Treatment Landscape in Pediatric Immune Thrombocytopenia: Addressing Unmet Needs
Joana Azevedo, Jennifer DiRaimo, and Cindy Neunert are the co-lead authors.
ABSTRACT
Pediatric immune thrombocytopenia (ITP) is associated with a multifaceted burden on children and their parents/caregivers due to bleeding, fatigue, activity restrictions, and psychological distress. Most children recover within 12 months, but up to 30% develop chronic ITP. While emergent therapies, such as steroids and intravenous immunoglobulin, are effective in many children and transiently raise platelet counts, 38–47% of children require subsequent therapies. The choice of subsequent therapy for individual children with ITP is often complex and the absence of head-to-head comparisons of available therapies and the use of nonstandardized outcomes in randomized clinical trials complicates treatment decisions. Furthermore, medication access varies globally and by age. Additional unmet needs in pediatric ITP include a lack of support and educational resources allowing children and parents/caregivers to effectively participate in treatment decisions, inadequate prediction of treatment response and disease chronicity, heterogeneous approaches to diagnostic evaluation of ITP, scarcity of novel treatments for children unresponsive to current therapies, and the need for a multispecialty approach to support the mental health of children and their families. This review summarizes the known impact of ITP on children and their families, current treatment strategies, and unmet needs in pediatric ITP.
Abbreviations
-
- 6-MP
-
- mercaptopurine
-
- ASH
-
- American Society of Hematology
-
- HRQoL
-
- health-related quality of life
-
- ICH
-
- intracranial hemorrhage
-
- ICR
-
- International Consensus Report
-
- ITP
-
- immune thrombocytopenia
-
- IVIG
-
- intravenous immunoglobulin
-
- MMF
-
- mycophenolate mofetil
-
- TPO-RA
-
- thrombopoietin receptor agonist
1 Introduction
Immune thrombocytopenia (ITP) is a rare, acquired autoimmune condition characterized by a low platelet count and an increased risk of bleeding, with a global incidence of 1.9–6.4 per 100,000 children annually [1, 2]. ITP is a diagnosis of exclusion, requiring other causes of thrombocytopenia to be considered first, including bone marrow disorders, disorders of platelet consumption, drug-induced thrombocytopenia, and inherited platelet disorders [3-5]. ITP may also be the first finding in children and adolescents with underlying immune dysregulation from a primary immunodeficiency or with evolving systemic autoimmunity to which the ITP is secondary [6].
ITP is a heterogeneous disease with variability in platelet counts, severity, disease course, and treatment response between individuals [5, 7]. As such, bleeding is not always predictable and can range from mild (i.e., bruising and petechiae) to more severe (i.e., hematuria or intracranial hemorrhage [ICH]) [1], with ICH occurring in approximately 0.4% of children with ITP [8]. Approved treatment options for newly diagnosed children with ITP include medications, such as corticosteroids and intravenous immunoglobulin (IVIG), that transiently raise platelet counts in the majority of children [9, 10]. ITP can spontaneously resolve within 1–2 years of diagnosis in many children [11], with one long-term follow-up study in children with ITP at 12 months reporting complete remission in 50% and 76% of children at 2 and 5 years after diagnosis, respectively [12]. Nevertheless, the impact of diagnosis and treatment on the health-related quality of life (HRQoL) for patients and their families can be severe, even during short disease courses [13].
2 Impact of ITP on HRQoL
The multifaceted burden of living with ITP can impact the overall HRQoL of children and their parents/caregivers. Children with ITP may experience fatigue, restricted activities due to concerns about bleeding risk, anxiety, and depression [14].
Fatigue is a common symptom reported in children and adolescents with both newly diagnosed and chronic ITP [15-17]. However, its etiology is unclear. Fatigue is associated with various patient characteristics, including low platelet count, corticosteroid use, bleeding symptoms, comorbidities, orthostatic symptoms, reduced physical activity, vitamin D deficiency, and physical, functional, emotional, and social well-being [18, 19]. Studies have failed to conclusively show beneficial effects of treatment on fatigue [16], and management of fatigue has not yet been standardized, remaining an unmet need in ITP [20].
