Association Between Rurality and Race/Ethnicity and Pediatric Cancer Early Mortality: A Population-Based Cohort Study Using SEER Data from 2000 to 2021
ABSTRACT
Background
Pediatric cancer mortality rates have steadily declined since 2009, but over a thousand deaths still occur annually. While existing research highlights the effects of race/ethnicity and rurality on overall survival, few studies have specifically analyzed these factors in relation to early mortality, defined as death within 12 months of diagnosis.
Procedure
This study utilized SEER Research Plus Limited-Field Data (2000–2021) to examine the association between race/ethnicity, rurality, and early mortality in pediatric cancer patients. A cohort of 138,648 individuals was analyzed using Cox proportional hazards regression models to calculate hazard ratios and 95% confidence intervals (CIs).
Results
The results demonstrated that both race/ethnicity and rurality were significantly associated with early mortality. Non-Hispanic Black patients were 70% (95% CI: 1.60–1.82) more likely to die within the first year of diagnosis compared to non-Hispanic Whites, and other racial/ethnic groups also saw significant associations. The adjusted hazard ratio for early mortality compared to urban counties greater than 1 million was significant (p < 0.05) for all degrees of rurality. Pediatric cancer patients in rural counties not adjacent to urban counties had the highest risk of early mortality, 27% (95% CI: 1.13–1.42), within 1 year of diagnosis. A subanalysis of adolescent patients also showed similar patterns.
Conclusions
These findings emphasize the need to address disparities in early mortality for pediatric cancer patients, particularly among racial/ethnic minorities and those in rural communities.
Abbreviations
-
- CI
-
- confidence interval
-
- CNS
-
- central nervous system
-
- DAG
-
- directed acyclic graph
-
- ICCC
-
- International Classification of Childhood Cancer
-
- OMB
-
- Office of Management and Budget
-
- RUCC
-
- Rural–Urban Continuum Code
-
- RUCC2
-
- Rural–Urban Continuum Code-2 Category
-
- RUCC5
-
- Rural–Urban Continuum Code-5 Category
-
- SEER
-
- Surveillance, Epidemiology, and End Results
1 Background
Each year, over 15,000 children are diagnosed with pediatric cancer, and while the mortality rate for pediatric cancers has decreased from 2009 to 2019 [1], cancer remains the leading cause of death by disease among children in the United States [2]. Despite advances in treatment protocols, clinical trial collaborations, risk stratification, and supportive care, the American Cancer Society estimates 1040 pediatric cancer deaths in 2024 [3]. Given the high annual death toll, research into factors associated with pediatric cancer mortality has become a critical focus in cancer epidemiology.
Racial and socioeconomic disparities are well-documented in the incidence and mortality rates of pediatric cancer. A 2018 study by Kehm et al. found that compared to White children, Black children had a statistically significant higher risk of mortality for all cancers except Wilms tumor, osteosarcoma, and germ cell tumors (GCT) [4]. The study also showed that socioeconomic status partly explained Black–White survival disparities for several cancers, including acute lymphoblastic leukemia, acute myeloid leukemia, neuroblastoma, and non-Hodgkin's lymphoma [4]. Lack of access to timely cancer diagnosis and treatment, particularly for rural and socioeconomically disadvantaged populations, poses a major barrier to survival [5]. Many pediatric cancer treatment centers are concentrated in urban areas, making it difficult for families in distant rural regions to access necessary care [6].
The impact of rurality on access to pediatric cancer care is significant, with numerous studies showing differences in survival rates between rural and urban pediatric cancer populations. For example, Ohlsen et al. completed a study on pediatric cancer patients within Washington state and saw that children living in rural areas (69.4%), or within socioeconomically disadvantaged areas (66.4%), or both (66.9%), had a lower 5-year survival rate compared to children from non-rural, non-socioeconomically disadvantaged neighborhoods (80.9%) [7]. Similarly, Carriere et al.’s meta-analysis found that those in rural areas were 5% less likely to survive cancer [8]. Geographic barriers to urban treatment centers, possible links between increased pesticide exposure in rural populations, and new cases of pediatric cancer further complicate care access in rural regions [9].
In addition to access issues, early mortality remains a significant challenge in pediatric cancer care, with 7.5% of deaths from cancer in children occurring within 1 month of diagnosis [10]. While early mortality for pediatric cancer is not clearly defined within the literature, many studies define early mortality as death within 1 month of diagnosis with cancer [10-13] or death within 12 months of diagnosis [14-17]. In a prior analysis of Surveillance, Epidemiology, and End Results (SEER) date, age younger than 1 year or 15–19 years, Hispanic ethnicity, Black race, and low income were independent predictors of early death [10] (Green et al.). A single-center, patient-level, retrospective cohort study found that children who died within 30 days of diagnosis with cancer were significantly less likely to be enrolled on a clinical trial [11]. Clinical trial participation is crucial at the individual patient level to provide access to novel therapeutics and broadly to continue to improve survival outcomes and decrease toxicity from treatment. A qualitative analysis of clinical trial participation in children with cancer found that major barriers to participation included transportation difficulties, as well as complex trial design and language discordance [18]. In addition, children with cancer and early death sought care later than those without early death (mean days of symptoms before first seeking care was 29.4 days in the early death group vs. 9.8 days in the control group) [11]. The impact of rural residence or distance from treating center on diagnostic delay in childhood cancer has been mixed in the literature [5, 19, 20]. The impact of rurality on early death from childhood cancer remains unknown.
