Effect of water on the thermal transition in chitosan films
Funding information: National Natural Science Foundation of China, Grant/Award Number: 21606138
Abstract
In this study, the influence of moisture content on the thermal behavior of chitosan was investigated by differential scanning calorimetry (DSC). An endothermic peak at around 100°C was generally observed for hydrated chitosan films. This endothermic event was attributed to the melting transition of chitosan in pans with good seal integrity, whereas it was assigned to the evaporation of water from sample in an open system. The melting temperature (T m) of chitosan was closely related to the water content. At low water contents up to 27 wt%, the T m increased with increasing hydration level. At moderate water contents up to 135%, T m decreased with further increasing water content. At high water contents superior to 135%, the melting transition was no longer visible in the DSC thermogram. In addition, the T m of chitosan in alcohol-aqueous mixed solvents increased with an increase in 1-propanol concentration. The addition of urea could significantly increase the T m of chitosan at high hydration levels, whereas it was independent on urea content for less hydrated samples. These observations indicate that the role of water is complex, depending on its state in polymer matrix.
1 INTRODUCTION
Chitosan is a cationic natural polysaccharide derived from alkaline N-deacetylation of chitin. It has been widely used in food and pharmaceutical industries due to its distinctive advantages of biocompatible, biodegradable, low toxicity, and availability.1, 2 In addition, it is a promising bioresource for packing material owing to its good film-forming ability, gas barrier property, and antimicrobial characteristics.3, 4 However, this biopolymer naturally interacts with water, which could result in undesired changes in its structural and dynamic properties.5-7 Consequently, accurate knowledge of its hydration is crucial to define the applications of this biopolymer.
The hydration properties of biopolymer depend on its primary and supramolecular structure. The presence of water can also result in significant changes in its structure as well as physical properties.8 Generally, the structure-property relationship of hydrated biopolymers can be better understood within the theoretical concept of glass transition, which has been intensively investigated in various hydrated systems, such as PVA,9 starch,10 and chitosan.11 In addition, the glass transition was also observed in some hydrated PEC systems.12 It is generally accepted that the water molecules act as plasticizers which can lead to a remarkable reduction of glass transition temperature (T g) of biopolymer. It is stated that nonfreezable water, which is strongly bound to polymer and shows no thermal transition by differential scanning calorimetry (DSC), is responsible for the plasticization in hydrated system.9 Although much attention has been paid to the glass transition in hydrated biopolymers, little is known about the role of water on their melting behaviors.
It is known that chitosan is a semicrystalline polysaccharide which cannot be melted and processed as a typical thermoplastic. The melting behavior of chitosan is very difficult to be observed before its decomposition due to the presence of the large number of intermolecular and intramolecular hydrogen bonds in this biopolymer.13 However, the situation is different for the plasticized biopolymer where these hydrogen bonds could be by destroyed by plasticizer molecules. As a result, the biopolymer may be melted at a low temperature. It was found that plasticizers like sorbitol could reduce the T m values to around 90°C to 100°C when incorporated into chitosan films.14 As for glycerol, the increase of plasticizer concentration led to a decrease of T m values.15 On the contrary, other studies showed that the melting temperature of chitosan films increased with increasing glycerol content.16 In these studies, the residual water as plasticizer in chitosan films could also affect their thermal behavior, which impaired the determination of the melting temperature. Therefore, the understanding of the influence of water content on thermal transitions of chitosan is of critical importance. Unfortunately, few work has been done on the melting behavior of chitosan in hydrated films. Bonilla et al observed a T m of chitosan films stored at 53% RH at around 70°C.17 A higher T m with values of 105°C for chitosan films stored at room temperature was reported by Liu et al.14 However, in most studies the endothermic events at around 100°C were generally attributed to the evaporation of water existed in chitosan films.18-24 Recently, Madeleine-Perdrillat et al stated that the endothermic peak at around 100°C could be assigned to the removal of the trapped acetic acid in chitosan films.25
The controversy in the melting temperature of chitosan may be related to its moisture content, crystalline structure, and polymorph nature together with chemical structure. In addition, the type of DSC pans used for the analysis may affect the determination of thermal behavior of chitosan. It was reported that the seal integrities of DSC pans could affect the peak temperatures and magnitudes of the endotherms of starch.26 Similar results were also observed by Mukherjee and Rosolen when DSC was applied to investigate the thermal transitions of gelatin.27 These results indicate that the choice of pan is one of the key issues in correct determination of the thermal behavior of such biopolymers.