Physical activity in children is an important part of their overall development [21], and limitations on daily activities can result in feelings of helplessness, disappointment, and frustration [14]. While evidence-based guidance on sports or activity participation in children with ITP is not currently available, physical activity is often intentionally restricted because of fear regarding risk of bleeding, particularly for contact sports [22]. However, it is unclear whether, or when, restrictions are necessary, as sports-related injuries are generally mild and infrequent in children with ITP, with most injuries not requiring ITP treatment [23].
ITP has an impact on mental health, with children and their family experiencing anxiety, stress, and depression [14]. For some children, an inability to predict their disease progression or response to treatment can significantly impact their mental health [14]. However, children and their parents/caregivers may have different priorities, with caregivers having greater concern over risk of bleeding and injury, adverse effects, and uncertainty over the future clinical course, whereas children are more concerned with hospital visits, blood tests, and restricted participation in activities [24].
3 Treatment Options for Primary ITP
3.1 Initial/Emergent Therapies
Many children with newly diagnosed ITP have no or mild (skin-only) bleeding symptoms that can be actively monitored without medication [1]. However, other children and adolescents will have moderate (overt mucosal bleeding) or severe bleeding (mucosal bleeding leading to a decrease in hemoglobin >2 g/dL or suspected internal hemorrhage bleeding) necessitating treatment, for which corticosteroids, IVIG, and anti-D immunoglobulin may be used initially [5].
Corticosteroids, IVIG, and anti-D immunoglobulin are generally effective in achieving platelet recovery in children with ITP, with the majority of children demonstrating a response [10, 25-27]. However, corticosteroids, IVIG, and anti-D immunoglobulin often only have a transient effect on platelet counts and are associated with potential adverse events [10]. Short courses of corticosteroids are generally well tolerated in children, but are associated with increased appetite/weight gain and changes in mood and sleep [26]. Longer-term corticosteroid use should be avoided due to risk of growth delay, bone disease, and adrenal suppression in children [28-30]. As such, American Society of Hematology (ASH) guidelines recommend a course of corticosteroids of 7 days or less [1]. Potential adverse effects of IVIG range from systemic, mostly infusion-related fever, chills, and headache, to severe reactions, such as anaphylaxis and aseptic meningitis, and United States Food and Drug Administration (US FDA) black box warnings for risk of thrombosis, renal failure, and renal dysfunction [31, 32]. Therefore, hydration with normal saline or premedication with antihistamines, acetaminophen/paracetamol, or corticosteroids are recommended, as they can prevent and reduce the severity of IVIG-induced adverse events [32, 33]. Potential adverse effects of anti-D immunoglobulin include moderate-to-severe hemolysis, infusion-related fever and chills, nausea, and pain [34]. The US FDA has issued a black box warning for anti-D immunoglobulin for intravascular hemolysis (IVH) [35]. IVH can be severe, leading to clinically compromising anemia, multisystem organ failure, acute respiratory distress syndrome, and death [35]. US FDA warnings for other serious complications of anti-D immunoglobulin include acute renal insufficiency/failure and thrombosis [35]. Moreover, anti-D immunoglobulin is only available in some regions (not available in Europe) and can only be used in children who are Rh positive, have an intact spleen, do not have a positive direct antiglobulin test, and have a hemoglobin level ≥9 g/dL [5].
A subset of children and adolescents with ITP will not respond to any of these initial treatments [36]. In addition, some children and adolescents will have only a transient response, with ongoing symptoms, or will have adverse effects precluding repeated courses [10]. Therefore, approximately 38–47% of children will require second-line therapies at 6–24 months [9].
Given the known adverse effects, transient responses, and possibility of treatment failures, and relapses associated with current first-line therapies, new treatment strategies are desirable in the pediatric population and merit further research.
3.2 Second-Line Therapies
As ITP is a heterogeneous disease, the reasons for initiating second-line treatment vary among individuals [37]. Reduced HRQoL and/or severity and frequency of bleeding are significant factors in choosing to initiate second-line treatment [38], but in some patients with low platelet counts, need for peri-procedural management and/or a desire to achieve remission may also be factors [37]. The choice of second-line treatment for children and adolescents with ITP is often complex, with therapeutic options varying due to access issues related to off-label use, insurance coverage, drug formularies, and patients’ ability to self-pay. The lack of comparative trials between different treatments and the use of nonstandardized outcome measures makes choosing the most appropriate therapy difficult [1, 5].
