Studies on why the heat deflection temperature of polylactide bioplastic cannot be improved by overcrosslinking
Funding information: Agriculture and Agri-Food Canada, Grant/Award Numbers: 052882, 051910; Canada Discovery Grants, Grant/Award Number: 401111; Ontario Ministry of Agriculture, Food and Rural Affairs, Grant/Award Number: 030054; Ontario Research Fund, Research Excellence Program Round-9, Grant/Award Number: 053970
Abstract
The high temperature application of polylactide (PLA) has been greatly limited by its low heat deflection temperature (HDT). As a slow crystallizing material, the HDT of amorphous PLA is mainly determined by its low T g value, near 55°C. In this work, PLA was chemically crosslinked, and the HDT of the crosslinked PLA was evaluated from two aspects—crystallization ability improvement and chain mobility limitation at T g of the PLA. The crystallization rate is improved by crosslinking because of the increased nucleation efficiency, and reached the highest level at moderate crosslinking. The final crystallinity is, however, still far lower than the crystallinity threshold for enhancing HDT of PLA. More importantly, T g of the PLA is not influenced by the crosslinking, even the average molecular weight between the crosslink points reaches as low as 1654 g/mol. The experimental research and theoretical calculations indicate that, to improve the HDT of the PLA, conformational restriction on the PLA chains should be focused on the segments with less than, at least, 14 repeating units. The fundamental reason why the HDT of PLA cannot be improved by crosslinking is investigated to guide the future structure design for high HDT PLA products.
1 INTRODUCTION
Heat deflection temperature (HDT) is significant in the application of polymeric materials because it determines the upper temperature at which the material can be used. Polymers with high HDT can find various applications in different fields that require high temperature stability, such as automotive and electrical board. Biodegradable polymers are believed to be able to reduce the burden on the environment via replacing certain traditional petrol-based plastics in our daily life.1 Polylactide (PLA), as one of the most important biodegradable plastics, has been widely researched and used in various applications.2 The application of this material at high temperature (>60°C) has, however, been greatly limited because of its poor thermal properties, for example, low HDT.3 The HDT of PLA is reported to be ~55°C, much lower compared to the incumbent petroleum-based plastics such as acrylonitrile-butadiene-styrene and poly(methyl methacrylate) (PMMA), and so forth. To improve the HDT of PLA, different methods have been applied by either optimizing the PLA characteristics such as molecular weight and crystallinity or chemical or physical modification like copolymer synthesis and melt compounding.4, 5
According to a reported study,6 the HDT behavior in amorphous and semicrystalline polymers is totally different, the former is decided by the glass transition temperature while the latter is mainly decided by the thermal history, crystallinity, and crystal size. Therefore, more and more efforts to improve the HDT of PLA are focused on improving the crystallinity, since melt processed PLA is normally in the amorphous state, and increasing T g of amorphous PLA via copolymerization is difficult to achieve.7, 8 Annealing,9 injection molding at high molding temperature,10 extrusion blending at high screw rotation speed,11 and structuring special stereocomplex (SC) poly(L-lactide)/poly(D-lactide) (PLLA/PDLA) crystals12 to obtain high crystallinity have been reported to be able to improve the HDT of PLA and, because of the slow crystallization rate of pure PLA, normally efficient nucleation agents are added in these research studies. However, it is difficult to achieve high crystallinity of PLA during the extrusion since the melt processing of PLA requires superfast crystallization during solidification and, to the best of our knowledge, no such high efficient nucleating agents for PLA have so far been developed. Melt blending PLA with high HDT polymers such as PMMA13 and PC14 has been reported to obtain high HDT blends, but with the sacrifice of the biodegradability of PLA. Normally, the PLA content in these blends is controlled at a low level, and sometimes annealing is also needed to increase the HDT.14, 15 Introduction of fillers such as layered silicate has been reported to be able to improve the HDT of PLA, but only for annealed samples.16
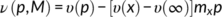 (1)
(1)The effect of crosslinking on physical and thermomechanical properties of PLA and its derivative has been reported.20-22 And in Yang et al.'s research, it is found that T g of PLA is not changed with the improving of crosslinking density.18 It is well known that the high temperature stability and modulus can be improved by crosslinking, as revealed by dynamic mechanical analysis (DMA) studies, even beyond the melting point of PLA. However, the influence of crosslinks on the HDT of PLA has not been reported so far to the best of our knowledge, although some research has reported that branching or crosslinking improved the crystallization rate of PLA as the branched chains can be acted as nucleation sites.23, 24 However, the relationship between the crosslink crystallinity and HDT was not discussed. A near linear increase in T g with the mole fraction of crosslinker has been reported for polystyrene and PMMA,25, 26 but the influence of crosslinking on improvement of T g of PLA has not been reported. T g of PLA is not influenced by long chain branching but depends on the differential scanning calorimetry (DSC) heating rate.27 Therefore, building a relationship between crosslinking, structure, and HDT performance is significant in understanding the HDT improvement of PLA by structural modifications.
