethnicity data [114], but racialized groups may better correlate with racism or discrimination [115]. A multi-variable approach to define ethnicity or racialized group in an informative manner could include: race-ethnic-cultural background/origin, nationality, birthplace, citizenship, language, migration background, religion, and belonging to minority group(s). The European Commission provides guidelines for data collection on racial and ethnic origin [113, 115]. Updated national censuses can serve to assess inclusion and terminology. Terms vary across communities (e.g., “black” [116], vs. “Afro descendants” or “African Europeans”); and recognized ethnic groups like the Roma or the Sámi (“Sápmi”) also differ across Europe [117]. Engaging with community representatives and citizen-research partners is encouraged to ensure respectful treatment and build trust.
Despite ethnicity (or race) having no biological basis, they were occasionally used to describe genetic differences explaining disease prevalence. However, independent of the frequency of genetic variations in different populations, their race, ethnicity and racialized groups should not be proxies for these. Otherwise, it could lead to discrimination and inappropriate treatment [118].
Collection of ethnicity data aids in identifying health disparities and inequities, assessing representation in studies and accounting for this determinant of health.
4.5 Socioeconomic Status, Education, Health Literacy
Socioeconomic status (SES) can be a barrier or boon to health, impacting behaviours and resources [119], access to healthcare, and research participation. It is typically defined by variables such as educational level, health literacy, occupation, income, living environment (e.g., rural/urban), and social benefits. Regional geographical indexes, like the UK Index of Multiple Deprivation [120], are used as proxies of SES but can have potential misclassification.
The impact of SES on healthcare access depends on structural population elements, such as power dynamics and the type of healthcare coverage/funding. Health literacy can affect the use of healthcare resources [121, 122] and medication adherence [123]. In Europe, low health literacy is more prevalent amongst persons with financial deprivation, low social status, low education, and old age [124]. Higher levels of education are associated with lower obesity rates, longer life expectancy [125], reduced smoking and alcohol consumption [126], better self-perceived health [127], and lower functional limitations [127]. Educational level may also be associated with structural power dynamics, which is especially relevant when social mobility is restricted. Additionally, digital literacy (or digital health literacy), relevant for access to research and health resources (e.g., eHealth), can be affected by SES, with education level and place of residence as indicators. The level of digital literacy varies between countries [128]; and higher levels of education are associated with greater digital literacy [128] and digital health literacy [129]. Disparities in digital health literacy may be due to educational opportunities and access to digital devices and internet connectivity [129].
SES and its related variables are often implemented in pharmacoepidemiology, typically as risk or protective factors. However, there are historically used socioeconomic categories that are no longer recommended, such as middle class, working class, or blue vs. white collar.
4.6 Health Status and Capabilities
In RCTs, the higher number of comorbidities increases the likelihood of exclusion [84]. Additionally, trials may unintentionally exclude vulnerable populations (e.g., persons with very active disease if this restricts physical/mental capacities). Suggestions to improve access include facilitating transport, ensuring facilities accessibility, allowing personal assistants, funding accommodations, and bringing research to the participants (e.g., decentralized trials).
In RWE, if exclusion due to disease or medication is needed but the target population would include those excluded, sensitivity or exploratory analyses should be considered. It is important to question how health and capabilities could affect representability in the data and data labels (e.g., late diagnosis due to stigma). Transparency of eligibility criteria is essential [130, 131].
5 Explore, Tailor, Implement, and Evaluate (ETIE) Framework
To successfully implement the diversity domains and ensure a fair pharmacological study, we recommend following the steps Explore, Tailor, Implement, and Evaluate, here referred to as the ETIE framework.
First, early in the designing process, question and explore the role or potential implications that each diversity domain (and their interplay, intersectionality) can have in the study. Second, tailor their definition to the study context. Third, implement them appropriately (e.g., disease modifier, confounder, discriminated group) and fourth, evaluate (or alternatively discuss) the study findings in the context of these domains.