Second-line treatments for pediatric primary ITP include thrombopoietin receptor agonists (TPO-RAs), rituximab, mycophenolate mofetil (MMF), sirolimus, azathioprine, mercaptopurine (6-MP), and combined treatments [37, 39]. ASH and International Consensus Report (ICR) recommendations for second-line treatments are summarized in Table 1. Less frequently used treatments include splenectomy, hydroxychloroquine, dapsone, danazol, and vincristine [37, 39-43].
| ASH | ICR | |
|---|---|---|
| TPO-RAs | TPO-RAs should be considered over rituximab and splenectomy in children with non-life-threatening mucosal bleeding and/or reduced HRQoL. | TPO-RAs should be used as preferred treatment in children with ITP for whom alleviating thrombocytopenia will likely have a clear clinical benefit, such as to reduce bleeding or improve HRQoL. |
| Rituximab | Rituximab should be considered over splenectomy in children with non-life-threatening mucosal bleeding and/or reduced HRQoL. | Rituximab should be considered for treatment in in children who do not respond to TPO-RAs, especially adolescent females. |
| Splenectomy | Splenectomy should be delayed until 12 months after diagnosis, where possible. | Splenectomy is rarely indicated in children with ITP and should be considered only in children who have failed all available medical therapies and who are at heighted risk of mortality or have substantially impaired HRQoL. Splenectomy should be avoided, if possible, before age 5 and within 1 year of disease onset. |
| MMF (immunosuppressant) | Mentioned but not explicitly recommended, citing a relatively slow effect in ITP and potential adverse effects. | Mycophenolate mofetil may be considered in combination with a TPO-RA in children who lose a response to a TPO-RA. |
| Sirolimus (immunosuppressant) | Not mentioned | Not mentioned |
|
Azathioprine and 6-MP (immunosuppressants) |
Mentioned but not explicitly recommended, citing the need for prolonged treatment to achieve platelet response and adverse events. | Azathioprine may be used in children failing other therapies but has a reduced role in patients who can try a TPO-RA or rituximab. |
| Hydroxychloroquine | Not mentioned | Not mentioned |
- Abbreviations: 6-MP, mercaptopurine; ASH, American Society of Hematology; HRQoL, health-related quality of life; ICR, International Consensus Report; ITP, immune thrombocytopenia; MMF, mycophenolate mofetil; TPO-RA, thrombopoietin receptor agonist.
3.3 Thrombopoietin Receptor Agonists
Two TPO-RAs, romiplostim [44] and eltrombopag [45], are approved for use in children aged ≥1 years and have been demonstrated as effective and well tolerated in randomized, placebo-controlled trials in children and adolescents [46-48].
In a randomized, double-blind, phase 3 study of children with ITP without rescue drug use in the preceding 4 weeks, a greater proportion of children achieved a durable platelet response (platelet count ≥50 × 109/L in ≥6 of the final 8 weeks of a 25-week treatment period) with romiplostim versus placebo (romiplostim, 52%; placebo, 10%; p = 0.002) [46].
In the phase 3 PETIT2 study, 40% of children who received eltrombopag compared with one (3%) patient who received placebo achieved a platelet count of at least 50 × 109/L for 6 of the last 8 weeks (weeks 5–12) of the treatment period (p = 0.0004) [48].
Clinical trial data and real-world studies have also demonstrated that romiplostim and eltrombopag may be associated with improved clinical symptoms and HRQoL in children and adolescents with ITP. In a prospective, multicenter, observational study of children (n = 120) initiating second-line therapies, only children receiving romiplostim and rituximab had a significant reduction in both skin-related and non-skin-related bleeding symptoms after 1 month of treatment [49]. All treatments were associated with significantly improved HRQoL, with children treated with eltrombopag having the greatest improvement at 1 month [49]. However, in a post-hoc analysis of clinical trial data from the PETIT study, eltrombopag was not associated with significantly improved HRQoL in pediatric ITP, although the study was not adequately powered for this outcome [50]. In a retrospective, multicenter cohort study of 79 children with ITP of varying duration and severity, the durability of responses to romiplostim and eltrombopag varied between patients, but response rates were similar between treatments, with 86 and 81% of patients who received romiplostim and eltrombopag, respectively, having a platelet response [51]. In the phase 2 PETIT study, fewer clinically significant bleeding events were observed in children who received eltrombopag compared with placebo [47]. However, in a systematic review of two placebo-controlled trials of children with ITP, romiplostim did not significantly reduce the risk of clinically significant bleeding versus placebo, but this may be related to the low frequency of bleeding in the study population [52].