In this study, the influence of the degree of crosslinking of PLA on its HDT is evaluated. It is found that the HDT of PLA is not influenced by crosslinking, even by overcrosslinking. From the point of view of crystallization and glass transition, the reason why the HDT of PLA cannot be improved by crosslinking is analyzed. The research illustrates a way to improve the HDT of PLA, especially by suppressing the segment mobility at the glass transition temperature.
2 EXPERIMENTAL
2.1 Materials
The PLA used in this research with the brand name Ingeo 4043D was purchased from Nature Works LLC, United States. Its number-average molecular weight (M n) is reported as 8.4 × 104 g/mol and its polydispersity index as 1.81, the L/D isomeric forms is reported to be 98/2.28, 29 1,3,5-Triallyl-1,3,5-triazine-2,4,6(1H,3H,5H)-trione (TAIC) used as crosslinker, and 2,5-bis(tert-butylperoxy)-2,5-dimethylhexane (Luperox 101) used as free radical initiator were obtained from Sigma-Aldrich (Canada) and used as received. Chemical structures of the polymer, crosslinker, and peroxide used in this research, as well as the crosslink reactions, are illustrated in Scheme 1.

2.2 Sample preparation
The crosslinking of PLA was completed in a microinjection molding machine (DSM Research, Netherlands) with a corotating twin screw system and 12 cm3 injection molding unit. The reaction was carried out at 180°C and 100 rpm for 2 minutes. The compounding procedure had two steps. First, Luperox 101 and TAIC were added to the PLA pellets in a plastic bag and mechanically mixed. Second, the blends with different compositions were added to the compounder and injection molded with a mold temperature of 30°C into ASTM standard specimens for HDT testing. The TAIC content was fixed at 3 wt% of PLA, while a range of concentrations of peroxide (0.02, 0.05, 0.1, 0.2, 0.5, and 1 phr) were added to prepare PLA with different degrees of crosslinking. The samples were labeled as PLA-TAIC3-Lx, where x = 0.02, 0.05, 0.1, 0.2, 0.5, and 1, indicating the concentration of peroxide.
The disk-shaped samples (diameter: 25 mm, thickness: 1 mm) for rheological testing and rectangle standard samples for three-point bending deflection testing were injection molded via a micro-DSM injection unit (DSM) at 190°C and 100 rpm for 2 minutes. The injection molding was done with a filling pressure of 12 bar, packing and holding pressure of 15 bar, the total injection time was finished in 30 seconds.
2.3 Characterization methods
2.3.1 Rheology
The rheological behavior of the crosslinked samples was studied with a dynamic mechanical rheometer (MCR-302; Anton-Paar, Germany). Frequency sweeping was carried out at 185°C with a small strain (1%) and under N2 protection.
2.3.2 Differential scanning Calorimetry
Nonisothermal and isothermal crystallization measurements were conducted by DSC (TA Q200) with N2 protection. For nonisothermal crystallization, the materials were heated to 200°C at 10°C/min, held for 3 minutes to ensure complete melting, and then cooled to 30°C at 10°C/min. To evaluate the effect of crosslinking on the thermal properties of PLA, a second heating scan was performed from 30°C to 200°C at 10°C/min. The melting temperature (T m), cold crystallization temperature (Tcc), and glass transition temperature (T g) were determined from the second heating scan.
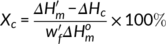 (2)
(2)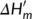 is the melting enthalpy and
ΔH
c is the cold crystallization enthalpy of PLA,
is the melting enthalpy and
ΔH
c is the cold crystallization enthalpy of PLA, 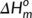 is the melting enthalpy of 100% crystalline PLA (93.7 J/g),30 and
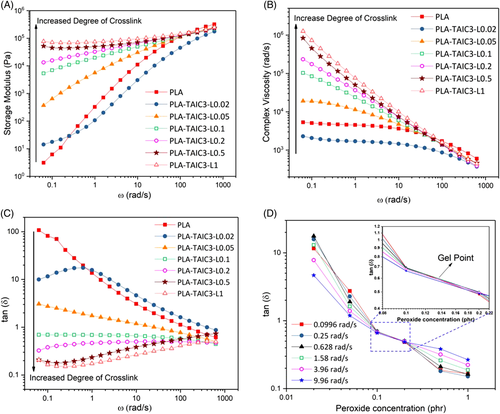
is the melting enthalpy of 100% crystalline PLA (93.7 J/g),30 and 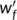 is the weight percentage of PLA in the sample. Two parallel experiments were performed for each sample to get the average crystallinity.
