Supramolecular self-assembly of compound β nucleating agent and effect on polypropylene microporous membrane
Funding information: Shanghai Synchrotron Radiation Facility; National Natural Science Foundation of China, Grant/Award Numbers: 21174092, 21674069, 51721091
ABSTRACT
In this work, the β crystalline morphology of polypropylene membranes and dual nucleating ability of compound nucleating agent (NA) are systematically invested. A kind of aryl amide-based compound TMB-5 and another kind of commercial rare earth-based β-NA WBG-II were chosen. It is intriguing that the supramolecular self-assembly aggregations of WBG-II changed from flower-like to needle-like frameworks with a lower supercooling temperature for recrystallization after the introduction of TMB-5. Considering that it is more difficult for TMB-5 to dissolve in polypropylene melt, the remaining undissolved TMB-5 aggregations serve as the heterogeneous nucleation sites, which can accelerate the recrystallization of WBG-II. Such a process prevents the WBG-II microfibers self-assembled into big flower-like frameworks. The needle-like aggregations of the compound NA are smaller and more uniform in size, which is helpful in obtaining cast films with more uniform crystal morphology. Thus, microporous membranes with high porosity could be obtained after biaxial stretching of oriented cast films. This work leads to a new understanding of the relationship between “NA framework size uniformity”- “crystallization uniformity”- “micropore uniformity,” which is helpful to find an appropriate crystalline morphology for high-performance microporous membranes of PP by biaxial stretching.
1 INTRODUCTION
The microporous polymer membranes have been widely adopted in the field of the liquid electrolyte lithium-ion separators due to their acceptable cost, mechanical strength, and thermal shutdown properties.1-4 In general, most separators in lithium-ion batteries are made of semi-crystalline polyolefins, predominantly polyethylene (PE)5-8 or polypropylene (PP).9-11 Methods for preparing commercial lithium-ion battery separator include “dry uniaxial stretching,”12-17“dry biaxial stretching,”11, 18-21 and “wet biaxial stretching”.22-24 The preparation method of “dry biaxial stretching” microporous membranes is generally biaxial stretching nonporous PP films with high content of β-crystals. Compared to the other two methods, “dry biaxial stretching” is more economical and environmentally friendly because it is free of solvent evaporation. Besides, the microporous membranes by “dry biaxial stretching” show better mechanical properties. So “dry biaxial stretching” is the most ideal route for the production of microporous membranes. However, most “dry biaxial stretching” separator cannot be used in some fields such as power battery because of its large and poor micropores distribution.
Many researchers explored the effect of processing parameters on the structure and performance of PP microporous membranes.13, 15, 18, 25-27 Tabatabaei et al.15 found that air cooling, cast roll temperature, draw ratio had a crucial rule on the orientation of the crystallization and using air cooling was helpful to improve the porosity and water vapor permeability of microporous membranes. Offord et al.27 found that the porosity of microporous membranes increased substantially when the cast films were annealed near the melting temperature of β-crystal. In addition to the processing parameters, the structure parameters of cast films also play a key role in the final performance of microporous membranes. Most of the previously mentioned studies aimed at explaining the mechanism for initial pores formation.
