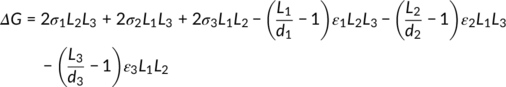Conjugated polymer single crystals and nanowires
Funding information National Key R&D Program of "Strategic Advanced Electronic Materials", Grant/Award Number: 2016YFB0401100; National Natural Science Foundation of China, Grant/Award Number: 51573185, 51890871, 91833306; Strategic Priority Research Program of the Chinese Academy of Sciences, Grant/Award Number: XDB12020300
Abstract
Conjugated polymer single crystals and nanowires show advantages for the study of intrinsic charge transport in conjugated polymers and achieve high device performance. However, preparation of conjugated polymer single crystals and nanowires, especially donor-acceptor (D-A) conjugated polymers, is challenging. In this review, the structural features of conjugated polymers are first described. Then, the mechanism of conjugated polymer crystallization in terms of thermodynamic equilibrium state, nucleation, and growth is discussed. Single crystals of poly(alkylthiophene)s (P3ATs) and nanowires of both P3ATs and D-A conjugated polymers are subsequently reviewed. Growth kinetics are a key issue in growing D-A-conjugated polymer crystals. Finally, the device performance based on conjugated polymer single crystals and nanowires is summarized.
1 INTRODUCTION
Conjugated polymers have many potential applications for preparation of organic optoelectronic devices (field-effect transistors [FETs], organic solar cells [OSCs], and organic light-emitting diodes) due to their advantages including flexibility, low cost, solution processing, and ease of achieving large area devices.1-13 Over the past two decades, the development of conjugated polymers underwent three generations: first-generation materials such as polyacetylene, second-generation materials such as poly(alkylthiophene)s (P3ATs) and third-generation materials such as donor-acceptor (D-A) polymers.14 The mobility of many D-A conjugated polymers has reached 10 cm2/vs in FETs,15-20 approaching the mobility of organic small molecules.21-25 The performance of OSCs based on D-A conjugated polymers have also reached 14%, making commercialization very promising in the near future.26, 27 To achieve high device performance, the aggregated structure of these conjugated polymers should be well controlled.28-31
For conjugated polymers, charge transport, which generally includes intrachain transport along the polymer chains and interchain transport between the backbones, is very complex and remains challenging. Conjugated polymer single crystals are important for the study of essential charge transport in conjugated polymers.32 For most semicrystalline conjugated polymers, the charge transport in crystalline regions is better than that in amorphous regions. Long polymeric chains that can bridge small crystalline domains, thus supporting charge transport on length scales, could also improve the charge transfer efficiency.33, 34 In addition, the pathways for charge transport are also very important. A long-range ordered structure of crystalline regions or polymer chains is beneficial for charge transport.35, 36 Based on the above principles, long and wide conjugated polymer nanowires or single crystals could facilitate charge transport due to their good molecular packing orientation and fewer amorphous regions.37, 38 Thus, preparing single crystals and nanowires of the right size is important for achieving high-performance TFTs. In OSCs, an interpenetrating network structure with a length scale of 10 to 20 nm may be formed by donor and acceptor materials to achieve high performance.2, 39 However, the actual morphology of OSCs generally contains a pure donor aggregated phase, a mixed phase, and a pure acceptor aggregated phase.40-44 The size, ratio, and composition of these phases greatly affect the device performance.44-49 Quantifying these parameters to acquire optimized morphology remains challenging. Conjugated polymer nanowires have advantages in OSCs due to their advantages of long charge transport channel, controllable crystal sizes, and lack of postprocessing. In addition, the morphology could be controlled accurately if both the donor and the acceptor materials use nanowires with controllable sizes. Thus, preparing conjugated polymer nanowires with controllable sizes is very important to achieve high-performance devices. However, the preparation of conjugated polymer single crystals and nanowires, especially D-A conjugated polymers, is still a challenge.
In this review, we focus on the preparation, structure, and device performance of conjugated polymer single crystals and nanowires. First, we compare the structural features of traditional flexible polymers, P3ATs, and D-A conjugated polymers. Second, we discuss the mechanism of conjugated polymer crystallization including the thermodynamic equilibrium state, nucleation, and growth. Third, we review the reported conjugated polymer singe crystals and nanowires in the literature, and discuss the relationship between the structure and polymer backbones. Growth kinetics are a key issue in growing D-A conjugated polymer crystals. The device performance based on conjugated polymer single crystal and nanowires is summarized. The purpose of this review is to summarize the research status of conjugated polymer single crystals and nanowires, as well as note the difficulties in growing D-A conjugated polymer crystals.
2 STRUCTURAL FEATURES OF CONJUGATED POLYMERS
The crystallization process of flexible polymers has been widely studied.50 Before discussing the crystallization of conjugated polymers, we note the differences between the structure of conjugated polymers and traditional flexible polymers. Their structural differences can be divided into two aspects: interchain interactions and backbone stiffness.
The chemical structure of polyethylene (PE), P3AT, and a D-A conjugated polymer is shown in Figure 1. The interactions between polymer chains can be divided into three aspects: one interaction is along the conjugated backbone and the other two are perpendicular to the conjugated backbone. For traditional flexible polymers such as PE, the two interactions perpendicular to the conjugated backbone have few differences due to the rotation of carbon-carbon bonds. Conjugated polymers generally contain a conjugated backbone and long alkyl side chains. The alkyl side chain can increase the solubility of conjugated polymers. For conjugated polymers, the two interactions perpendicular to the conjugated backbone are along the π-π stacking direction and the side chain direction. In general, the interaction in the π-π stacking direction is much larger than that in the other two directions. We discuss how the differences in interchain interactions affect the resulting crystals in Section 3.1.
 (1)
(1)
The backbone stiffness determines whether polymer chains can fold when forming crystals. The persistence length of PE is only approximately 0.7 nm.51 Thus, PE chains are folded in crystals.55 The persistence length of P3ATs is not very long, approximately 2.8 nm.51, 56 P3AT chains can fold during the crystallization process if Mn is large enough. However, for most D-A conjugated polymers, the persistence length is much longer. For example, the persistence length of OD-PDBT-TT can reach 8 nm, and the persistence length of PDPP2TBDT can be more than 1 μm.51 The energy needed for chains to bend would be much larger in this situation. Generally, D-A conjugated polymers do not fold during the crystallization process. The structure of conjugated polymer crystals is discussed in Section 4.