is the weight percentage of PLA in the sample. Two parallel experiments were performed for each sample to get the average crystallinity. (3)
(3) (4)
(4)2.3.3 Thermomechanical and HDT testing
The HDT under load was performed with the DMA analyzer (TA Q800) according to ASTM D 648-18. The test was performed by heating from 20°C to 80°C at 2°C/min. Three-point bending deflection mode was applied with loading stress of 0.455 MPa. Three specimens were tested for each sample to indicate the repeatability. The HDT was recorded as the temperature when a deflection of 250 μm was reached.
2.3.4 Gel fraction and swelling ratio calculation
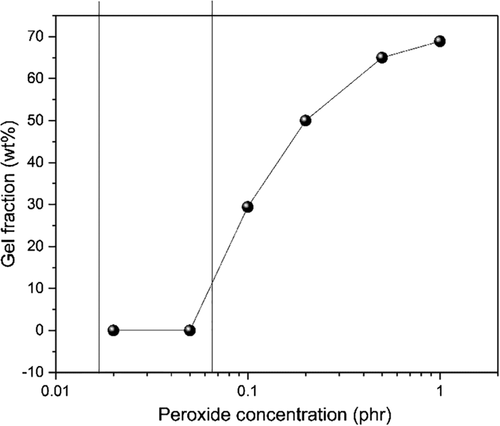
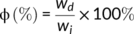 (5)
(5) (6)
(6)3 RESULTS AND DISCUSSION
3.1 Crosslinking reaction of the PLA
The crosslinking reaction of PLA, TAIC, and peroxide is depicted in Scheme 1. The chemical crosslinking reaction between PLA and TAIC is initiated by the Luperox peroxide. The free radicals decomposed from peroxide (─RO) abstract the hydrogen on the backbone chains of PLA, leading to the generation of free radicals (─CO). Meanwhile, the double bonds of allyl groups of TAIC were broken by the -RO. free radicals. Consequently, the free radicals from TAIC reacted with ─CO on the backbone of PLA chains, resulting in the chemical crosslinked PLA.20 As a fast and effective method to crosslink the PLA, the in-situ reactive extrusion crosslinking can be completed in 2 minutes in the extruder and the degree of the crosslinking density is easily to be manipulated by controlling the concentrations of peroxide. More important is that the whole reaction is completed in the extruder in melt state without using any solvent. The successful crosslinking of PLA by TAIC and peroxide was proved by the rheological and gel fractions studies. The strong interaction between the polymer chains would result in different rheological properties of the melt, such as increased modulus and viscosity.
The dependence of rheological properties of the crosslinked PLA samples on the peroxide concentration is depicted in Figure 1. Compared to pure PLA, both the storage modulus and complex viscosity of the reacted PLA with 0.02 phr peroxide is reduced, indicating the facility for chain movement at lower peroxide concentrations. The typical Newtonian liquid behavior ( G′~ω2, G″ ~ω) observed at sufficiently low frequencies for neat PLA disappears, the slope of G′ deviates from 2 with a small exponent of 0.60 for PLA-TAIC3-L0.02. The deviation is probably caused by the increased polydispersity of the PLA because of the thermal degradation of PLA in the presence of peroxide. Besides the thermal degradation effect, short branching chains of the reacted PLA at low dosage of peroxide is suggested to be also able to cause the decrease of the storage modulus and viscosity.32 With further increase of the peroxide concentration to 0.05 phr and higher, both the storage modulus and complex viscosity increase, as shown in Figure 1A,B, indicating long chain branching or increased degree of crosslinking of the PLA. The long chain branching and high crosslinking are supposed to greatly improve the melt modulus and viscosity rather than the decreasing effects brought by the short chains.33 Especially, for PLA-TAIC3-L0.5 and PLA-TAIC3-L1, G′ is independent of frequency over the whole frequency range, demonstrating that PLA with a high amount of peroxide is overcrosslinked.34 Also, the slope of tan δ of the PLA decreased with increasing degree of crosslinking, as shown in Figure 1C. Moreover, the values of tan δ with 0.1 phr peroxide and above are lower than 1.0, whereas those of PLA and PLA-TAIC3-L0.05 are >1.0, reflecting a sol-gel transition of the reacted PLA samples. By plotting the dependence of tan δ on the peroxide concentration at different frequencies, the critical peroxide concentration for gel formation in the PLA/TAIC/peroxide system could be determined. Based on the theory that tan δ is independent of frequency at critical gel formation content,35 the critical peroxide concentration of gelation was found to be 0.12 phr, as shown in Figure 1D. The PLA was highly crosslinked and transferred into gel-like material when the peroxide concentration was higher than 0.1 phr at 3 wt% TAIC. The gel fraction of the reacted PLA shown in Figure 2 also follows the above rheological predications. It increases noticeably from 0 to ~29 wt% with peroxide concentration increased from 0.05 to 0.1 phr. There is no doubt that the high gel fractions are due to the increased degree of crosslinking of the PLA. Therefore, by using different peroxide concentrations, PLA with different degrees of crosslinking are produced. According to the different gel fractions, the degree of crosslinking in the PLA samples was divided into three regimes: light crosslinking (the gel fraction is less than 10%), medium crosslinking, and overcrosslinking (the gel fraction is over 50%).