In this article, some key factors that affect the results of thermal behavior of chitosan, such as type of DSC pans, measurement conditions and moisture content, will be discussed. The influence of water mixtures with 1-propanol and urea on the T m was also examined. The purpose of this work is twofold: to identify whether chitosan exhibit a melting temperature in hydrated films, and to better understand the melting behavior of chitosan underlying the role of water. Understanding the relationships between the thermal properties and moisture content is vital for the use of such a complicated biopolymer in a variety of industrial applications.
2 EXPERIMENTAL
2.1 Materials
Chitosan (90% DD, degree of deacetylation), acetic acid (100% pure), 1-propanol and urea were all supplied by Sinopharm Chemical Reagent Co., Ltd, China. The average molecular weight of chitosan is about 260 000. All aqueous solutions were prepared using ultrapure water (18 MΩ/cm) from a Milli-Q system (Millipore). All chemicals were of analytical grade.
2.2 Film preparation and conditioning
A chitosan solution of 2.0 wt% was prepared by dissolving chitosan powder in 1% (vol/vol) aqueous acetic acid solution, stirred overnight at room temperature. Then, the polymer solution was filtered through 0.45 μm pore-size poly(tetrafluoroethylene) (PTFE) membrane filters to remove impurities. Subsequently, this film-forming solution were cast onto a PTFE mold (150 × 200 mm2) and dried at room temperature for 72 hours, yielding chitosan films.
To perform the conditioning experiment, the as-prepared polymer films were predried under vacuum at 80°C for 12 hours and subsequently equilibrated in different relative humidities at 25°C for 1 week. Six saturated salt solutions, LiCl (11.3RH), CH3-COOK (21.6RH), CaCl2 (32.8RH), K2CO3 (44RH), KI (68.9RH), and (NH4)2SO4 (81RH) were used to maintain the desired relative humidities in sealed chambers. To prepare samples with water (or mixed solvents) contents more than 30 wt%, dry chitosan film (about 5 mg) was placed in an aluminum hermetic pan and a known amount of water (or mixed solvents) was added by a microsyringe. In this work, the mixed solvents refer to water/1-propanol mixture or aqueous urea solution. The pan was sealed hermetically to prevent solvent loss and the mixture was equilibrated at 25°C for 24 hours.
2.3 Methods
2.3.1 Thermogravimetric analysis
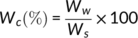 (1)
(1)2.3.2 Differential scanning calorimetry
A Q2000 DSC (TA Instruments, New Castle, Delaware) was used in this study, which was equipped with a cooling accessory RCS90. Three kinds of pans from TA instruments were used: high pressure capsule (withstand an internal pressure of 12 atm), Tzero hermetic pan (withstand an internal pressure of 3 atm) and Tzero pan. For each kind of DSC pan employed, an identical reference pan was used.
To eliminate heat flow anomalies from the reaction of aluminum and moisture, all the aluminum pans were treated in boiled ultrapure water for 2 hours, and dried thoroughly prior to use. To investigate the effect of the integrity of the seal of the DSC pans on the thermal behavior of chitosan films, samples with mass about 5 mg were sealed in the three kinds of pans with lids and heated in the DSC cell from 5°C to 180°C at a heating rate of 5°C/min, respectively. For the determination of the melting transition of chitosan in hydrated films, samples with mass about 5 mg were sealed in Tzero hermetic pan with lids and heated in the DSC cell from 5°C to 140°C at a heating rate of 5°C/min. In addition, three heating rates of 3, 5, and 10°C/min were applied to study the influence of heating rates. Dry nitrogen was used as the purge gas at a flow rate of 50 mL/min. Thermal data were determined at least by triplicate on each film sample.
2.3.3 X-ray diffraction
X-ray diffraction (XRD) patterns of control film and sample immersed in 1-propanol were measured by a BDX3300 X-ray diffractometer equipped with a multichannel detector by use of a Cu Kα1 (λ = 0.15406 nm) monochromatic X-ray beam. The samples were measured within 2θ range of 4° to 50° with a scan rate of 1°/min.