Isotactic polypropylene is of polymorphic composition, having at least four modifications: α, β, γ, and smectic.28-31 As is known, the most reliable and feasible way to control β-crystals content and morphology of polypropylene product is introducing β nucleating agent (NA).32-40 It has been widely reported and accepted that β-crystals tend to transform into α-crystals when cast films are stretched. Micropores formed owing to the volume contraction during β-α transformation, because density of α-crystals polypropylene is higher than that of β-crystals.26, 41 However, recent research developments have revealed that β-α transformation may not be the primary reason for the pore formation. While, the existence of weak areas between β lamellae was the direct cause of pore formation.12, 42, 43 Also, others thought that initial cavitation were derived from plastic deformation of the weak boundaries between β lamellar blocks.44, 45 Grant et al.18, 27 illustrated that initial micropores occurred between vertical big β-lamellae when they separated before yielding and there was no β-α transformation during the separation process. β-α transformation occurred as the lamellae further separated when less stable β-lamellae began to transform to α phase. From these works, the crystalline morphology is also playing decisive parts in the formation of micropores. Because annealing made distribution of β lamellar thickness narrower, performances of stretched annealed polypropylene membranes were improved.46 Our previous work19 also found that polypropylene cast films, which consisted of β-transcrystals induced by self-assembly β-NA had a good porosity after biaxial stretching and discussed the effect of different cast films crystalline structure on the performance of microporous membranes. According to our previous works, the preparation of the microporous membranes is not necessarily limited to a precursor cast film with a high β-crystal content.19, 47 The foremost factor for the microporous membranes preparation is heterogeneous crystalline structure. Improving the uniformity of the size distribution of NA and further obtaining uniform crystalline structures of the cast films are critical to the preparation and performance of the microporous membranes.
Due to hydrogen bonding or dipole-dipole interactions, dissolved NA in PP melt could recrystallize/self-assemble into unique frameworks.48, 49 And unique NA supramolecular self-assembly frameworks with large surface area could induce polymer to achieve several particular crystalline morphologies. Varga et al.49 reported that β-NA NJS self-assembled into needle-like, cone-like, and shish-kebab-like frameworks with different nucleating abilities. They also established that the dual nucleating ability was caused by lateral surface of NJS needle-like frameworks. Since the polymer crystalline lamellae grows vertically along the surface of NA fibrils, regulating the self-assembly behavior of NAs is regarded as an useful method to manipulate crystalline morphology.39, 50 In order to improve the uniformity of pore-size distribution, more uniform crystalline structure could be obtained by controlling the NA self-assembly behavior. Fu et al.51 found that under the effect of hydrogen-bond between ultrafine full-vulcanized power rubber and β-NA WBG-II, the introduction of EA-UFPR could change crystalline morphology of PP from spherulites into β-transcrystals. Owing to the finer β-transcrystals, the membranes with high porosity and uniformly distributed pores were prepared. Thus, the regulating of NA self-assembly by means of compounding nucleating agents may be a way to obtain more uniform needle-like frameworks. In this work, two different types of β nucleating agents named WBG-II and TMB-5 were chosen. The self-assembly process of compounded β-NA and the interaction between them in PP melt were systematically investigated. Finally, PP membranes with uniform β-transcrystals structure induced by the compound NA self-assembly were achieved. It can help us to make a good understanding of the relationship between cast films crystallization uniformity and microporous membranes micropores uniformity.
2 EXPERIMENTAL SECTION
2.1 Materials and sample preparation
The matrix polymer used in this wok was commercial grade iPP, S801, with melt flow index of 3 g/10 minutes, Mw = 3.23 × 105 and Mw/Mn = 4, purchased from Korea petrochemical Ind. Co, Ltd. The rare earth β-nucleating agent (β-NA), trademark as WBG-II, was provided by Guangdong Winner Functional Materials Co. (Foshan, China). The nucleating agent N,N′-dicyclohexylterephthalamide (TMB-5) was supplied by Fine Chemicals Department of Shanxi Provincial Institute of Chemical Industry (China).
Master batch with 1 wt% WBG-II and 1 wt% TMB-5 was prepared firstly using a SHJ-20 twin- screw extruder followed by water cooling and pelletizing. β-iPP containing different NAs were pelletized containing 0.2 wt% WBG-II, 0.1 wt% WBG-II + 0.1 wt% TMB-5, 0.2 wt% TMB-5 after melting iPP and master batch. The temperature profile along the barrel was set at 150/180/190/200/200/210°C. The β-PP cast films were then prepared by extrusion using a slit die of 1 mm × 100 mm (thickness × width). The die temperature was set at 220°C, and the calender rolls were maintained at 90°C. Cast films having a uniform thickness of 0.09 mm and a draw ratio of 10 were used. The specimens were denoted as I/W/T, where I, G, and T mean iPP, WBG-II, and TMB-5, respectively.