3.2 HDT evaluation of the Crosslinked PLA
The HDT of the crosslinked PLA samples was recorded by the DMA three-point bending curves and listed in Table 1. It is found that the HDT value of the PLA stays at ~53°C and is not influenced by crosslinking, even for PLA-TAIC3-L1 with the highest degree of crosslinking. The glass transition temperature of the crosslinked PLA samples was obtained from the tan δ peaks in the DMA dual cantilever beam testing shown in Figure S1. The glass transition temperature is independent of the degree of crosslinking, indicating that the segment mobility is not influenced by crosslinking, which will be discussed in the following sections.
| Samples | HDT (°C) | T g (DMA) |
|---|---|---|
| PLA | 52.0 | 68.6 |
| PLA-TAIC3-L0.02 | 52.2 | 68.7 |
| PLA-TAIC3-L0.05 | 51.8 | 68.6 |
| PLA-TAIC3-L0.1 | 52.5 | 68.3 |
| PLA-TAIC3-L0.2 | 52.3 | 68.6 |
| PLA-TAIC3-L0.5 | 52.4 | 70.6 |
| PLA-TAIC3-L1 | 52.9 | 69.6 |
- Abbreviations: DMA, dynamic mechanical analysis; HDT, heat deflection temperature; PLA, polylactide; TAIC, 1,3,5-triallyl-1,3,5-triazine-2,4,6(1H,3H,5H)-trione.
3.3 Crystallization evaluation of the crosslinked PLA
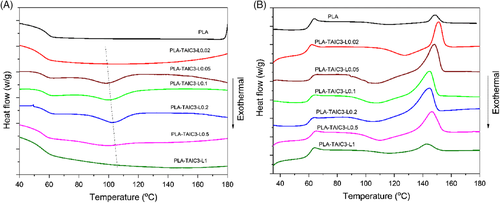
For semicrystalline thermoplastics, the crystallinity greatly influences the HDT of the materials.6 Because of its super slow crystallization rate, pure PLA shows an amorphous state when cooling from the melt, which is the main reason why the HDT of PLA products is rather low. To investigate the influence of crosslinking on crystallization, especially the crystallinity of PLA, the non-isothermal and isothermal crystal kinetics studies were conducted. The nonisothermal crystallization curves of the crosslinked PLA are depicted in Figure 3 and the related thermal parameters are summarized in Table 2. The amorphous pure PLA shows no crystallinity peaks at a cooling rate of 10°C/min. The improvement of the crystallization rate is not obvious for the reacted PLA with low peroxide concentration (PLA-TAIC3-L0.02), with no crystallinity peaks in the cooling process, while a cold crystallinity peak emerged in the following heating process. This indicated that the crystallization of PLA could not be improved at low peroxide concentrations, mainly because the main reaction is thermal degradation and not branching or crosslinking. At medium peroxide concentrations from 0.05 to 0.2 phr, the reacted PLA shows increased crystallization rate with display of crystallinity peaks in the cooling process. The melt crystallization peaks also shift to higher temperatures with increasing peroxide concentration, indicating a faster crystallization rate of the PLA after crosslinking. With further increasing of the peroxide to 0.5 and 1 phr, the crystallization peaks during cooling gradually disappear and are undetectable for PLA-TAIC3-L1. This is because of the high crosslinking of the PLA, as revealed by the rheological studies. Full crosslinking greatly limits the movement of the PLA chains during crystallization, leading to reduced crystallization rate and crystallinity.