While effective, both romiplostim and eltrombopag have limitations; romiplostim is administered by weekly subcutaneous injection, often at a physician's office [44, 53], whereas orally administered eltrombopag is associated with an increased risk of iron deficiency and generally mild and reversible hepatotoxicity and has dietary restrictions [44, 54, 55]. Treatment with TPO-RAs may also be associated with increased risk of thrombosis and generally mild and reversible bone marrow fibrosis [51, 56].
Avatrombopag is an oral TPO-RA approved for the treatment of adults with chronic ITP [57, 58]. In a phase 3 randomized, placebo-controlled trial, once-daily avatrombopag was superior to placebo in increasing platelet counts over a 12-week treatment period in children and adolescents with ITP of at least 6 months duration (n = 75) [59]. In a real-world study of children with persistent or chronic ITP (n = 34), 79.4% achieved a platelet response within 4 weeks, with a median response time of 7 days, and 88.2 and 76.5% achieved a response or complete response, respectively, at 12 weeks [60]. Of 22 children who achieved a response and were followed-up for 24 weeks, 54.55% achieved a sustained response [60]. In contrast to eltrombopag, avatrombopag is not associated with significant hepatotoxicity and has no dietary restrictions [61]. Additionally, based on the dosing guidelines, a reduction from once-daily to several times weekly dosing may be possible in some children with stable platelet counts [62]. As such, if approved in children, avatrombopag would represent another option for the use of oral TPO-RAs.
Limited real-world studies have demonstrated switching TPO-RAs to be effective in the majority of children [63, 64], and studies in small populations of adults have also found switching TPO-RAs to be effective in the majority of patients [65-68]. Therefore, access to multiple TPO-RAs is of benefit to children with ITP. Tapering of treatment with TPO-RAs should be considered in children with platelet counts >150 × 109 and/or a following a stable period with a platelet count >50 × 109/L to determine the potential for treatment discontinuation or dose reduction [5, 69].
3.4 Rituximab
ICR guidelines recommend that rituximab should be considered in children who fail to respond to, or relapse on, TPO-RAs [5]. Similarly, ASH guidelines recommend TPO-RAs over rituximab but that rituximab should be considered instead of splenectomy in children with ITP who have non-life-threatening mucosal bleeding and/or diminished HRQoL and do not respond to first line treatment [1]. Rituximab and biosimilars are often used off-label to treat children with ITP [70]. Rituximab is commonly administered as four doses of 375 mg/m2 per week, but the optimal dose to treat ITP in children remains unclear, with different dosing strategies (including fewer weekly exposures or lower weekly doses) also being shown to be effective [70, 71]. Several observational studies support the use of rituximab in children and adolescents with chronic ITP, with response rates of approximately 30–80%, but less than 30% of children maintain a long-term response [71]. In a systematic review of studies including children and adolescents, rituximab had a similar rate of overall platelet response as TPO-RAs but differed in other important measures; for example, rituximab is also associated with higher rates of rescue therapy use [72]. Notably, compared with other second-line therapies, rituximab has the advantage of not requiring ongoing treatment [71]. Moreover, in a small observational study of second-line therapies (including romiplostim, oral immunosuppressants, and eltrombopag) in children and adolescents with ITP, rituximab was the only individual treatment to significantly improve fatigue, with reductions at 1 month in children and at 12 months in adolescents [16].
Adverse events associated with rituximab include risk of infection associated with B-cell depletion, infusion reactions, and impaired response to vaccination [71]. Persistent hypogammaglobulinemia has also been observed in a small number of children with ITP following use of rituximab [1, 73]. It is unclear if different dosing strategies would reduce these risks [71].
3.5 Other Immunosuppressants
Various other immunosuppressive agents may be used to treat ITP, including MMF, [74] sirolimus [75], azathioprine [76], 6-MP [77], and hydroxychloroquine [78]. However, evidence for the efficacy of these treatments is limited due to a lack of studies.