Biaxial stretching of cast films was performed using an MTS universal tensile testing machine equipped with a temperature chamber. The initial size of cast films before stretching was 40 mm × 40 mm. The samples were transversely stretching (TD) to a predetermined strain (ε) = 200% at 90°C with a constant speed of 10 mm/min. And the films after stretching (TD) were cut into films with size of 20 mm × 20 mm. A sequential biaxial stretching to the same strain (ε) = 200% at 125°C with the same constant speed of 10 mm/min in machine direction (MD). After heat setting at 130°C for 5 minutes, final microporous membranes products were prepared.
2.2 Experimental methods
2.2.1 Polarizing optical microscopy
The supramolecular self-assembly behaviors and aggregations of NAs were observed using an Olympus BX51 microscope (OM) (Olympus Co., Tokyo, Japan) equipped with a Linkam FTIR600 hot stage and a MicroPublisher 5.0 RTV CCD. The crystalline morphologies of different specimens after isothermally crystallization at 135°C for 1 minute were observed by polarizing microscope (POM).
2.2.2 In situ Fourier transform infrared spectroscopy
In situ Fourier transform infrared spectroscopy spectra were performed over the wavenumber range 800 ∼ 3500 cm−1 with a resolution of 4 cm−1, using a Nicolet 6700 FTIR spectrometer equipped with a Linkman hot stage in dry air atmosphere. β-PP cast films were placed between two ZnSe plates that were used as IR windows.
For thoroughly exploring the supramolecular self-assembly behavior of nucleating agents in PP melts, different specimens were heated to Tfs at a heating rate of 30°C min−1 and kept for 5 minutes. Next, samples were cooled to 150°C at the rate of 10°C min−1. In situ IR spectra were collected as temperature cooled down to a certain temperature between Tf and 150°C with the decrement of 10°C.
2.2.3 Differential scanning calorimetry
The calorimetry experiments were performed with the DSC (Q20, TA Instruments, New Castle, DE) instrument calibrated with indium under nitrogen atmosphere (50 mL min−1). In addition, around 4-mg samples were weighted. Firstly, the sample was heated up to 200°C for 5 minutes to erase any previous thermal history. Next, it was cooled to 50°C at a cooling rate of 10°C min−1. Then it was heated to different Tfs (200-250°C) at a heating rate of 10°C min−1 and held for 5 minutes to control the NA self-assembly structures in the melt. At last, the sample was cooled down again to 50°C at a cooling rate of 10°C min−1, followed by heating up to 200°C at a heating rate of 10°C min−1.
2.2.4 2D-WAXD and SAXS measurements
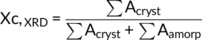 (1)
(1)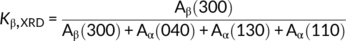 (2)
(2)In the formula (2), where Aβ (300) was the area of the (300) reflection peak of β phase at 2θ =16.1°, Aα (110), Aα (040), and Aα (130) were the areas of the (110), (040), and (130) reflection peaks of α phase and correspond to 2θ = 14.1°, 16.9°, and 18.6°, respectively. The fitted areas of the amorphous and the crystal region were Aamorp and Acryst.
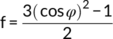 (3)
(3) (4)
(4)I(φ) in the above formula (4) was the scattering intensity along the angle φ, which is the azimuthal angle. The orientation parameter was calculated mathematically using Wilchinsky's method.53
2.2.5 Scanning electron microscopy
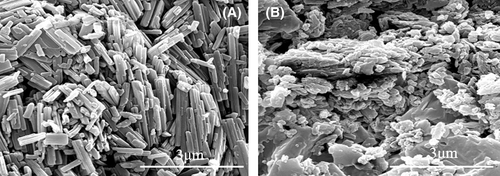
The morphologies of WBG-II and TMB-5 powder were characterized by field-emission scanning electron microscope (JSM-5900LV, JEOL, Japan) with an accelerating voltage of 20 kV.