| Samples | T g (DSC) | T c | Tcc | T m | X c (%) |
|---|---|---|---|---|---|
| PLA | 60.6 | — | 114.2 | 148.0 | 1.1 ± 0.1 |
| PLA-TAIC3-L0.02 | 60.1 | — | 112.0 | 150.9 | 1.9 ± 0.2 |
| PLA-TAIC3-L0.05 | 60.6 | 98.4 | 107.2 | 148.2 | 4.5 ± 0.2 |
| PLA-TAIC3-L0.1 | 61.3 | 101.3 | 107.6 | 145.1 | 8.0 ± 0.2 |
| PLA-TAIC3-L0.2 | 61.2 | 102.5 | 107.0 | 144.9 | 10.3 ± 0.3 |
| PLA-TAIC3-L0.5 | 62.2 | — | 110.1 | 144.3 | 5.8 ± 1.9 |
| PLA-TAIC3-L1 | 61.2 | — | — | 142.3 | 4.7 ± 0.1 |
- Abbreviations: DSC, differential scanning calorimetry; PLA, polylactide; TAIC, 1,3,5-triallyl-1,3,5-triazine-2,4,6(1H,3H,5H)-trione.
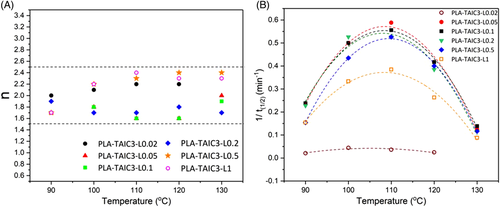
The increased crystallization rate of the PLA caused by the crosslinking can be evaluated more clearly by the isothermal studies. The isothermal curves of the sample with different crosslinking densities are shown in Figure S2 in supplementary information, and the two important parameters, nucleation constant n and reciprocal of the isothermal crystallization time 1/t1/2, were calculated by Equations 2 and 3 from the isothermal curves, and shown in Figure 4 and Table S1. The nucleation constants shown in Figure 4A lie between 1.5 and 2.5, independent of the crystallization temperature. The nucleation constants are close to 2, which means that the crystal structure should be spherical. After crosslinking by TAIC and peroxide, n decreased compared to that of pure PLA, and deviated from 2, especially at high crosslink density. This is because the growth of the crystals is still three-dimensional, but the growth is suppressed by the increased nucleation sites, resulting in imperfect crystals which possess low nucleation constants. The fact that nucleation constants were lower than 3 was due to the space confined growth of crystals limited by the crosslinking points, resulting to the loss of the growth dimensions.24 The reciprocal of the half-crystallization time of the samples is shown in Figure 4B. Compared to pure PLA and PLA-TAIC3-L0.02, which show no branching effect, the other samples exhibit much lower half-crystallization times, indicating faster crystallization rates. At different crystallization temperatures, it is found that the crystallization rate is the highest at 0.2 phr peroxide (PLA-TAIC3-L0.2). This is because the crystallization rate is determined by the nucleation sites and chain mobility together.36 At 0.2 phr peroxide, the highly branched PLA chains ensure increased nucleation sites and higher chain mobility compared to the crosslinked PLA samples with higher peroxide concentrations. At higher peroxide concentrations, the chains are highly crosslinked and lead to weakened chain mobility. Therefore, the crystallization rate is highest at 0.2 phr peroxide where there is moderate crosslink density, ensuring large numbers of nucleation sites and high chain activity in crystal growth. The fastest crystal growth temperature of the crosslinked PLA locates at 110°C is independent of the crosslinking density, as shown by the reciprocal of the half-crystallization time in Figure 4B. The reported temperature is same to our former research on the PLA nanocomposites30 but different from the previous study which shown that the fastest crystal growth temperature of the branched PLA was 120°C.24 This is probably due to the different PLAs used in these studies, the properties of the raw materials such as the molecular weight and L/D isomeric forms will influence the crystallization properties of the PLA.