3 RESULTS AND DISCUSSION
3.1 Thermal behavior of chitosan in different types of DSC pans
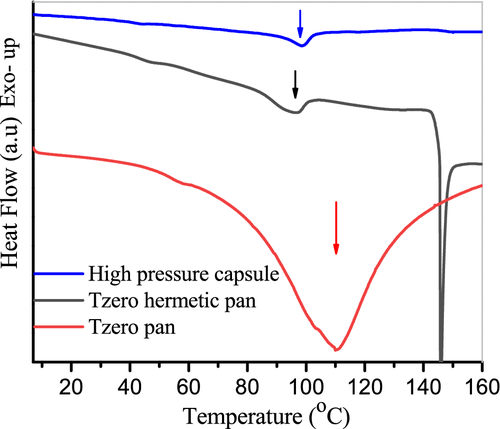
Pan selection is very important for analyzing high moisture samples, and the experimental results can be affected by the seal integrities of DSC pans. Generally, the wetted materials could not be characterized at high temperatures in typical DSC pans, due to the inability to contain the internal pressure.29 Figure 1 shows the DSC thermograms of chitosan containing 20 wt% moisture measured with different types of DSC pans. It can be seen that the endothermic peaks have different shapes with different pans. The sample run in the Tzero pan presented a very large, broad endothermic peak in the temperature region of 60°C to 150°C. As the Tzero pan was a completely open system, the moisture could easily escape from the sample during the heating process. Therefore, this broad endothermic peak is attributed to water evaporation, which has been validated by many studies.18-24 It should be noted that these studies were also performed in an open system, such as pin-hole aluminum pan. An endothermic peak appeared at around 100°C for both the Tzero hermetic pan and the high pressure capsule. Obviously, this peak was not related to water evaporation, because there were no leaks in the sample crucibles at this temperature, as evidenced by their unchanged mass. An alternative reason for this thermal event is the structural relaxations of polymer films stored at room temperature. However, this peak basically remained unchanged after the annealing of the sample at temperature higher than its T g (Figure S1). Thus, this possibility was also ruled out. Moreover, this endothermic peak was nearly invariable with the changing of heating rates (Figure S2). On the basis of these results, it can be deduced that, this endothermic peak can be assigned to a cooperative water mediated melting of chitosan. It should be noted that this endothermic peak disappeared in the second heating scan (Figure S3). The absence of the thermal event was not associated with the loss of water molecules. In DSC experiment, chitosan with rigid molecular chains was very unlikely to crystallize in the cooling process, due to the limited mobility of polymer chains. Consequently, this melting transition was not observed in the second heating scan. A similar result was reported by Liu et al.14 In addition, a lower T m with values of 70°C was observed for chitosan films stored at 53% RH.17 These results indicate that the melting behavior of chitosan can be observed in pans with good seal integrity. The discrepancy among these results may be caused by different water contents in the tested samples. The influence of water on the melting behavior of chitosan will be discussed in the following section. In addition, this pan leaked at about 147°C, as demonstrated by the appearance of a sharp peak in the DSC endotherm (Figure 1) with weight loss of sample. Thus, the setting temperature did not exceed 140°C in the subsequent DSC experiments to avoid moisture leakage.
3.2 Effect of water content on the melting behavior of chitosan films
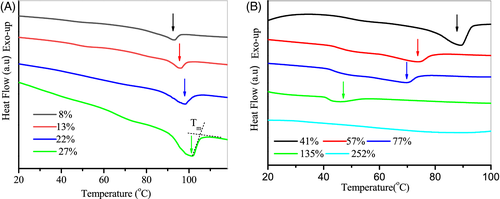
Figure 2 presents the DSC thermograms of chitosan films with various water contents. It is evident that water content has a notable influence the melting behavior of chitosan. T m value increased on increasing water content up to 27 wt% and then it decreased with further increasing water content (Table 1). At high water contents of 135%, the melting behavior of chitosan was no longer visible in the DSC thermogram. In addition, the melting enthalpy (ΔH) had the same moisture dependence as melting temperature.