3 MECHANISM OF CONJUGATED POLYMER CRYSTALLIZATION
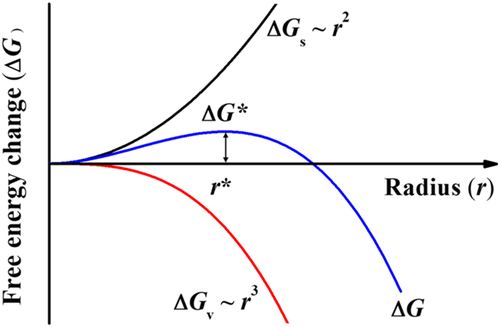
As with small molecules, the crystallization process of polymers includes nucleation and growth. For flexible polymers, folded chains and bundle-like nuclei may form during the nucleation process. Lauritzen and Hoffman showed that a loop-type crystal grown by folding of the polymer chains is more stable than a bundle-like crystal in a sufficiently dilute solution.57 Based on thermodynamic equilibrium theory, polymer chains tend to continue going into the crystal as it is formed. However, only the crystals with the right folded length have the maximal growth rate in kinetics.58 Thus, flexible polymers tend to form lamellar structures with a certain thickness. For conjugated polymers, the situation is more complex. The chains of some conjugated polymers can fold and others do not fold when forming a crystal, as discussed in Section 2. The molecular interactions also differ, and the π-π interaction is much larger than other interactions. In this section, we first discuss the crystal sizes at the ultimate thermodynamic equilibrium state. Because of the complex interactions among polymer monomers, the free-energy landscape is very rich when polymer chains try to gather together.59, 60 The equilibrium state could hardly be reached and the observed crystal size is deeply determined by kinetics. We then discuss the kinetics of conjugated polymers crystallization including nucleation and growth.
3.1 Thermodynamic equilibrium state
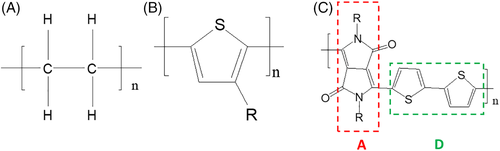
Considering the formation of a crystal from single chains in the solution, as shown in Figure 3, the length of the crystal along the π-π stacking, conjugated backbone and alkyl directions are L1, L2, and L3, respectively. Let σ1, σ2, and σ3 be the interfacial energy per unit area of surfaces perpendicular to the π-π stacking, conjugated backbone, and alkyl directions, respectively. Within the crystal, the interaction energies per unit area between two adjacent molecules in the π-π stacking, conjugated backbone, and alkyl directions are ε1, ε2, and ε3, respectively. The lengths of a single polymer chain in the π-π stacking, conjugated backbone, and alkyl directions are d1, d2, and d3, respectively. Then, the number of polymer molecules in these three directions can be written as n i = L i/d i, i = 1, 2, and 3.
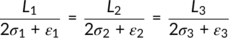 (3)
(3)For conjugated polymers, the molecular interaction in the π-π stacking direction is much larger than that in the other two directions. Thus, conjugated polymers tend to form one-dimensional fibrils or nanowire structures.64-67
3.2 Nucleation and growth kinetics
The crystallization kinetic process includes nucleation and growth. In solution crystallization, understanding the nucleation process is very important because it largely determines the resulting crystal structure and size distribution. Before the nucleation process occurs, unimer coil conformation in solution is required for crystallization, and aggregates should be minimized.68, 69 In the crystallization process of most conjugated polymers, the chains generally planarize first and then form intermolecular aggregates.70-73 We focus on the subsequent nucleation process. The nucleation process can be divided into homogeneous and heterogeneous nucleation according to different mechanisms, which will be introduced below. We also focus on self-seeding heterogeneous nucleation because it is an important method for growing single crystals.
3.2.1 Homogeneous nucleation
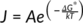 (4)
(4)
where A is the pre-exponential factor, k is the Boltzmann's constant, and T is the temperature.
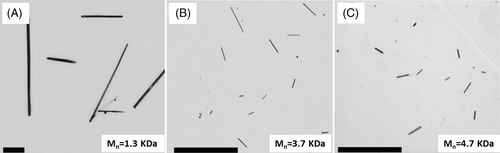
The increase in interfacial energy occurs along three dimensionalities for homogeneous nucleation. This leads to a rather large interfacial energy. Homogeneous nucleation dominates when there are fewer impurities and undissolved crystals. Ludwigs et al. obtained poly(3-hexyl thiophene) (P3HT) spherocrystals by appealing P3HT films with carbon disulfide vapor.77 They found that the seed density was low when the vapor pressure was large. In this situation, nucleation was dominated by homogeneous nucleation. Reiter et al. prepared P3HT single crystals through homogeneous nucleation, as shown in Figure 5.78 The low molecular weight samples were dissolved in tetrahydrofuran (THF) or 3-alkylthiophene. P3HT single crystals were obtained by maintaining these solutions at the correct temperature for several hours. The size of the P3HT single crystals varied. Thus, they proposed the crystals were grown through homogeneous nucleation.78
3.2.2 Heterogeneous nucleation
Heterogeneous nucleation generally occurs at the interface or surface of impurities, nucleating agents, and other crystals. This leads to less interfacial energy than homogeneous nucleation. Thus, heterogeneous nucleation is generally easier than homogeneous nucleation. The critical nuclei size is associated with the contact angle between the solution and impurities.79 Many groups have used small molecules, carbon nanotubes, and nanowires as nucleating agents to improve the crystallization of conjugated polymers.80-83 Heterogeneous nucleation of P3ATs has also been observed during isothermal crystallization.84, 85
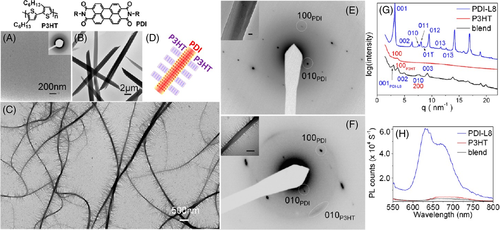
Hayward et al. found that P3HT could form fibrillar structure on the surface of perylene tetracarboxydiimide (PDI) nanowires, as shown in Figure 6.86 During the solvent casting process, PDI formed nanowire structures. These PDI nanowires could act as heterogeneous nuclei for P3HT, resulting in a shish-kebab structure. They also found that the dimensions of these nanostructures could be tailored by varying the P3HT/PDI ratio or solvent quality.