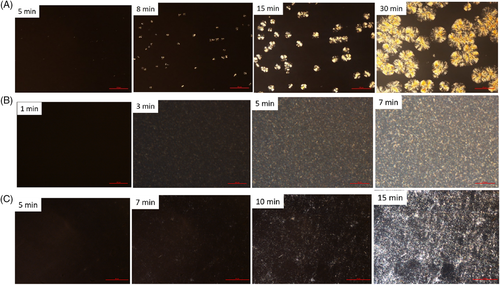
The above hypothesis on n and increased crystallization rate is proven by the optical microscopy images shown in Figure 5. The crystallization rate is highest for 0.2 phr peroxide material, which exhibits tiny crystals and crystallization completed in 7 minutes. Because of the absence of nucleation sites, the crystals appear about 5 minutes later and grow slowly to spherical crystals with high perfection after 30 minutes for PLA-TAIC3-L0.02 samples, similar to the crystal growth of pure PLA.30 After being highly crosslinked by TAIC and peroxide, the nucleation effect of the PLA has been greatly improved. As shown in PLA-TAIC3-L0.2, large amounts of tiny crystals are found in the crystallized samples because of the nucleation effects of the branched chains. The nucleation induction period has been dramatically decreased with crystals appearing in 3 minutes and the growth completed in 7 minutes. Compared to PLA-TAIC3-L0.2, the degree of crosslinking of the PLA-TAIC3-L1 is much higher. The higher crosslinking suppresses the chain movement during crystal growth, resulting in decreased crystallization rate. The POM images of PLA-TAIC3-L1 are shown in Figure 5C, where the nucleation sites are more than in PLA-TAIC3-L0.02 without crosslinks or branching, and the nucleation induction time is decreased. Large amounts of tiny crystals are formed in the final crystallized PLA-TAIC3-L1. On the other hand, the crystallization time of PLA-TAIC3-L1 is double that of PLA-TAIC3-L0.2, also indicating that the crystallization of the PLA is slower because of the suppressed chain growth caused by the overcrosslinking.
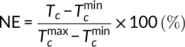 (7)
(7)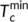 and
and 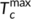 are the minimum and maximum crystallization temperatures of the homopolymers on cooling from different melt temperatures, which are determined from a self-nucleation experiment. For the pure PLA, the cooling crystallization curves from different melt temperatures are shown in Figure 6A with crystallization temperatures and exothermic enthalpy marked. The crystallization temperature increases with decreased melting temperature because the crystals which are not melted can serve as self-nucleation sites during the subsequent cooling. It was determined that
are the minimum and maximum crystallization temperatures of the homopolymers on cooling from different melt temperatures, which are determined from a self-nucleation experiment. For the pure PLA, the cooling crystallization curves from different melt temperatures are shown in Figure 6A with crystallization temperatures and exothermic enthalpy marked. The crystallization temperature increases with decreased melting temperature because the crystals which are not melted can serve as self-nucleation sites during the subsequent cooling. It was determined that 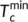 and
and 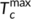 of PLA are 108.6°C and 128.5°C, respectively. Using the same cooling rate (2°C/min), the crystallization temperatures (T
c) of different crosslinked samples were determined and the NE calculated.
of PLA are 108.6°C and 128.5°C, respectively. Using the same cooling rate (2°C/min), the crystallization temperatures (T
c) of different crosslinked samples were determined and the NE calculated.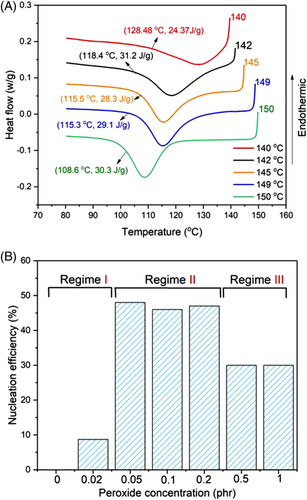
The NE of the crosslinked samples is given in Figure 6B. It is found that the NE of PLA is improved after crosslinking by TAIC and peroxide. According to the relationship between the NE and peroxide amount, three different regimes were identified. In the less or uncrosslinked PLA with small amounts of peroxide, NE is improved a little and very similar to that of the pure PLA melt, which is designated as regime I. With further increase in peroxide concentration to 0.05 to 0.2 phr, the PLA is crosslinked but still with chain mobility, and NE is improved greatly to 48%, which is designated as regime II. At high peroxide (0.5 and 1 phr), the PLA is overcrosslinked and NE is decreased to 28%, a lower NE regime which is designated here as regime III. There is no doubt that the NE can be improved by branching/crosslinking of the PLA chains, as discussed before, but the NE of crosslinked PLA is also closely related to its chain structure, especially the degree of crosslinking. Overcrosslinking will lead to suppressed NE, as shown by PLA-TAIC3-L1. Compared to previously reported nucleation agents for PLA, such as talc and SC of PLLA/PDLA, the NE of the crosslinked PLA is higher than talc (32%)38 but lower than the SC of PLLA/PDLA.39
Although the crystallization rate of the PLA can be increased by branching/crosslinking reactions, the final crystallinity of the samples calculated from the first heating nonisothermal crystallization curves is still not high enough to increase the HDT. The final crystallinity is shown in Table 2. The highest crystallinity is only 10.3% for PLA-TAIC3-L0.2 (as shown in Table 2), too low to increase the HDT of PLA as a semicrystalline polymer. Previous research indicates that the crystallinity of PLA should be higher than 25%, at which level the PLA can be regarded as a semicrystalline polymer and the HDT will be influenced by the crystallinity.9
In summary, the crystallization ability of PLA can be improved by crosslinking as the branched chains can act as nucleation sites. Both the crystallinity (X c) and NE of PLA are increased after crosslinking. However, the increases, particularly the increase of crystallinity, are not enough to improve the HDT of the PLA. Therefore, the crosslinked PLA samples are regarded as amorphous materials, and the HDT is mainly influenced by the chain movement at the glass transition temperature.6 The glass transition of the crosslinked PLA and the relationship between the chain segment movement and chain length between the crosslink points are more important, and are discussed in the following section.