| W c (%) | 8 | 13 | 22 | 27 | 41 | 57 | 77 | 135 | 252 |
|---|---|---|---|---|---|---|---|---|---|
| T m (°C) | 96 | 99 | 103 | 105 | 93 | 78 | 73 | 52 | — |
| ΔH m (J/g) | 4.8 | 6.2 | 9.3 | 17.9 | 8.1 | 2.8 | 2.0 | 0.3 | — |
The nonmonotonic dependence of T m on water content suggests that the role of water in chitosan films is complex. At low water contents up to 27%, only nonfreezable water existed in chitosan films.28 These water molecules could interact strongly with biopolymer via hydrogen bonds which stabilized the crystalline structure of chitosan.30 With the increase of water content, the strength of these hydrogen bonds increased, resulting in a rise of T m of chitosan. This increase observed on T m with increasing plasticizer concentration was also reported for glycerol-plasticized chitosan films.16 In addition, a similar trend was observed by Rivero et al working on glycerol-plasticized gelatin films.31
Generally, a small amount of water in chitosan could favor the chains mobility and thus the formation of crystalline domains from amorphous polymer chains, leading to an increase in the crystallinity of chitosan films.6, 28 Consequently, at such low hydration levels, the melting enthalpy, an indirect measurement of the crystallinity, increased with an increase in water content. Similar results were also found for glycerol-plasticized chitosan films.15, 16, 32 It is interesting to note that the T g could also be increased by the hydration of chitosan at low water contents.25 These observations suggest that small amounts of water may exhibit an antiplasticization effect on chitosan films, which has also been observed in the case of chitosan-propylene glycol and starch-glycerol systems.33, 34
At moderate water contents up to 135%, the T m of chitosan decreased remarkably with the increase of moisture content. For the sample at a water content of 135%, the melting transition shifted to a lower temperature around 48°C, which appeared to be merged with its glass transition. It is reasonable to expect that the interchain and intrachain hydrogen bonds which act to stable the crystalline structure of chitosan can be destroyed by water at such high contents. As a result, the crystalline regions in chitosan films are progressively dissolved with increasing water content, resulting in a decrease in T m as well as the melting enthalpy. A similar phenomenon was observed in water plasticized starch, where the T m of starch films decreased upon hydration.35 At high water contents above 135%, the melting behavior of chitosan was no longer visible in the DSC thermogram. This result suggested that the sample became completely swollen, tending towards 100% amorphous content. Furthermore, the glass transition was also invisible in this experiment.
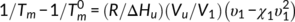 (2)
(2)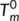 ) with values of 181°C. To our knowledge, the values of
) with values of 181°C. To our knowledge, the values of 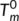 have seldom been reported in the previous literatures. There are two main reasons for this: it is a challenge to obtain the anhydrous form of chitosan due to the difficulty to complete removal of adsorbed water, and the fact that an essentially dry chitosan usually undergoes thermal degradation prior to melting due to its rigid-rod polymer backbone with strong interchain and intrachain hydrogen bonds. The
have seldom been reported in the previous literatures. There are two main reasons for this: it is a challenge to obtain the anhydrous form of chitosan due to the difficulty to complete removal of adsorbed water, and the fact that an essentially dry chitosan usually undergoes thermal degradation prior to melting due to its rigid-rod polymer backbone with strong interchain and intrachain hydrogen bonds. The  values obtained in this work are in agreement with reported results for chitosan powder,22 which possess more perfect crystalline structure and less water comparing with chitosan film. In addition, Equation (2) has been applied successfully to predict the
values obtained in this work are in agreement with reported results for chitosan powder,22 which possess more perfect crystalline structure and less water comparing with chitosan film. In addition, Equation (2) has been applied successfully to predict the 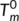 of starch.37 It should be noted that in this work the T
m depression of chitosan by moisture could be observed only when the water content was higher than 27%.
of starch.37 It should be noted that in this work the T
m depression of chitosan by moisture could be observed only when the water content was higher than 27%.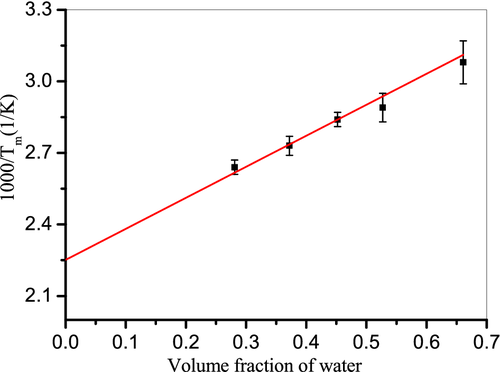
3.3 Melting behavior of chitosan films in mixed solvents
To further understand the role of water, the melting behavior of chitosan in the mixed solvents of 1-propanol and water was investigated. Compare to chitosan, the hydroxyl group of the 1-propanol molecule could be easily hydrogen-bonded to water molecules, leading to disruption of the H-bonding between chitosan chains and water. Thus, this solvent can be used to regulate the strength of the hydrogen bonds in the hydrated polymer films. Figure 4 shows the effect of water mixtures with 1-propanol on the melting behavior of chitosan films. The T m values of chitosan films with various water contents in alcohol-water mixtures are listed in Table 2. It was generally observed that T m increased with decreasing water/1-propanol ratios. For example, at a constant mixed solvents content of 77 wt%, T m increased from 73°C to 92°C as the water content in the mixed solvents decreased from 100% to 20%. These observations suggest that 1-propanol does not plasticize chitosan. With the increasing of 1-propanol content, the density of hydrogen bonds between water and chitosan was significantly diminished. As a result, the plasticization effect of water for chitosan was weakened and the melting temperature of polymer was increased. In addition, it has been demonstrated that the monoalcohols act as poor solvents for chitosan,38 which do not hydrate the polymer matrix. It is likely that the crystallization of chitosan may be hindered by alcohol additions in polymer films (Figure S4). Further work is needed to exploit the role of alcohol.