MMF has been shown to be effective in the treatment of pediatric ITP, with one retrospective study of 40 children reporting a platelet response in 58% of patients [79]. Similarly, in an observational study of 16 children with ITP refractory to first-line treatment, MMF was associated with a response in 73% of patients and mostly mild and tolerable adverse events [80]. A randomized trial of upfront corticosteroids versus corticosteroids plus MMF in adolescents and adults aged ≥16 years with newly-diagnosed ITP demonstrated a higher rate of platelet response in the corticosteroids plus MMF cohort (91.5 vs. 63.9%, p < 0.001) [74].
Sirolimus has been shown to be safe and efficacious in observational studies. In a study of 19 children with ITP refractory to MMF, 68% achieved a platelet response with a median time to response of 50 days [81]. In a retrospective study of sirolimus in 20 adults (n = 18) and children (n = 2) with ITP, sirolimus was associated with similar response rates, with no observed association between age and treatment efficacy [75]. In both studies, sirolimus was well tolerated [75, 81].
Limited retrospective studies have provided evidence for the benefit of 6-MP and hydroxychloroquine in children with ITP, with 85 and 57% demonstrating a platelet response, respectively [43, 77]. Hydroxychloroquine has been shown to be of particular benefit in children with ITP with a positive antinuclear antibody test [82].
3.6 Supportive Care
Supportive care options in ITP include antifibrinolytics to control mucosal bleeding and vasoconstrictors for epistaxis [5]. For the control of heavy menstrual bleeding in adolescents, which is a common and often distressing symptom of ITP that can substantially impact HRQoL, hormonal therapies should be considered [83, 84]. Though not evaluated for use in ITP, regular use of tranexamic acid should also be considered in patients with heavy menstrual bleeding [5]. Platelet transfusions may be used in cases of life-threatening bleeding [5].
3.7 Splenectomy
Splenectomy is generally avoided in children and is usually reserved for those who have failed all available pharmacologic therapies, have life-threatening bleeding, or are in resource-limited regions [5, 85]. Both ASH and ICR guidelines recommend splenectomy be delayed until 12 months after diagnosis given the possibility of spontaneous remission [1, 5]. Additionally, it is advised to avoid splenectomy in children aged <5 years due to infection risk [5]. Adverse effects of splenectomy include surgical complications, thrombosis, and a life-long increased risk of infection [86], with one observational study reporting 11% and 3% of children being admitted to hospital due to fever or infection and sepsis, respectively, during long-term follow-up after splenectomy [87].
3.8 Treatments in Development
Multiple clinical trials are ongoing and are reported in Table 2.
| Treatment | Avatrombopag | Eltrombopag | Hetrombopag | Anti-CD38 antibody | Obinutuzumab | Rilzabrutinib |
|---|---|---|---|---|---|---|
| Class | TPO-RA | TPO-RA | TPO-RA | Monoclonal antibody | Monoclonal antibody | Bruton tyrosine-kinase inhibitor |
| Primary outcome measures | Proportion of patients achieving at least 6 out of 8 weekly platelet counts ≥50 × 109/L during the last 8 weeks of the 12-week treatment period in the core phase, in the absence of rescue medication | The proportion of patients achieving a platelet count ≥50 × 109/L with eltrombopag compared with standard first-line treatments |
Part A: Peak plasma concentration (Cmax) from baseline to week 2 Part B: Proportion of patients with a platelet count ≥50 × 109/L at week 10 |
Proportion of patients with a platelet count ≥50 × 109/L within 8 weeks after initial administration in absence of rescue therapy, and without having had dose increment of TPO-RA or corticosteroids during the study period Incidence, severity, and relationship of treatment emergent adverse events after anti-CD38 antibody treatment |
Overall response rate, defined as the proportion of patients with a platelet count ≥30 × 109/L and at least a twofold increase from baseline within 12 weeks after initial administration in absence of rescue therapy, and without having had dose increment of TPO-RA or corticosteroids during the study period | Durable response rate, defined as the proportion of patients who achieve platelet counts ≥50 × 109/L for ≥two-thirds of at least 8 nonmissing weekly scheduled platelet measurements during the last 12 weeks of the 24-week treatment period in the absence of rescue therapy, provided that at least 2 nonmissing weekly scheduled platelet measurements are at or above ≥50 × 109/L |
| Patient population | ||||||
| Age | 1–17 years | 1–17 years | 6–17 years | 12–17 years | 12–18 years |
Children: 10–17 yearsa Adults: ≥18 years |