3.4 Glass transition of the crosslinked PLA
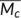 ) between crosslink points in our samples. It is widely accepted that the glass transition temperature can be regarded as the onset temperature of internal mobility or where “segmental mobility frequency” increases to the value which is characteristic of the time scale of the experiment.19 The segmental mobility frequency is significant in determining T
g of the polymer and it will be influenced by the crosslinking. The crosslinking probably influences the segmental mobility frequency by limiting its mobility and decreasing the specific volume in polymers.19 First, the unchanged T
g indicates that the free volume of the PLA is not influence by the crosslinking even with high crosslinking density; second, the average chain length or molecular weight (
) between crosslink points in our samples. It is widely accepted that the glass transition temperature can be regarded as the onset temperature of internal mobility or where “segmental mobility frequency” increases to the value which is characteristic of the time scale of the experiment.19 The segmental mobility frequency is significant in determining T
g of the polymer and it will be influenced by the crosslinking. The crosslinking probably influences the segmental mobility frequency by limiting its mobility and decreasing the specific volume in polymers.19 First, the unchanged T
g indicates that the free volume of the PLA is not influence by the crosslinking even with high crosslinking density; second, the average chain length or molecular weight (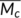 ) between crosslink points is important in determining the limitation effect on the chain mobility in the crosslinked materials, and can be calculated by Equation 8
41 using equilibrium swelling data from Equation 6.
) between crosslink points is important in determining the limitation effect on the chain mobility in the crosslinked materials, and can be calculated by Equation 8
41 using equilibrium swelling data from Equation 6.
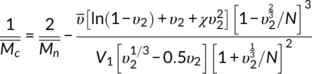 (8)
(8)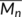 is the number average molecular weight of the uncrosslinked PLA, which is 8.4 × 104 g/mol,
is the number average molecular weight of the uncrosslinked PLA, which is 8.4 × 104 g/mol,  is the density of PLA taken as 1.24 g/cm3,
V1 is the molar volume of CHCl3 (
V1 = 277.7 cm3), and
χ is the solvent interaction parameter for PLA swollen in CHCl3, taken as 0.62.31 N is calculated by
is the density of PLA taken as 1.24 g/cm3,
V1 is the molar volume of CHCl3 (
V1 = 277.7 cm3), and
χ is the solvent interaction parameter for PLA swollen in CHCl3, taken as 0.62.31 N is calculated by 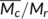 in which
M
r is the molecular weight of repeating units of PLA (72.1 g/mol),
υ2 is the equilibrium PLA volume fraction swollen in CHCl3, and is calculated from the equilibrium volume degree of swelling q by Equation 9.
in which
M
r is the molecular weight of repeating units of PLA (72.1 g/mol),
υ2 is the equilibrium PLA volume fraction swollen in CHCl3, and is calculated from the equilibrium volume degree of swelling q by Equation 9.
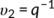 (9)
(9)The calculated data based on the swelling characterization are shown in Table 3.
| Equilibrium degree of swelling (q) | Equilibrium polymer volume fraction ( υ2) | Experimental 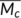
|
Experimental repeating units number | |
|---|---|---|---|---|
| PLA-TAIC3-L1 | 9.0 | 0.11 | 1654 | 23.0 |
- Abbreviations: PLA, polylactide; TAIC, 1,3,5-triallyl-1,3,5-triazine-2,4,6(1H,3H,5H)-trione.