3.2.3 Self-seeding
Self-seeding is a particular case of heterogeneous nucleation.87 In the solution, there may be some polymer crystals when most of the polymers are dissolved. In this case, these crystals could act as nuclei when the temperature decreases. Unlike common heterogeneous nucleation, the resulting crystal grown from self-seeding is pure. Thus, self-seeding is a successful method for growing polymer single crystals.88-90
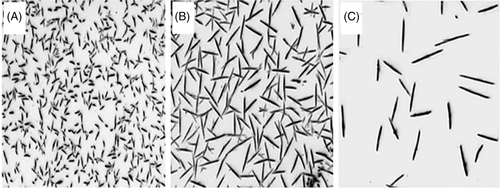
Reiter et al. prepared P3HT single crystals and controlled the number density size through self-seeding, as shown in Figure 7.78 The solutions were heated to a so-called seeding temperature (TSS) for 6 hours and then maintained at a lower crystallization temperature (TC) for 24 hours. The TSS is lower than the total dissolved temperature, and some undissolved crystals could survive and act as nuclei. They found that the crystal sizes depended on the TSS. A higher TSS caused fewer nuclei, resulting in larger and fewer single crystals. Homogenous nucleation was not observed on the timescale of their experiment. As we referred before, the single crystals of low molecular weight samples were grown through homogenous nucleation, possibly because crystals in low molecular weight samples are more likely to totally dissolve in the primal solution.
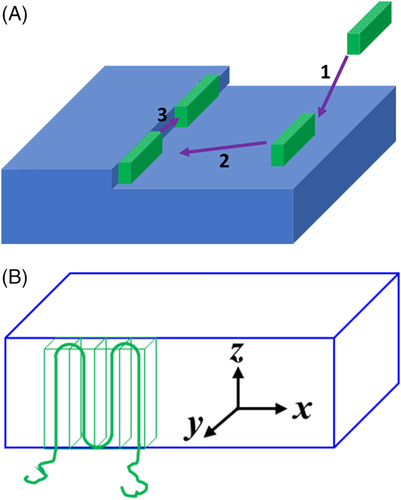
Once the nuclei form, polymer chains continue to grow on the surface of the nuclei and eventually form crystals. Based on the crystallization of small molecules, the growth of crystals can be divided into three steps: (1) molecules move to the surface of a crystal by diffusion, (2) surface diffusion moves the molecules toward the steps, and (3) the molecules are integrated into the crystal along the steps, as shown in Figure 8A.91 Based on these steps, the mechanism of crystal growth can be divided into diffusion-controlled growth and interface-controlled growth. The growth rate is associated with the time when the crystal growth is controlled by diffusion,92-94 and the growth rate is not associated with the time when the crystal growth is controlled by the interface.92, 95-97 Based on this theory, Lauritzen and Hoffman (LH) proposed LH theory to describe the growth of polymer crystals, as shown in Figure 8B. They regarded the growth process of polymer crystals as a secondary nucleation process of polymer chains, and the chains folded along the steps with a constant length.57, 98 Similarly, the growth direction of crystals is along x if the diffusion process is much faster than the nucleation process. In this situation, the growth is controlled by the interface. Conversely, the growth can be controlled by diffusion and the growth direction of crystals is along y.
For conjugated polymers, P3AT chains could fold similar to flexible polymers during the crystallization process. Thus, LH theory could be used to analyze the growth process of P3AT crystals.83, 84 However, the chains of most D-A conjugated polymers cannot fold when forming a crystal (see Section 2). The diffusion of D-A conjugated polymer chains is more difficult than for small molecules, and D-A conjugated polymer chains could not assemble themselves into crystals gradually through chain folding. How these long rigid chains form a crystal is still unclear. Understanding the growth process more is an issue in preparing D-A conjugated polymer crystals.
Using these basic thermodynamic and dynamic theories, many conjugated polymer single crystals and nanowires have been prepared. We summarize them in the next section.
4 CONJUGATED POLYMER SINGLE CRYSTALS AND NANOWIRES
Many P3AT single crystals and nanowires have been prepared. However, few nanowires of newly emerging high-mobility D-A conjugated polymers have been reported, and single crystals of D-A conjugated polymers have been rarely reported. Single crystal of D-A conjugated oligomers, whose structure is approaching that of conjugated small molecules, has been reported.99, 100 Here, we first discuss the preparation and structure of single crystals (P3ATs) and nanowires. Then, we summarize the literature and the key facts in preparation of D-A conjugated polymer single crystals and nanowires. Many factors affect the crystallization of conjugated polymers, such as the temperature, solvent, concentration, polymer species, and growth conditions. However, these factors could be well controlled and their effects on crystallization have been largely reported.63, 78, 101 Here, our purpose is to offer advice for preparing D-A conjugated polymer single crystals and nanowires. Thus, we focus on the specific factors that should be considered in growing D-A conjugated polymer single crystals and nanowires.