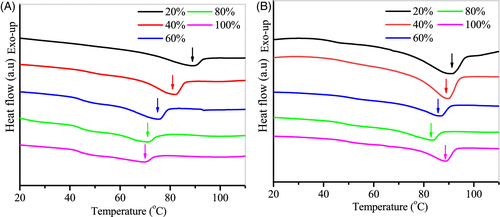
| W m (%) | 100 | 80 | 60 | 40 | 20 |
|---|---|---|---|---|---|
| T m,1 (°C) | 93 | 86 | 89 | 94 | 95 |
| T m,2 (°C) | 73 | 75 | 79 | 85 | 92 |
Interestingly, for the sample films at a mixed solvents content of 41 wt%, the T m decreased from 93°C to 86°C when the concentration of water in the mixed solvents decreased from 100% to 80%. This decrease of T m may be related to the state of water. It was reported that the minimum amount of water required to hydrate chitosan was around 42%.28 Therefore, the moisture in these films mainly exists as nonfreezable water, which takes part in stabilizing the stabilization of the crystalline structure of chitosan.30 The decrease of water content could result in a change in microstructure of chitosan films as well as the melting behavior of chitosan.
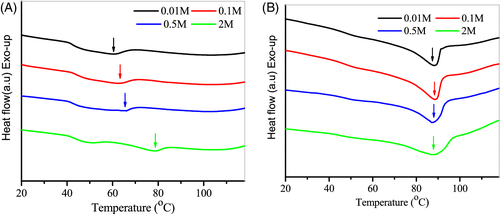
To further exploit the effect of hydrogen bonds on the thermal transition, we measured T m in the presence of urea. Urea is known as a hydrogen bonds inhibitor. It was found that the T m for chitosan at 77 wt% urea-water mixture increased with an increase in urea content (Figure 5A). In mixtures of water and urea, small fraction water molecules could be tightly associated with urea, forming specific urea-water complexes.39 The dynamics of these water molecules were strongly hindered and the “apparent” water content was reduced. As a result, the presence of urea causes an increase of T m. However, the influence of urea is minor when the content of mixture is decreased to 41%, and the T m remains nearly unchanged with the variation of urea content (Figure 5B). A likely explanation is that the nonfreezable water interacting with polymer is unable to form hydrogen bonds with urea, which has a negligible effect on the crystalline structure of chitosan films. In addition, close examination of thermograms in Figure 5B revealed that the endotherm peak became broad with increasing urea content. This may be a consequence of the fact that the crystalline structure is not homogeneous.
4 CONCLUSIONS
Thermal behavior in hydrated chitosan films is determined by calorimetry, which is affected by the integrity of the seal of the DSC pans. The endothermic peak occurred at around 100°C in hermetically sealed pans is assigned to the melting transition of chitosan, while this thermal event appeared in open systems is attributed to moisture evaporation. The hydration plays an important role in the melting behavior of biopolymer. At low water contents up to 27%, the T m of chitosan increased upon hydration. On the contrary, water at high contents appears to have a plasticizing effect by which T m decreases with increasing hydration level. In addition, the T m of chitosan in 1-propanol/water mixture increases with the increasing of alcohol concentration. In more hydrated chitosan films, the T m is improved significantly with the increase of the content of urea, whereas it remains basically unchanged with urea concentration for less hydrated samples.
ACKNOWLEDGMENT
The work was supported by the National Natural Science Funds of China (No. 21606138).