Moreover, surface micrographs of PP microporous membranes after stretched and the crystalline morphologies of cast films were observed using SEM with an acceleration voltage of 10 kV. The amorphous region of polypropylene cast films was etched on the surface using the method developed by Olley.54 Prior to SEM characterization, etched films, the NA power, and microporous membranes were sprayed gold on surfaces.
2.2.6 Porosity determination
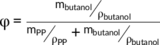 (5)
(5)In the formula (5), mbutanol was the value of quality after immersing minus initial sample weight and ρbutanol was 0.808 g∙cm−3.
3 RESULTS AND DISCUSSION
3.1 Dissolution and recrystallization behavior of compound nucleating agent in PP matrix
We introduced NA into PP with a low-temperature approach without dissolving NA in the matrix, which can facilitate understanding the dissolution and self-assembly behavior of NA in the polymer matrix. TMB-5 and WBG-II are composed of polar amide bonds, while polypropylene has nonpolar molecular chains. Considering the similarity and impermissibility, aromatic amide NAs could not completely dissolve in the nonpolar PP due to their mismatched polarity. The dissolution state of NAs in the matrix has an important influence on the self-assembled supramolecular structures of NAs and the crystalline morphology of polymer during subsequent cooling and crystallization process. TMB-5 and WBG-II are melt-sensitive nucleators, whose melting point is beyond the normal processing temperature for PP-based resins. As shown in Figure 1, for the NAs WBG-II and TMB-5, at lower Tf, the NA was not dissolved in the polymer matrix and existed in the form of primary microrods-like structure. At lower melting temperature, the NA hardly dissolved and self-assembled in the polymer matrix and kept its initial appearance.
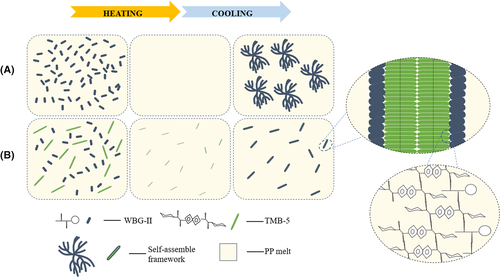
As shown in Figure S1, when the additive amount was 0.2 wt%, WBG-II was partially dissolved in the PP melt at a melting temperature Tf of 230°C. When temperature cooled down to approximately 200°C, it self-assembled into needle-like aggregations in the melt after recrystallization. The supercooling temperature of recrystallization as needle-like aggregations was about 30°C. However, when the Tf rose up to 240°C, WBG-II was completely dissolved in the matrix. It could be seen from Figure 2A that needle-like self-assembly frameworks of NA changed to flower-like frameworks. As temperature cooled down to 210°C, NA self-assembly frameworks could not be observed (shown in Figure 2A2). Flower-like frameworks could be seen at about 170°C with a higher supercooling temperature of 70°C (shown in Figure S1). As shown in Figure 2B, for another NA TMB-5, at the Tf of 240°C, TMB-5 in PP melt was mostly dissolved. The remnant undissolved TMB-5 aggregations played as nucleation sites and accelerated the recrystallization/self-assemble of NA dissolved part. As a result, the dissolved NA precipitated from PP melt as needle-like frameworks. We could observe the appearance of needle-like aggregation at 210°C with a supercooling temperature of 30°C (shown in Figure 2B2). It could be concluded that the recrystallization process of flower-like aggregations required a higher supercooling temperature than that of needle-like aggregations. This difference might be due to the decrease of nucleation sites and lower crystallization kinetics of flower-like aggregations at a higher melting temperature.49
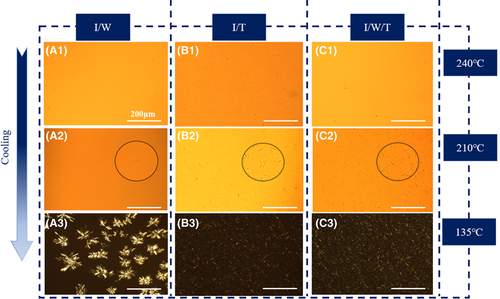
However, when we added the other β-NA TMB-5 to constitute compound NA and kept the total content of NA constant, we observed a different self-assembly phenomenon (shown in Figure 2C). At a Tf of 240°C, the compound NA did not self-assemble into flower-like frameworks like pure WBG-II in PP melt, but self-assembled into short and fine needle-like frameworks. Similar to specimen I/T, needle-like self-assembly frameworks began to appear when temperature cooled down to 210°C with the same supercooling temperature 30°C. The introduction of TMB-5 accelerated recrystallization of WBG-II and reduced its supercooling temperature. This phenomenon suggested that different crystalline morphologies and the size-distribution of NA self-assembly frameworks could be speculated by compound NA.