Seeking an actionable recommendation to ease the implementation of the ETIE framework, we provide a checklist (Table 1) to be used in addition to common reporting guidelines, such as those of the equator network [131, 132].
| Section and item Nr. | Recommendations | Page | |
|---|---|---|---|
| Methods | |||
| 1/E | Explore |
|
|
| 2/T | Tailor |
|
|
| 3/I | Implement |
|
|
|
|||
|
|||
|
|||
| 4/E | Evaluate | Evaluate the fairness of the study, addressing: | |
|
|||
|
|||
|
|||
| Results/discussion | |||
| 5 |
Communicate findings in relation to diversity domains with extra caution, especially when the topic is subject to misinterpretation as different rights or capabilities. Examples: In some cases, mentioning “sex influences” instead of “sex differences” may be preferable. Differentiate between racialized groups and self-identified ethnicity and race. When evaluating behavioural changes, keep in mind that choice of behaviour can only be feasible when there is the option, and this may be more challenging in vulnerable populations, or in the absence of control on these factors. Use respectful terminology. |
||
| 6 | In the absence of relevant diversity domains in the data, highlight this limitation and its consequences (including consequences to the study findings and its implementation or expected benefit in the target population). | ||
6 Conclusions and Call for Action
Pharmacoepidemiology informs pharmaceutical industry strategies, health policies, regulatory decision-making, and clinical practice. It is therefore our duty to ensure fair care to everyone in the target population. Implementing diversity and fairness in pharmacoepidemiologic research serves to target equal opportunity, inclusion and representation, and equal benefit across members of the population. To address this, we elaborated within this article on diversity domains relevant for pharmacoepidemiologic research, and we suggest following the ETIE framework (i.e., Explore, Tailor, Implement, and Evaluate) in every pharmacoepidemiologic study (Table 1).
The described diversity domains encompass sex, gender, age, life stages, ethnicity, race, migration, nationality, SES and related variables (education, health literacy), health status and capabilities (Figure 1). All of these are determinants of health, either through biological pathways (e.g., sex), socially constructed norms (e.g., gender) or behaviors (e.g., discrimination), and structural barriers to healthcare (e.g., SES and health literacy) and/or research participation (e.g., health status and capabilities). Their impact on pharmacoepidemiologic research can be topic- and setting-dependent. Although we divided these domains by topic, we acknowledge their intersections and encourage researchers to be conscious of the heterogeneity within and the interplay between population groups. Acknowledging intersectionality (relation between systems of privilege or oppression, like sexism, racism, classism, ageism, etc.) in pharmacoepidemiology implies considering the complexities of individual experiences, with several layers interplaying advantages or disadvantages, shaped by multiple systemic and dynamic forms of social inequity.
Diversity domains encompass sensitive data. Transparency about the purpose of gathering this information, ensuring confidentiality, and detailing how the data will be utilized is expected to foster trust and cooperation amongst participants. Different population groups may require different recruitment and engagement strategies, thus, adapting processes accordantly is needed. Involving the community and embracing patient-centred research may lead to more relevant research and improve inclusivity. For example, after historical overlook of women in human immunodeficiency virus (HIV) research, inclusion of women in research teams led to better inclusion of their needs in the studies [133].
We advocate for higher presence of diversity domains in RWD, recognizing that data collection may need tailored strategies to address regional/cultural variations. Emerging technologies increase availability of patient-generated data, which are very relevant for diversity domains data. Working in diverse teams is expected to yield better and more inclusive findings. Continuous learning, evaluating, and improving in the field is key.
We call for action from pharmacoepidemiologists to implement this framework and raise awareness of equity and fairness in pharmacoepidemiology (Table 2). Additionally, we call on data holders and custodians to enlarge diversity data. Regulators are also invited to take an active role (e.g., participatory policy making [134]). Likewise, research funding bodies are encouraged to include equity and fairness aspects within their evaluation criteria (e.g., Multicomponent interventions by the Canadian Institutes of Health Research (CIHR) to enhance the uptake of sex and gender in funding calls [20]).
| Call for action | |
|---|---|
| Researchers |
|
| Data holders/data custodians |
|
| Regulators |
|
| Funding bodies |
|
Moving forward, progression of the fairness and equity in pharmacoepidemiologic research may be assessed by quantitative indicators (e.g., diversity assessment of study populations, disparities in health outcomes, funding allocation, and bias assessments in data-driven healthcare models), combined with qualitative metrics (e.g., assessment of community engagement in research and integration of equity findings into policy).
Implementing diversity domains in pharmacoepidemiology using the ETIE framework is expected to aid identifying health disparities and inequities, address D&I and evaluate the fairness of the data-driven analyses and study findings. Whilst this framework targets pharmacoepidemiologists, every stakeholder should join forces to improve health equity and fairness in pharmacoepidemiology.