4.1 P3AT single crystals
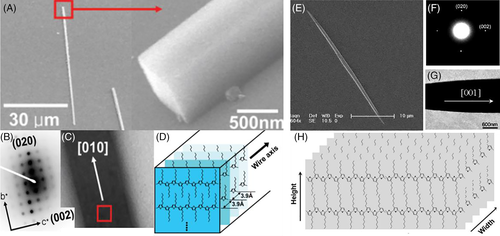
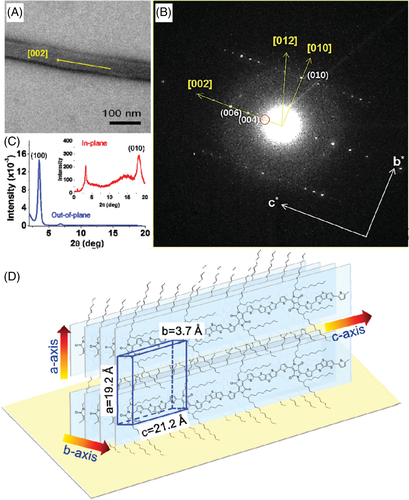
In general, the observed morphology of single crystals can be correlated to the molecular structure by electron diffraction and the lattice parameters can be determined by X-ray.63 Based on the difference in molecular orientations, P3AT single crystals can be divided into edge-on and flat-on orientations. P3HT single crystals were first prepared by Cho et al. using the self-seeding method, as shown in Figure 9A-D.102 P3HT solution in chloroform (CF) with a concentration of 0.1 mg/mL was seeded at 40°C first and then the crystallization process occurred at 10°C. The single crystal adopted an edge-on orientation on the silicon substrates modified with the CH3-functionalized self-assembled monolayers. The typical dimensions of the single crystal were 0.7 to 1.3 μm high, 1 to 3 μm wide, and 30 to 500 μm long. The length direction of the P3HT single crystal was along the π-π stacking, and the width direction was along the conjugated backbone. Later, He at al. reported a poly(3-octylthiophene) (P3OT) single crystal with the same structure by controlling the solvent evaporation.103 He et al. also prepared P3HT and P3OT single crystals with the length direction along the conjugated backbone and the width direction along the π-π stacking, as shown in Figure 9E-H.103, 104 A drop of P3HT solution in CF was deposited onto the substrate and the container was covered with a lid. The obtained film was placed in an open container full of THF vapor at 35°C under ambient pressure. After removal of the residual solvent, P3HT single crystal film was obtained. The resulting P3HT single crystals were mainly needle-like with a length of 20 to 60 μm and width of 1 to 2.2 μm.
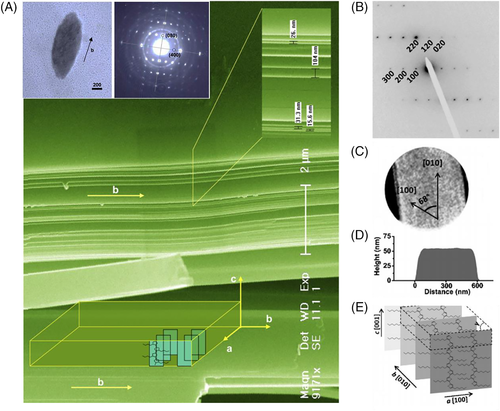
Yan et al. first prepared poly(3-butylthiophene) (P3BT) single crystals with a flat-on orientation, as shown in Figure 10A.105 P3BT films on the surface of glass substrate or mica coated with amorphous carbon thin films were obtained by drop-casting P3BT solution in THF and controlling the slow solvent evaporation. The film was dipped into a mixture of nitrobenzene and THF (3:1 in volume) for several days until the solvents were evaporated. The resulting P3BT single crystals showed lamellar structure with a lamellar thickness of 15.6 to 104 nm. The different lamellar thicknesses were caused by the gradient crystallization of the macromolecules with different molecular weights. They observed alkyl side chains in the growth front during crystallization. Later, Yan et al. grew poly(9,9-dioctylfluorene)s (PFOs,) single crystals with a flat-on orientation using the same method.106-109 They found that the orientation of PFO crystals could be adjusted from edge-on to flat-on by changing the solvent.106 They also found that both monodisperse and polydisperse PFOs could form this kind of lamellar crystal. The crystallization process was mainly affected by the saturation solubility of different molecular weight components.107-109
P3HT single crystals with a flat-on orientation were also prepared by Reiter et al. through self-seeding, as shown in Figure 10B-E.78 The resulting single crystals were approximately 59 nm high, which was consistent with the molecular weight. The length direction of these single crystals was along the π-π stacking. Using self-seeding, the single crystals were grown in solution. The molecular interaction in the π-π stacking direction is much stronger than that in the other two directions. Thus, the length direction was along the π-π stacking. When the single crystals are transferred to the substrate, the crystal size and the substrate determine the orientation. This could explain why Cho et al. and Reiter et al. obtained P3HT single crystals with different orientations by using the same method. Using the self-seeding method, P3AT or P3AT block copolymer single crystals have also been prepared by several groups.110-112
Using the appropriate methods, P3AT single crystals with different orientations have been prepared. The growth direction also depends on the growing conditions. However, the structure of P3AT nanowires is quite simple.
4.2 Nanowires with different polymer backbones
Nanowire is generally used to describe a nanostructure with an aspect ratio larger than 1000. The diameter of nanowires could be tens or hundreds of nanometers and the length of nanowires is always larger than 1 μm. The structure of D-A conjugated polymers is rather complex. Nanowires or nanofibers of several D-A conjugated polymers had been reported.113-120 For example, Mullen et al. prepared cyclopentadithiophene-benzothiadiazole copolymer (CDT-BTZ) nanowires by solvent vapor enhanced drop casting.113 Park et al. prepared poly[2,6-(4,4-bis-(2-ethylhexyl)-4H-cyclopenta[2,1-b;3,4-b0]-dithiophene)-alt-4,7-(2,1,3-benzothiadiazole)] nanowires by a surfactant-templating process.116 Zhan et al. prepared nanowires of a D-A conjugated polymer based on bithiazole-thiazolothiazole (PTz) by self-assembly.117 Interestingly, the length direction of all the D-A conjugated polymer nanowires is along the conjugated backbone. However, it is difficult to make a conclusion on how to prepare D-A conjugated polymer nanowires. Here, we reviewed the success in preparing P3AT nanowires and chose diketopyrrolopyrrole (DPP) DPP-based polymer as an example to illustrate the challenges in preparing D-A conjugated polymer nanowires.