The average molecular weight between the crosslink points of the overcrosslinked PLA (PLA-TAIC3-L1) was calculated to be 1654 g/mol. It can be inferred that the average number of repeating units between the crosslink points is 23.0 by dividing the mole of repeated units of PLA (~72.1 g/mol). Because PLA-TAIC3-L1 is the sample which possesses the highest crosslink density, it is believed that the average molecular weight between the crosslink points of the other samples should be higher than 1654 g/mol. The calculated number of repeating units between the crosslink points indicates that the length of the segment mobility for glass transition relaxation is below 23.0 for PLA, since T g of the PLA is not changed by the overcrosslinking. Therefore, T g of PLA can only be improved by introducing conformational restriction of the PLA chains within less than 23 lactoyl repeating units. Taking the fluctuation theory prediction that the characteristic length at T g of polymer was estimated to be 1 to 2 nm,42, 43 and the average length of lactoyl repeating unit as consisting of three freely rotating real bonds was reported to be 0.141 nm,44 the calculated repeating unit number at T g of PLA is 7 to 14 (lower that the value of 23 in our research), which is perfectly in line with the predictions and experimental results in this work. To improve T g of the PLA, it is predicted that the average molecular weight between the crosslink points should be lower than 504 g/mol, up to 1009 g/mol, as illustrated in Scheme 2.
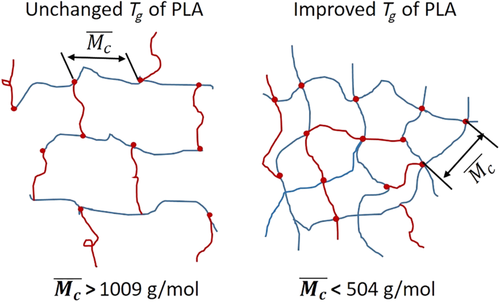
The whole studies on the relationship between crosslinking and HDT have shown that the chain relaxation of PLA at T g should be limited if the HDT of PLA is to be increased. In this case, the number of repeating lactoyl units between the crosslink points should be lower than, at least, 14. However, the PLA will lose its flowability when the number of lactoyl units between crosslink points is lower than 14 due to overcrosslinking. Therefore, conformational restriction such as introduction of large, conformational restricted groups via copolymerization with molecular weight of the PLA segments lower than 1009 g/mol, to decrease the free volume of PLA via introducing bulky side groups should be the main approaches to improve the HDT of PLA based on the current reported research.
4 CONCLUSIONS
The low HDT of PLA is closely related to its T g values, the methods to increase T g of PLA probably have a positive effect on improving the HDT of PLA. Therefore, a series of PLA materials with different levels of crosslinking was prepared via reactive extrusion with TAIC and peroxide, expecting to improve the HDT via suppressing the chain mobility on heating. However, it was found that the HDT was not influenced by crosslinking and was independent of the crosslink density. The reason why HDT of PLA cannot be improved by crosslinking was discussed from the point of view of crystallinity and glass transition temperature. Through this fundamental investigation, possible technological methods to improve the HDT of PLA were discussed and predicted.
Two fundamental factors were taken into consideration to clarify the relationship between crosslinking and HDT—crystallization and glass transition. Both the nonisothermal and isothermal crystallization studies showed that the crystallization rate was increased with chain crosslinking because of the nucleation effects of the branched chains. According to the degree of crosslinking, the nucleation effects of the crosslinking on PLA were divided into three regimes. The NE was highest in samples with moderate crosslinking. However, even with improved NE, the highest crystallinity of the crosslinked PLA was only 10%, too low to influence the HDT. Therefore, the influence of the glass transition on the HDT of amorphous materials was investigated. Both the DMA and DSC studies showed that T g of the PLA had not been influenced by the crosslinking, remaining constant and leading to unchanged HDT. It means that the free volume of PLA is very difficult to decrease via crosslinking method, different from the traditional polymer materials. To clarify the reason why T g cannot be improved by crosslinking, the average molecular weight between crosslink points for the samples with the highest degree of crosslinking (PLA-TAIC3-L1) was also calculated. It was found the chain length (or number of repeated units) between the crosslink points was higher than the chain length that was responsible for the segment mobility at the glass transition. To improve the HDT of PLA via increasing T g, it is suggested that two methods can be taken into consideration: (1) copolymerization with bulky side groups to decreasing the free volume could have possibility to solve this problem and (2) confirmation restriction should be imposed on the lactoyl repeating units to number less than 14.
ACKNOWLEDGMENTS
The authors would like to acknowledge the Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA)/University of Guelph - Bioeconomy for Industrial Uses Research Program (Project # 030054); Agriculture and Agri-Food Canada (AAFC) and Competitive Green Technologies through AgriInnovation Program project (Project # 052882 and 051910); the Ontario Research Fund, Research Excellence Program Round-9 (ORF-RE09) from the the Ontario Ministry of Economic Development, Job Creation and Trade (Project #053970); and the Natural Sciences and Engineering Research Council of Canada (NSERC), Canada Discovery Grants (Project # 401111) for their financial support to carry out this research work.