Finally, although this framework is developed for the European context, its contents may be valuable and applicable elsewhere, and we invite other regions to consider and adapt our recommendations to their needs.
This manuscript was endorsed by the ISPE.
6.1 Plain Language Summary
Pharmacoepidemiology studies the use and effects of medical products and interventions in big groups of people, thus, generally using large health data. As a health scientific discipline, it should be fair and embrace diversity and equity. This article provides evidence and operational guidance to fulfil this. We describe key diversity domains that can influence health through biological mechanisms and/or social pathways, and should therefore be considered in pharmacoepidemiologic research. These include biological sex, socially constructed gender, age, life stages (e.g., pregnancy, menopause), ethnicity, race, migration, nationality, socioeconomic status, education, health literacy, and health status and capabilities. These domains are interlinked and context-dependent. Applying diversity domains enables research to be more inclusive and fairer. To successfully implement diversity domains, we recommend researchers to follow the Explore, Tailor, Implement, and Evaluate (ETIE) framework: explore the role/implication of the diversity domains in the study, tailor their definitions to the study context, implement them appropriately and evaluate the study results in their context. Moving forward, there is a need to increase information on diversity domains in health databases, for which support from stakeholders is crucial. This manuscript was endorsed by the International Society for Pharmacoepidemiology (ISPE).
Author Contributions
Study idea: E.V.Y. and collaborators from the Health Equity Special Interest Group from the International Society for Pharmacoepidemiology (ISPE). Study conceptualization, organization and coordination of the taskforce: E.V.Y. Scope and key themes of the manuscript were decided during panel discussions, including: E.V.Y. (chair); S.T.d.V., A.D., E.A., A.M.-D.L.T., and H.G. (panel I); I.v.V., J.W., M.L.F., and T.O. (panel II); S.G., A.F.M., and D.L.P.C. (panel III); D.C.R., A.M., and J.R.N.G. (one-to-one discussions). Input by participation in initial survey: S.T.d.V., D.L.P.-C., E.A., H.G., M.L.F., I.v.V., A.D., S.G., A.M.-D.L.T., J.W., T.O., H.N., A.M.S.D., C.C., I.M., F.I., M.S.G., O.K., M.K., S.G., D.C.M., A.F.M., and M.S.G. Writing of the draft manuscript: E.V.Y. All authors reviewed the manuscript, provided input, edited, and/or provided references, and all authors approved the final version before submission.
Acknowledgments
On behalf of every author, the members of the Health Equity Special Interest Group from the International Society for Pharmacoepidemiology (ISPE) are thanked for their input on the project. Special thanks to Syd Phillips, Alexander Cole, Elisabeth S Russell, and Serena Jingchuan Guo for their input at early phases of the project. Also, Katoo Muylle and Marine Hoche are thanked for participating in the study survey. The International Society for Pharmacoepidemiology (ISPE) is thanked for endorsing this manuscript. Open access publishing facilitated by Universitat Bern, as part of the Wiley - Universitat Bern agreement via the Consortium Of Swiss Academic Libraries.
Disclosure
This project did not receive funding and work was conducted on personal time. Views expressed in this article are the personal views of the authors and not those of their employers, associations and institutions; thus, they may not be understood or quoted as being made on behalf of or reflecting the position of the authors' affiliations. E.V.Y. is co-president of the Swiss Society for Gender Health. E.A. is employee and a shareholder from Novartis Pharma AG. I.v.V. is board member of the Dutch Society Gender & Health. S.G. was an employee of Ferring Pharmaceuticals A/S when this review was conducted. A.M.D.L.T. is shareholder of Methodds GbmH. I.M. is employee and stockholder of Regeneron Pharmaceuticals. F.I. employee and shareholder of CSL Behring LLC. M.S.G. is an employee at Bayer A.G., Germany. A.M. works at SANNO. D.R. works at the European Alliance of Associations for Rheumatology, EULAR. M.K. is employee of IQVIA, which performs commissioned pharmacoepidemiological studies for several pharmaceutical companies. A.F.M. is employee and stockholder of Regeneron. J.R.N.G. is former co-president of the African Students Association of Zurich. Other authors have nothing to disclose in relation to this submission.
Ethics Statement
The authors have nothing to report.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.