4.2.1 P3AT nanowires
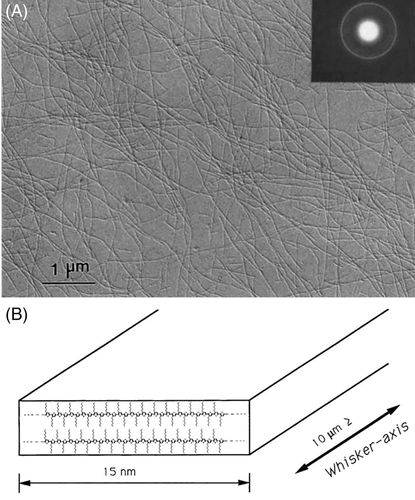
P3HT nanowires were first prepared by Ihn et al. using a so-called whiskers method, as shown in Figure 11.121 P3HT nanowires were grown from a dilute solution of cyclohexanone, prepared at 50°C, by slowly cooling to room temperature. The resulting P3AT nanowires are highly crystalline and approximately 15 nm wide and 10 μm long. The length direction of P3HT nanowires was along the π-π stacking and the width direction was along the conjugated backbone. Considering that the number-average contour length of the macromolecule is 65 nm, they proposed the macromolecules must fold back and forth along the lengthwise edges of the nanowires. They also showed that the formation of whiskers depends on the solvent quality (solubility), temperature, and alkyl side-chain length.
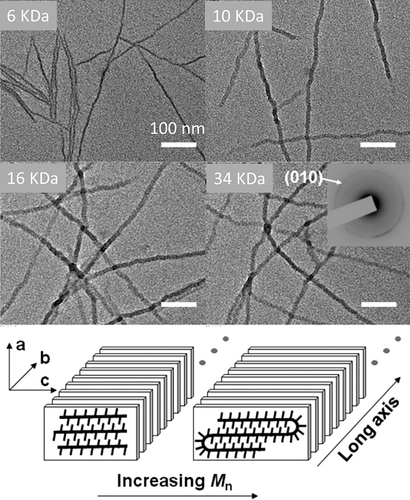
Zhai et al. researched the effect of the number-average molecular weight (Mn) on the structure of P3HT nanowires, as shown in Figure 12.66 They found that when the Mn was less than a critical value (10 kDa), the width of P3HT nanowire corresponded to the length of the extended polymer chain. However, the width of nanowire remained constant when the Mn was higher than 10 kDa. This result suggested that the P3HT chain changed from extended to folded at the critical Mn. They also found that the length of the folded chain increased when the temperature is increased. This is consistent with the behavior of flexible chains.57, 62
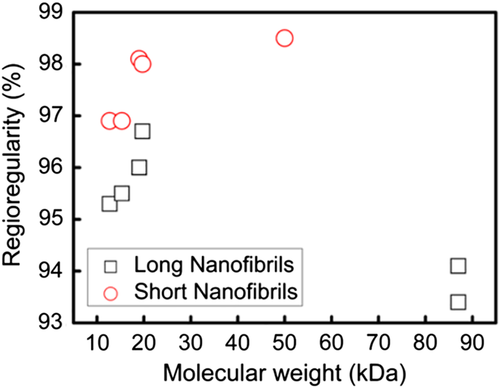
Jeong et al. found that the regioregularity (RR) of the P3HT molecular structure also affected the resulting P3HT nanowires, as shown in Figure 13.122 By choosing P3HTs with different RR and molecular weights (MW), they concluded that low-RR P3HTs generated neat long nanofibrils (LNFs) and high-RR P3HTs formed short nanofibrils (SNFs). They also identified a critical RR (96%-98%) depending on their MW, below which P3HT grew into LNFs and above which P3HT grew into SNFs.
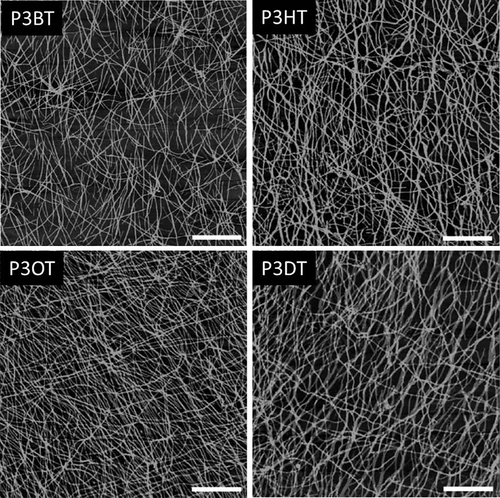
Using the whiskers method, Samitsu et al. prepared P3AT nanowires with four different alkyl chain lengths (4, 6, 8, and 10 carbon atoms), as shown in Figure 14.123 They found that the nanowires had an anisotropic cross section 3 to 4 nm high and 24 to 27 nm wide, which slightly increased with the alkyl chain length. Later, Vanderzande et al. prepared P3AT nanowires with alkyl chain lengths between three and nine carbon atoms.124
In addition to the whisker method, P3AT nanowires can also be prepared by other methods. Reichmanis et al. prepared P3HT nanowires using ultraviolet irradiation.125 Hayward et al. obtained water-processable P3HT block copolymer nanowires by photocrosslinking and click functionalization.126 Sung et al. produced P3HT nanowire arrays using a direct printing method.127 Jeong et al. controlled the nucleation process by using a cycle of cooling and heating in solution, and produced P3HT nanowires with a spin-coating process.128
4.2.2 DPP-based polymer nanowires
Among many promising D-A conjugated polymers, DPP-based polymers are one of the most widely investigated polymers due to their excellent device performance.5, 129-134 Unlike P3AT nanowires, the width of DPP-based polymer nanowires can be changed,135 which is pivotal in the improvement of device performance.136-140 Choi et al. first prepared DPP-based polymer nanowires based on a DPP-bearing copolymer (PDTTDPP), as shown in Figure 15.141 The PDTTDPP nanowires were prepared using the whiskers method in very dilute solution (0.001 wt%). The resulting nanowires were approximately 50 nm wide, 80 nm high, and more than 10 μm long. The nanowires adopted an edge-on orientation, and the length direction was along the conjugated backbone. They also tried to prepare other DPP-based polymers nanowires, but failed.141, 142 They proposed that the more planar DTT donor moiety could promote intermolecular interactions to propel the self-assembly of polymer chains.