3.2 The mechanism of compound nucleating agent self-assembly
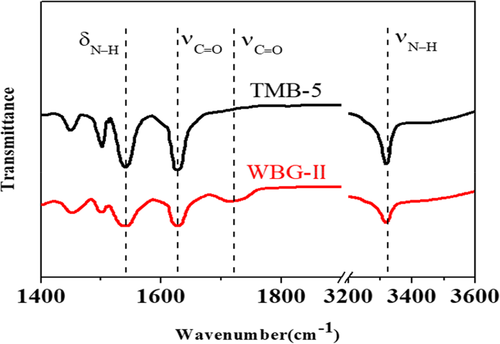
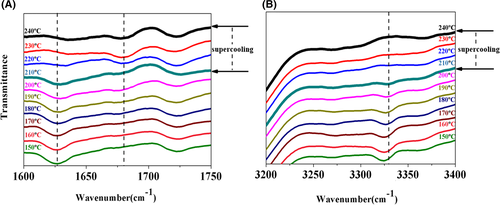
In situ FT-IR analysis was further carried out to verify the molecular interaction between TMB-5 and WBG-II in the self-assembly process. And FT-IR spectra were performed to detect surface functional groups of WBG-II and TMB-5. The main peaks shown on the spectra of TMB-5 and WBG-II were roughly similar as shown in Figure 3. They both had three characteristic absorption bands of the NH, CH, and CO groups: ν (NH) at ∼3317 cm−1, ν (CO) at ∼1625 cm−1 and ∼1720 cm−1 and δ (NH) bands at 1530 cm−1. The intermolecular hydrogen bond between the amide group and the carboxylic acid group supported the self-assembly behavior of the two kinds of NA in the PP melt.
Hu et al.48 found that the self-assembly of DCTH was related to the dissociation/association of hydrogen bonds and the conformational change of molecules. And the band with the lower frequency aroused from associated CO⋯HN hydrogen bond, while that with higher frequency was due to the “free CO” group. What's more, NH stretching frequency would have a red shift when hydrogen bonding association or strengthening and a blue shift when hydrogen bonding disassociation or weakening. For the specimen I/W melted at a Tf of 230°C (shown in Figure 4A), we observed the appearance of the associated “CO” peak at 1625 cm−1 when the temperature cooled down to 200°C. And the supercooling temperature was about 30°C. As for the specimen I/W melted at a Tf of 240°C showed in Figure 4B, temperature at which associated “CO” peak was observed had a further decrease to 170°C. It had a higher supercooling temperature of NA recrystallization about 70°C. The result was consistent with the self-assembly phenomenon observed as shown in Figure S1 and Figure 2.