| Region | France, Germany, Hungary, Poland, Russia, Turkey, Ukraine, United Kingdom, United States | United States | China | China | China | Argentina, Australia, Austria, Brazil, Canada, Chile, China, France, Germany, Hungary, Israel, Italy, Japan, Republic of Korea, Mexico, Netherlands, Norway, Poland, Russia, Singapore, Spain, Thailand, Turkey, Ukraine, United Kingdom, United States |
| ITP history | Primary ITP for ≥6 months and an insufficient response to previous treatment | ITP for <3 months |
ITP for ≥6 months (Part A) or ≥12 months (Part B) and refractory to, or relapse after, ≥1 prior ITP therapy |
ITP for ≥3 months with failure to achieve a response to, or relapse after, ≥1 prior second-line ITP therapy | Persistent or chronic ITP with failure or recurrence of previous hormonal therapy or hormone dependence. | Primary ITP >6 months (children) or >3 months (adults) with an unsustained response to IVIG, anti-D immunoglobulin, or corticosteroid and documented intolerance, insufficient response, or any contraindication to any appropriate course of standard of care ITP therapy |
| Target/actual N | 75 | 122 | 117 | 20 | 60 | 194b |
| Phase | 3 | 3 | 3 | 2 | 2 | 3 |
| Trial status | Active, not recruiting | Active, not recruiting | Unknown | Recruiting | Recruiting | Active, not recruiting |
| Estimated completion | November 2025 | December 2025 | May 2023 | December 2025 | November 2026 | November 2026 |
| NCT number | NCT04516967 | NCT03939637 | NCT04737850 | NCT06168851 | NCT06094881 | NCT04562766 |
- Abbreviations: ITP, immune thrombocytopenia; IVIG, intravenous immunoglobulin; NCT, national clinical trial; TPO-RA, thrombopoietin receptor agonist.
- a Children aged 10–11 years will be enrolled in European Union countries only.
- b Includes both pediatric and adult patients.
4 Unmet Needs of Children with Primary ITP
4.1 Education and Shared Decision-Making
Clinical guidelines stress that treatment decisions should be based on shared decision-making between the physician and patient/caregiver, recognizing that concerns and preferences regarding treatment choice differ between individuals [1]. As such, children with ITP and their caregivers require improved communication with healthcare professionals and greater involvement in decision-making (Figure 1). Children with ITP may feel uninvolved in the process of their own care due to their age. Many parents note that while treatment options may be presented to them, the extent to which they feel included in the decision is variable, with many feeling they are not important in the decision-making process [88]. Additionally, some parents report being inadequately educated on the options presented [88].
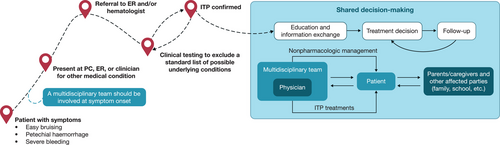
Physician, patient, and caregiver treatment preferences are influenced by multiple factors, including bleeding frequency and severity, impact of treatment on bleeding and HRQoL, potential for long-term remission, toxicity and adverse effect profiles, route of administration, dietary considerations, need for monitoring/frequent clinic visits, impact on activity levels, and costs [37, 89]. Clinicians must consider which goals of treatment are most critical. Resolution of active bleeding symptoms and historical bleeding events should be considered when selecting the most appropriate treatment for each individual. However, choice of treatment should also consider possible differences between age groups, for example, that younger children and their caregivers may have different priorities and perspectives in comparison with adolescents.
Shared decision-making tools, such as the UK ITP Support Association and ITP Forum Shared Decision-Making Toolkit [90], and educational materials on bleeding risks, activity and dietary restrictions, route of administration, cost, and safety concerns associated with treatment must be standardized in clinical practice to enable children and their caregivers to engage in treatment decisions. To ensure all children with ITP and their caregivers can benefit from these tools and materials, they must be made cross-cultural and available in multiple languages.
4.2 Predicting Treatment Response and Disease Progression
Improved methods of predicting treatment responses are required to avoid an extended “trial-and-error” approach to finding an effective and tolerable treatment. A more refined approach of personalized and targeted medicine would reduce exposure to unnecessary risk of adverse effects and treatment costs that are ultimately ineffective. Predicting whether individuals with ITP are likely to resolve or become chronic would further aid in determining whether long-acting therapies should be considered earlier in the treatment course. The prediction of future significant bleeding would aid in the identification of children who require a more intensive approach, given that a lower platelet count is permissive but insufficient as a surrogate marker for bleeding [91].