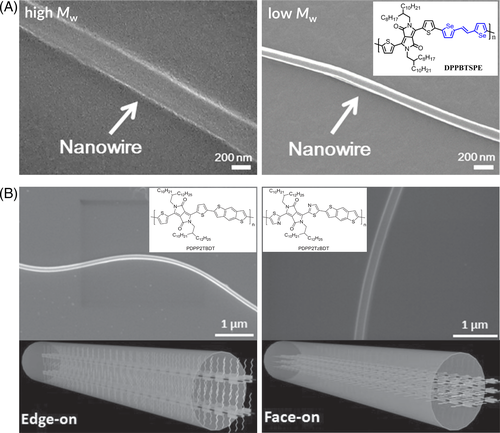
Later, Choi et al. prepared nanowires of a more planar DPP-based polymer bearing 1,2-bis(5-[thiophen-2-yl]-selenophen-2-yl)ethene in the repeating group, as shown in Figure 16A.135 They used high- and low-Mw polymers to grow nanowires and found that the resulting nanowires made of high-Mw polymer were much shorter and wider than those made of low-Mw polymer. The nanowires also adopted an edge-on orientation, and the length direction was along the conjugated backbone. Wang et al. prepared nanowires of two DPP-based polymers with different orientations, as shown in Figure 16B.143 For the DPP-based polymer consisting of decyltetradecyl substituted DPP, benzodithiophene repeat units, and thiophene (T) units as the linker (PDPP2TBDT), the nanowires adopted an edge-on orientation. However, when the link units were replaced with thiazole (PDPP2TzBDT), the resulting nanowires adopted a face-on orientation. The length direction of both PDPP2TBDT and PDPP2TzBDT nanowires is along the conjugated backbone.
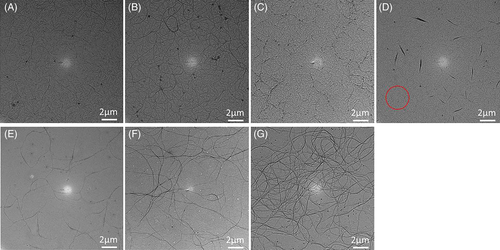
Unlike P3AT nanowires whose width depends on the folded length, the width direction of DPP-based polymer nanowires is always along the π-π stacking, which makes it easier to adjust the width. However, DPP-based polymer nanowires have only been successfully prepared in very dilute solution, which limits the applications. Recently, Han et al. was the first to prepare DPP-based polymer nanowires in a higher concentration (1.2 mg/mL) by decreasing the aggregate speed of the polymer in solution, as shown in Figure 17.144 The solvent consisted of a good solvent, CF, as the main solvent and a marginal solvent, 1,2-dichlorobenzene, as the cosolvent. They controlled the evaporation of CF at 30 μL/hours. During the evaporation process, the polymer crystals gradually grew from short rod-like fibrils to long nanowires. They also showed that the number of nuclei could be controlled by changing the concentration of the pristine solution.144 As the length direction of the DPP-based polymer nanowires is along the conjugated backbone and the probability that short nanowires meet the ends of other molecules to grow longer is small, the aggregate speeds of the molecules should be controlled slowly to increase the probability. This may explain the results of Choi et al. For high-Mw polymers, the probability that short nanowires meet the ends of other molecules is lower, resulting in shorter and wider nanowires. Thus, the diffusion of chains is an important factor to control for in growing D-A conjugated polymer crystals. More research is needed to prepare good-quality DPP-based or D-A conjugated polymer nanowires.
4.3 Key factors in preparing D-A conjugated polymer single crystals and nanowires
According to the literature, we conclude that the structures of P3AT and D-A conjugated polymer crystals differ greatly. P3AT chains can fold during the crystallization process, whereas D-A conjugated polymer chains cannot fold. This finding can be explained by their different backbone stiffness, as discussed in Section 2. Moreover, the arrangement of P3AT chains can be adjusted by changing the crystallization conditions, and the length direction of almost all of the conjugated polymer nanowires is along the conjugated backbone. The structure of D-A conjugated polymer nanowires may be caused by three factors: (1) the crystallization conditions approach the thermodynamic equilibrium state, and structures with a length direction along the conjugated backbone is more stable; (2) the nanowires tend to grow along the conjugated backbone in kinetics; and (3) the nanowires can grow along the conjugated backbone and the π-π stacking direction simultaneously, but the length of the conjugated backbone is much longer than the π-π stacking distance. However, the factors determining the structure of D-A conjugated polymer nanowires are still unknown. Understanding the formation of D-A conjugated polymer crystals is indispensable for preparing D-A conjugated polymer single crystals and nanowires.
Another difference is that the preparation of D-A conjugated polymer crystals is much more difficult. Please check the sentence “Since the chain conformation and nucleation” for clarity. Since the chain conformation and nucleation can generally be well controlled by changing the crystallization conditions such as the solvent and temperature.118, 145 Growth kinetics may be an important reason why the crystallization of D-A conjugated polymers is much more difficult than that of P3ATs. P3AT chains are semirigid, and the movement of P3AT chains could be realized by segmental motion. However, D-A conjugated polymer chains are stiff, and the movement of such a long rod-like chain is difficult. The average time for a single chain arranging itself into the crystal can be written as tC, and the average time for a single chain move to the growth front of crystals or nuclei can be written as tM. As shown in Figure 18, polymers in the supersaturated solution may form aggregates or crystals. To form crystals, polymer chains must move to the growth front of crystals. Thus, when tC ≪ tM, polymer chains have enough time to move to the growth front of crystals to form an ordered structure. Polymer chains would form unordered aggregate when tC ≫ tM, which could be the reason why nanowires or single crystals of D-A-based polymers are harder to prepare than those of P3ATs. By decreasing the concentration or the aggregate speed of a D-A-based polymer, polymer chains have enough time to move to the growth front of crystals, resulting in a nanowire structure despite the low-mobility chains. Thus, increasing the chain diffusion is an issue in growing D-A conjugated polymer nanowires.