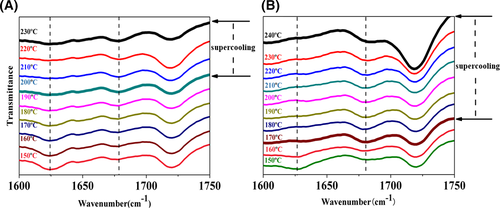
Interestingly, a different self-assembly phenomenon was observed when we changed WBG-II to compound NA. Different from Figure 4A, the associated “CO” peak at 1625 cm−1 appeared at 210°C with a lower supercooling temperature 30°C during the cooling process. Furthermore, ν (NH) at 3317 cm−1 also occurred at the same temperature and the NH stretching frequency had a red shift during the cooling process as shown in Figure 5A,B. This phenomenon suggested that when we introduced another NA TMB-5 into the matrix, a lower supercooling temperature for NA recrystallization was observed at the same melting temperature of 240°C. The decrease in supercooling temperature indicated that the remaining TMB-5 particles accelerated the recrystallization process of dissolved WBG-II. Based on previous discussion and analysis, a vivid schematic diagram of different NAs supramolecular self-assembly mechanism in PP melt was proposed in Figure 6.
3.3 Effect of melting temperature on heating curve of TMB-5 and WBG-II
Some research found that the melting temperature could affect the activity of β-NA and morphology of β-crystals.55 Varga et al.49 found that diverse supramolecular structures could form as a result of the solubility and dual nucleating ability of NJS. And it was proposed that the lateral surface of NJS needle-like frameworks has α-nucleating ability. The formation of needle-like aggregation would weaken the ability of some β nucleating agents like NJS. From the DSC heating curve as shown in Figure 7A, the height of the α-crystals peak and the β-crystals peak were substantially unchanged. It indicated that the dual nucleating ability of WBG-II was independent of the melting temperature and self-assembly framework. As for heating curve of PP nucleated with TMB-5 shown in Figure 7B, the height of β-crystals melting peak decreased and α-crystals peak increased with Tf rising. When Tf came to 250°C, the melting peak of β-crystals nearly disappeared. But it did not mean that TMB-5 had no β nucleating ability at this temperature because there was a β-α transformation during the heating process of DSC characterization. According to our previous work,19 when the melting temperature increased from 240°C to 250°C, the self-assembly framework of TMB-5 changed from needle-like to flower-like framework. Therefore, we speculated that most of TMB-5 self-assembled into flower-like frameworks, which had fairly low β nucleating ability when Tf rose to about 250°C. While for the heating curves of compound NA as shown in Figure 7C, it did not show the same phenomenon-like WBG-II observed in Figure 7A. Instead, it showed the same tendency with the curves of TMB-5. And the temperature in which β-crystal melting peak disappeared was 10°C lower than that in Figure 7B.
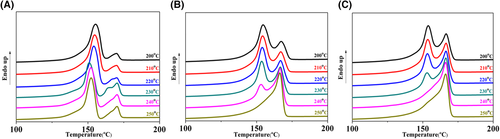
The result proved the existence of the interaction between WBG-II and TMB-5, TMB-5 acted as heterogeneous nucleation sites for the recrystallization of WBG-II. If there was no interaction, the β-crystal melting peak curves in Figure 7C would not disappear as Tf increased according to Figure 7A. Owing to a higher temperature, at which the dissolution of TMB-5 in the PP matrix required, the residual undissolved TMB-5 aggregations played a role as heterogeneous nucleation sites and accelerated the recrystallization/self-assembly of the dissolved WBG-II, and then the dissolved WBG-II precipitated as needle-like frameworks during the subsequent cooling process. When the melting temperature Tf increased, completely dissolved TMB-5 self-assembled into flower-like frameworks and WBG-II recrystallized onto the surface of it. As a result, the WBG-II did not recrystallize into its original state, instead precipitated by epitaxial crystallization of TMB-5 as a nucleation site. This was the possible reason for this phenomenon that β-crystal melting peak curve was hardly discovered at a higher melting temperature in Figure 7C. Under the influence of interaction between them, the crystallization ability of WBG-II depended on the self-assembly aggregations of TMB-5. Besides, compared with specimen I/T, I/W/T was completely dissolved at a lower temperature due to the lower content of TMB-5 in compound NA. TMB-5 in compound NA self-assembled into flower-like frameworks in a lower temperature. Therefore, the temperature in which β-crystal melting peak disappeared (shown in Figure 7C) was 10°C lower than that in Figure 7B.