4.3 Additional Treatment Options
As fewer pediatric treatments are available compared with adult ITP, further clinical trials are required to provide rapid access to novel treatments for children with ITP. In light of the relatively small sample sizes in previous clinical trials of second-line treatments for pediatric ITP [46-48, 59], inclusion of children meeting weight requirements in adult trials rather than age-based inclusion criteria would improve statistical power and allow for greater access to investigational study drugs in pediatric populations. To offer effective treatment to children who are not responsive to current therapies, additional treatments with novel mechanisms of action, in particular targeted immune treatments versus more general immunosuppression, are required. Further research into the underlying biology of ITP will aid the development of targeted therapies. Clinical trial design must also be standardized, including outcomes and response definitions, to enable comparisons across different therapies and therapy types using parallel cohort studies and/or meta-analyses. To maximize treatment adherence, increased availability of oral options with pediatric formulations with simple administration guidelines would be of benefit.
4.4 Disease Management and Patient and Caregiver Support
Many children with ITP do not have severe bleeding; however, children who do exhibit bleeding symptoms require consistent, effective management and counseling. Resources aiding children with ITP to discuss their disease with family members and their peers may be useful in mitigating some of the impact of ITP on children's mental health. However, children and caregivers must also be adequately supported outside of the clinical environment, including at school, where staff should be educated on necessary adjustments required by children with ITP while avoiding overly severe daily activity restrictions. In regard to contact sports, survey data have demonstrated substantial variation in physician's perception of risk associated with different sports and acceptable platelet count thresholds for sports participation [22]. A high proportion of physicians felt that evidence-based guidelines on the topic of sport participation would be useful in clinical practice; however, it may be difficult to establish guidelines given the variables involved [22].
5 Conclusions
The impact of ITP on HRQoL is multifaceted, affecting both children and their caregivers. Emergent therapies are generally effective in raising platelet counts in the short-term, but up nearly half of children with ITP require second-line therapy at 12-months following diagnosis. For these children, a range of treatment options are available, including TPO-RAs, rituximab, and other immunosuppressive agents. These treatments may differ in mode of action, route of administration, potential side effect profile, management, impact on HRQoL, and costs. Therefore, though treatment guidelines frequently recommend TPO-RAs over other second-line therapies, clinicians must consider the priorities of their patients and caregivers. The lack of involvement of patients and their caregivers in treatment decisions is a major unmet need in pediatric ITP. To better inform these discussions, the ability to predict treatment response and disease course needs to be improved, and attention must be given to the progression of bleeding symptoms or changes in patient's usual bleeding symptoms. Additional unmet needs include the development of novel second-line treatment options for patients unresponsive to current therapies and improved support for the mental health impact of ITP on patients and their caregivers. These unmet needs must be addressed to improve outcomes in children with ITP.
Acknowledgments
This review was funded by Sobi. Publication coordination support was provided by Simona Vertuani, an employee of Sobi. Medical writing and editorial support, funded by Sobi, was provided by Daniel Spindlow, MSc, CMC Connect, a division of IPG Health Medical Communications, based on the authors’ input and direction, and in accordance with Good Publication Practice (GPP) 2022 guidelines (https://www.ismpp.org/gpp-2022).
Conflicts of Interest
Joana Azevedo has received consultancy fees and nonfinancial support from Amgen, Takeda, and Sobi; and nonfinancial support from Novartis, Sanofi, AbbVie, Janssen Cilag, and Gilead. Nichola Cooper has received consultancy fees from Amgen, Argenx, Novartis, Sanofi, and Sobi; and research support from Grifols and Novartis; and is partly funded by the Imperial NIHR Biomedical Research Centre. Jennifer DiRaimo has not personally received any payment, but the Platelet Disorder Support Association has received grants and consultancy fees to the institution from Novartis, Sobi, Sanofi, Amgen, and Argenx. Rachael F. Grace has received research funding from Agios, Novartis, Sobi; and is a consultant for Agios, Sanofi, and Sobi. Cindy Neunert has received research support from Novartis and is a consultant for Argenx, Novartis, Sanofi, and Sobi.
Open Research
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.