Other differences between P3ATs and D-A conjugated polymers, such as the backbone structure, may also affect the crystallization process. Two different units may have different intermolecular packing styles compared with a single D unit.146-148 However, the effect of unit composition on the cyclization process remains unknown.
Besides controlling the crystallization process, other physical or chemical methods could also prepare conjugated polymer single crystals and nanowires. For example, electrospinning is a simple method to prepare conjugated polymer nanowires.149, 150 Topochemical polymerization is an effective method to prepare linear conjugated polymer single crystals.151-153
5 DEVICE APPLICATION
Conjugated polymer single crystals were mostly applied to FETs,1, 22, 65, 154 while conjugated polymer nanowires were used in both FETs and OSCs.37, 65, 155-157 We introduce FETs and OSCs below.
5.1 Field-effect transistors
FETs are transistors that use an electric field to control the electrical behavior of the device. FETs have three terminals: a source (S) for the carriers to enter the channel, a drain (D) for the carriers (holes and electrons) to leave the channel and a gate (G) to control the channel conductivity.7 The mobility of the carriers is the most important factor in FETs.
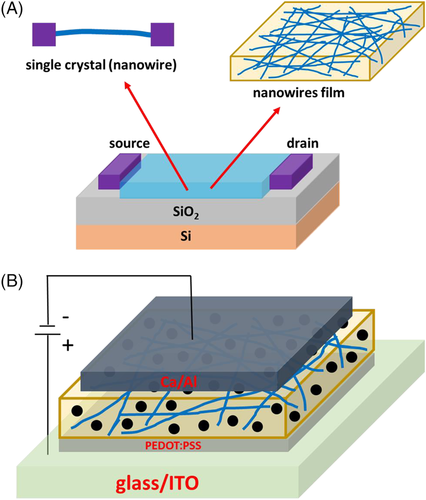
FETs based on conjugated polymer single crystals, single nanowires, nanowire films, and ordered nanowire films have been manufactured by several groups. A schematic diagram of FETs based on single crystals (nanowires) and nanowires film is shown in Figure 19A. Their orientations of the length axis and mobilities are summarized in Table 1. The mobility of P3AT single crystal or nanowire with the length axis along the π-π stacking is much higher than that of P3AT single crystal or nanowire with the length axis along the conjugated backbone. However, D-A conjugated polymer single nanowire can achieve high mobility although the length axis is along the conjugated backbone. The strongest electronic coupling in a polymer is along its backbone, but intermolecular transport is necessary because no individual chain can provide macroscopic transport.33 Compared with D-A conjugated polymer crystals, the charge transport along the conjugated backbone direction appears to be more difficult for P3AT crystals. Park et al. showed that the mobility of P3HT films could be improve several times if the chains were well linked.158 Thus, the charge transport among chains is difficult for P3AT crystals or nanowires with the length axis along the conjugated backbone. P3HT chains with similar length tend to crystallize together when the length axis is along the conjugated backbone.105 This could be the reason for the bad charge transport among chains.
| PolymerRef | Orientation of the length axis | Mobility (cm2/vs) |
|---|---|---|
| P3HT SCs102 | π-π stacking | ∼10 |
| P3OT SCs104 | Conjugated backbone | 1.54 × 10−4 |
| P3HT SCs103 | Conjugated backbone | 1.57 × 10−3 |
| P3OT SCs103 | π-π stacking | 0.62 |
| P3HT SNWs167 | π-π stacking | 0.06 |
| PDTTDPP SNWs141 | Conjugated backbone | 7.0 |
| DPPBTSPE SNWs135 | Conjugated backbone | 24 |
| PDPP2TBDT SNWs143 | Conjugated backbone | 7.42 (h), 0.04 (e)a |
| PDPP2TzBDT SNWs143 | Conjugated backbone | 5.47 (h), 5.33 (e) |
| PDPP2T-BT-co-NDI SNWs168 | Conjugated backbone | 0.98 |
| CDT-BTZ SNWs113 | Conjugated backbone | 5.5 |
| PTz SNWs117 | Conjugated backbone | 0.46 |
| P3HT NWFs169 | π-π stacking | 0.15 |
| P3HT O-NWFs170 | π-π stacking | 0.06 |
| P3HT O-NWFs159 | π-π stacking | 0.32 |
| P3HT O-NWsF160 | π-π stacking | 0.24 |
- Abbreviations: CDT-BTZ, cyclopentadithiophene-benzothiadiazole copolymer; DPPBTSPE, DPP-based polymer bearing 1,2-bis(5-[thiophen-2-yl]-selenophen-2-yl)ethane; FETs, field-effect transistors; NWFs, nanowire films; O-NWFs, ordered NWFs; P3HT, poly(3-hexyl thiophene); P3OT, poly(3-octylthiophene); PDTTDPP, DPP-bearing copolymer; PTz, bithiazole-thiazolothiazole; SCs, single crystals; SNWs, single nanowires.
- a The mobility of holes (h) and electrons (e).
For DPP-based polymers, the highest mobility of single nanowire FET was achieve 24 cm2/vs.135 Interestingly, Wang et al. found that both edge-on and face-on orientations of DPP-based polymer nanowires showed high mobility.143 They noted the usefulness of studying the intrinsic charge transport properties in organic FETs. Other D-A conjugated polymer nanowires could also achieve high mobility. Pisula et al. prepared CDT-BTZ nanowires and achieved a mobility of 5.5.113 Zhan et al. prepared a D-A conjugated polymer based on PTz nanowires and achieved a mobility of 0.46.117
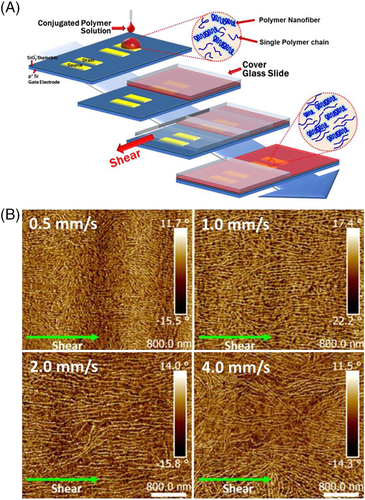
The nanowire film also contains some amorphous polymers. The charge mobility in amorphous polymers is much lower than that in crystalline polymers. Thus, the mobility of nanowire film TEF is much lower than that of single crystal or nanowire FET. Egap et al. and Reichmanis et al. prepared ordered P3HT nanowires almost simultaneously by offering a shear force during the film forming process.159, 160 Figure 20A shows the schematic diagram of nanowire films deposited on an FET device substrate with a shear force.159 Egap et al. found that P3HT nanowires achieved the best order when the shearing speed was approximately 2 mm/s, as shown in Figure 20B. The mobility of the best ordered P3HT nanowire films was approximately doubled than that of unordered nanowire films. The preparation of D-A conjugated polymer nanowires was only realized in low concentration, which causes a low density of nanowires in the film. Preparing high concentration D-A conjugated polymer nanowires that can be processed into nanowire films is still a challenge. FET devices made of D-A conjugated polymer nanowire films have rarely been reported.