3.4 Crystallinity and orientation analysis of cast films
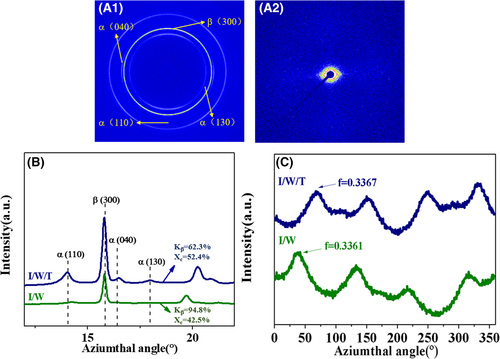
The 2D-WAXD and 2D-SAXS pattern of I/W/T cast film were given in Figure 8A. From the 2D-WAXD pattern, the diffraction rings of α (110), α (040), β (300), and α (130) were observed from edge to center. It could be concluded that I/W/T cast films comprised high β-crystal content from the brightest diffraction ring of β (300). In addition, the diffraction ring of cast films was not a homogeneous circular but two arcs symmetric distributing on meridian. This indicated that the β phase lamellae of the sample were aligned perpendicular to the machine direction. Moreover, the SAXS scattering pattern of I/W/T specimen was on the meridian and weak scattered signals were also observed in the equator. From this phenomenon, it could be concluded that peculiar orientation morphology was due to the epitaxial growth of chain-folding lamellae.56, 57 β-NA needle-like frameworks would orient to the flow direction due to the hydrodynamic force prior to the crystallization of PP. PP molecular epitaxially crystallized onto the surface of the needle crystals.58, 59 Therefore, the β phase lamellae of the sample were oriented vertical to the machine direction. From the 1D-WAXD intensity profiles, the relative amount of the β phase (Kβ) and the crystallinity (Xc) of I/W/T and I/W cast film were presented in Figure 8B.We can see that there still remain a high Kβ value (62.3%) and crystallinity (52.4%) of specimen I/W/T. Furthermore, Hermans' orientation functions of α (040) along azimuthal angle of different β-PP samples can be gained from the I-A curves (as shown in Figure 8C). The orientation degree of the I/W/T sample was 0.3367, which was very close to that of the I/W sample (0.3361). Therefore, oriented polypropylene cast films consisted of β-transcrystals can be obtained through compound NA self-assembly.
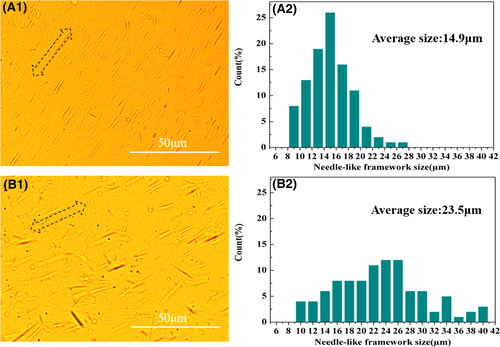
Polarizing optical microscopy and particle size statistics were employed to provide information on size of needle-like NA self-assembly frameworks of two kinds of β-PP cast films. As shown in Figure 9, needle-like self-assembly frameworks were oriented along the machine direction, which was consistent with the result shown in Figure 8. The particle size statistics result was shown in Figure 9A2,B2. Compared with specimen I/W, the framework size distribution of specimen I/W/T was narrower and more uniform. Besides, the framework average size of specimen I/W/T (14.9 μm) was smaller than that of specimen I/W (23.5 μm). The result indicated that crystalline structure and morphology could be regulated and controlled through NA self-assembly. Smaller and more uniform needle-like crystals could be obtained induced by the compound NA. In addition, intuitive β crystalline morphology could be observed as shown in SEM graphs of Figure 10. We could see clear isolate β-transcrystals induced by NA self-assembly frameworks and amorphous region between them. Typical β-transcrystals existing remarkably induced by β-NA microfibers aligned along the machine extrusion. Cast films with the same crystal morphology, similar orientation, and different crystal size were obtained. It provided a good opportunity to study the effect of cast films crystal size on microporous membranes.