5.2 Organic solar cells
OSCs are a type of photovoltaic that uses organic materials as the light absorption layer. The operation of OSCs is as follows: (1) the photoactive layer absorbs photons, (2) photoinduced charge separation occurs and carriers form, and (3) the holes are collected in the anode and the electrons are collected in the cathode.2 The power conversion efficiency is generally used to describe the performance of OSCs.
P3AT nanowires have been used to improve the performance of OSCs by many groups,46, 128, 161, 162 but D-A conjugated polymer nanowires have seldom been used in OSCs. For OSCs, the nanowires were generally blended with an electron acceptor as the light absorption layer, as shown in Figure 19B.
Guillerez et al. first fabricated a photovoltaic device based on P3HT nanowires.46 The blend film of P3HT and PCBM had a good fibrillar interpenetrating network structure. The average performance of pristine nanostructured P3HT:PCBM film reached 3.2%. Because not all of the molecules form nanowires in the solution, they purified P3HT nanowires by centrifugation. They also fabricated OSCs with different percentages of P3HT nanowires by adding well-dissolved P3HT while maintaining the total amount of P3HT constant. The device performance was the best (average 3.4%, maximum 3.6%) when the percentage of P3HT nanowires was 75%. They proposed that amorphous material played a role of cement in the nanostructured layer, ensuring good interconnection between the nanofibers or between the nanofibers and PCBM domains. The result is consistent with the deeper understanding of the effects of amorphous phase in the OSCs. OSCs with polymer and fullerene aggregated phase as well as the mixed phases (amorphous polymer and fullerene) can achieve good performance.40, 41 The mixed phase has been reported to have energy levels that are shifted with respect to the aggregated phases, forming an energy cascade that assists the generation of free charge.42, 45, 140, 163 Later, P3AT nanowires with different lengths of alkyl chain were used in OSCs, and their performance was excellent.161, 162, 164 The long nanowires structure has much better charge transport than spin-coated films in which the polymer fibril length is generally less than 1 μm. Chen et al. added 7 wt% P3BT nanowires to a solution of P3HT and PCBM, and found the performance of a device made of spin-coated films improved approximately 40%. The improved performance was associated with enhanced hole and electron mobilities, due to the formation of interconnected pathways along the crystallites of P3HT and P3BT-nws for efficient long-range charge transport.80
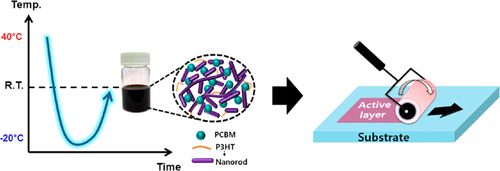
Besides better charge transport, OSCs made of nanowires also have better stability than conventional bulk-heterojunction (BHJ) devices. Jeong et al. have found that the performance of nanowires-based solar cells maintained more than 80% after a month in the air, while the performance of conventional BHJ devices only maintained 20% in the same conditions.165 Jeong et al. also found that nanowires have advantages to manufacture large-area printed OSCs, as shown in Figure 21.166 They found that nanowires-based solar cells had higher performance and better stability against large bending strains than conventional BHJ devices. Nanowires-based solar cells are also less sensitive to the film thickness than conventional BHJ devices.
6 SUMMARY AND OUTLOOK
The crystallization of conjugated polymer is significantly different from that of traditional flexible polymers such as PE. In thermodynamics, the crystal size is associated with the interfacial energy and intermolecular interaction energies. For conjugated polymers, the molecular interaction in the π-π stacking direction is much stronger than that in the other two directions, resulting in a tendency to form one-dimensional fibrils or nanowire structures. The backbone stiffness, which can be described by the persistence length, determines the crystallization behavior of polymer chains. Conjugated polymers such as P3ATs have a low persistence length, and the chains fold when crystallization occurs if the Mn is large enough. Most D-A conjugated polymers have a larger persistence length, but their chains do not fold during the crystallization process. The crystallization of less stiff chains is easier, and P3AT single crystals and nanowires have been prepared by many groups. The performance of TEFs and OSCs made of P3HT single crystals or nanowires is excellent. However, the preparation of D-A conjugated polymer single crystals and nanowires is much more difficult. Few D-A conjugated polymer nanowires have been prepared in low concentration and used in TEFs. The crystallization kinetics process of D-A conjugated polymers requires further study. In addition, conjugated polymer single crystals and nanowires have advantages in TEFs and OSCs due to their long charge transport channel, controllable sizes, lack of postprocessing, and ease of processing large areas. Control over the sizes, degree of crystallinity, and orientation of conjugated polymer single crystals and nanowires is crucial to achieve high-performance and low-cost devices. In addition, the solvents used to prepare conjugated polymer single crystals and nanowires should be optimized to decrease environmental damage.
ACKNOWLEDGMENTS
This work was partly supported by the National Key R&D Program of “Strategic Advanced Electronic Materials” (no. 2016YFB0401100), the National Natural Science Foundation of China (51573185, 51890871, and 91833306), and the Strategic Priority Research Program of the Chinese Academy of Sciences (grant no. XDB12020300).