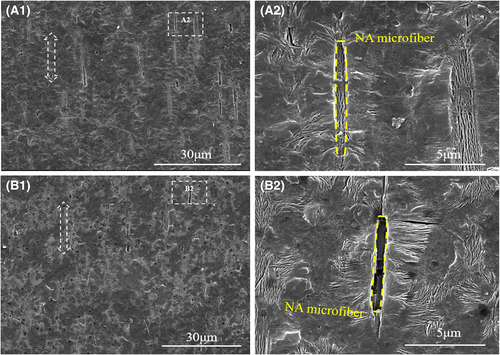
3.5 Pore structure of stretching film
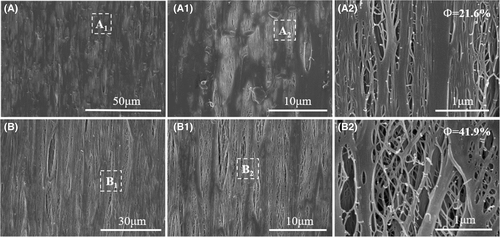
Figure 11 shows surface morphology of microporous membranes obtained through uniaxial and biaxial stretching to ε = 200%. Interestingly, many uniformly distributed crack-like pores could be observed. After biaxial stretching the β-PP specimen nucleated with compound NA, most area of cast films surface was stretched into crack-like micropores, and the micropores diameters were not much different. Additionally, the porosity value of I/W/T sample after biaxial stretched was 41.9%. This phenomenon suggested compound NA was a meaningful way to accurate NA crystallization and prepare β-PP cast film with more uniform crystal-size distribution. Microporous membranes with higher porosity and more uniform pore-size distribution could be obtained through stretching β-PP cast film nucleated with compound NA self-assembly.
4 CONCLUSIONS
In this work, the potential interaction between two kinds of β-NA WBG-II and TMB-5 was confirmed, and the mechanism of their supramolecular self-assembly was systematically investigated. DSC heating curves showed the addition of TMB-5 changed the dual nucleating ability of WBG-II. The in situ FTIR and POM results indicated that the addition of TMB-5 reduced the supercooling temperature for β-NA recrystallization and changed WBG-II self-assembly frameworks from flower-like to needle-like aggregations. Interestingly, cast films nucleated with compound NA had more uniform needle-like self-assembly frameworks and more uniform β-transcrystals distribution. It is tentatively proposed that the uniformity improvement could be caused by the synergistic effects of WBG-II and TMB-5. Dissolved WBG-II can be recrystallized onto the surface of undissolved TMB-5 frameworks due to hydrogen bond interaction. Furthermore, SEM images showed microporous membranes with high micropore uniformity could be obtained after biaxial stretching of oriented cast film with more uniform crystal-size distribution. Therefore, compound β-NA had certain significance in adjusting the uniformity of needle-like NA frameworks and β-transcrystals sizes of cast films, which helps to obtain microporous membranes with uniform micropores distribution and pore-size distribution.
ACKNOWLEDGMENTS
The authors sincerely acknowledge the financial support of National Natural Science Foundation of China (Grant number 51721091, 21674069, 21174092). The authors also express sincere thanks to the Shanghai Synchrotron Radiation Facility (SSRF, Shanghai, China) for the kind help on 2D-SAXS and 2D-WAXD measurement.