Adolescents ages 15–19 years is a sub-area of oncology literature that is lacking analysis of early-mortality risks including rurality and race/ethnicity. Previous studies have shown that access to care for adolescent pediatric cancer patients is an issue that remains unsolved [21]. Several studies have proposed that access to care for adolescent pediatric cancer patients is impacted by a variety of factors including access to social support, transportation, and insurance status [21, 22]. A SEER study from 2016 to 2021 on incidence and mortality of adolescent and young adult pediatric cancer patients (15–39 years) found increased incidence of cancer over the time period for all adolescents and non-Hispanic Blacks with the highest overall mortality rate [23]. Current literature groups pediatric adolescent patients with young adult patients, and therefore analysis of adolescents alone provides an understudied population within early mortality [22-26]. A subanalysis of pediatric cancer patients ages 15–19 years was also completed within this study.
This study investigates rurality and race/ethnicity as risk factors for early mortality—defined as death within 12 months of diagnosis—in pediatric cancer. The objectives are to (i) examine the effect of rurality on pediatric cancer early mortality; and (ii) examine the effect of race/ethnicity on early mortality. Analyzing these factors will help shed light on previously understudied risks in pediatric cancer outcomes.
2 Methods
This population-based cohort study uses data from the SEER Program (2000–2021). The study was deemed non-human subjects research by the University of Nebraska Medical Center Institutional Review Board. Children and adolescents (ages 0–19) diagnosed with primary malignant cancers were included. Only first matching records were analyzed to avoid counting second malignancies, and cases with incomplete data were excluded.
2.1 Data Source
The SEER Research Plus Limited-Field Data (2000–2021) was used, providing access to additional variables typically excluded for confidentiality reasons. SEER Data includes cancer incidence data, demographics, survival data, treatment data, socioeconomic data, and geographical data. SEER Research Plus has more specific data on cause of death, survivorship, rural/urban designations, and Hispanic ethnicity. The SEER Research Plus Limited-Field Data (2000–2021) includes data from specific urban areas and territories, as well as data from the states of California, Connecticut, Georgia, Hawaii, Iowa, Kentucky, Louisiana, New Jersey, New Mexico, and Utah.
2.2 Outcome Variable
The outcome variable was survival time. Survival time was calculated in months from the date of diagnosis to the date of death or last follow-up, whichever came first. For cases with 0 survival months, 0.5 months was added to retain these cases in the analysis. Early death was defined as survival duration less than or equal to 12 months from the time of diagnosis. One year was chosen as the time point of interest, because this timeframe has been used in several other early-mortality studies, and allows for access to treatment if possible [14-17].
2.3 Exposure Variables
The exposure variables are rurality based on the residential data and race/ethnicity. Rurality at the time of diagnosis was determined based on the Rural–Urban Continuum Code (RUCC). The SEER database provides Code-2 category (RUCC2) and Code-5 category (RUCC5) variables. The RUCC2 designates counties as either metropolitan (urban) or nonmetropolitan (rural) based on the 2023 Office of Management and Budget (OMB) delineation of metro areas. The RUCC5 breaks down counties as urban greater than 1 million, urban counties 250,000–1 million, urban less than 250,000, rural adjacent to urban, or rural non-adjacent to urban. Individuals diagnosed from 2000 to 2007 were recorded with 2003 RUCCs, while individuals diagnosed from 2008 to 2021 were recorded with 2013 RUCCs. Race and ethnicity were classified as non-Hispanic White, non-Hispanic Black, non-Hispanic American Indian/Alaska Native, non-Hispanic Asian or Pacific Islander, and Hispanic (all races).
2.4 Other Variables
The International Classification of Childhood Cancer (ICCC) codes for each patient were recategorized into three cancer types: hematological malignancy, central nervous system (CNS) tumors, and solid tumors. The median household income adjusted to 2022 dollars was classified as ≤$40,000, $40,000–$99,000, or ≥$100,000.
2.5 Statistical Analysis
Descriptive characteristics of the patient cohort were reported using frequencies and percentages. Differences in demographic characteristics between individuals diagnosed in rural and urban areas were examined with chi-square tests. Kaplan–Meier curves with a log-rank test were used to examine survival probabilities over time for the first year following diagnosis by rurality and race/ethnicity. Cox proportional hazards regression models were developed to compute unadjusted and adjusted hazard ratios and 95% confidence intervals (CIs), and a directed acyclic graph (DAG) was used to identify potential confounding variables to adjust for in the analysis. Potential variables considered included age, sex, type of cancer, rurality, median household income, and race/ethnicity. Based on DAG, adjustment of race/ethnicity, sex, and type of cancer was necessary to estimate the total effect of rurality and race/ethnicity on early mortality (see Figure S1).
The proportional hazards assumption for the regression models were tested using a Goodness-of-fit test with Schoenfeld residuals. Because of the known differences in survival by cancer type, we planned a priori to stratify the results by cancer type. Statistical analyses were completed in R (version 2024.04.2+764). Tests were two-sided, and p < 0.05 was used to determine statistical significance.
An additional subanalysis of adolescent pediatric patients ages 15–19 years was completed following the same methods and adjustments.
3 Results
After excluding incomplete cases, 138,648 individuals remained in the cohort. The majority of cases involved hematologic malignancies (n = 58,681), followed by solid tumors (n = 56,653) and CNS tumors (n = 23,314). Table 1 summarizes the demographic characteristics of the final cohort. There was a significant difference in racial/ethnic distribution (p < 0.0001)—71.4% of individuals living in rural areas were non-Hispanic White, while 47.1% of individuals living in urban areas were non-Hispanic White. There was a significant difference in the median household income between rural and urban residents (p < 0.0001)—a higher proportion of rural residents had $40,000 or lower median household income than urban residents (8.2% vs. <0.05%) (Table 2).
|
Rural (n = 13,581) |
Urban (n = 125,067) |
||||
|---|---|---|---|---|---|
| Variable | Number | % | Number | % | p-value |
| Age group (years) | |||||
| <1 | 869 | 6.4% | 8536 | 6.8% | 0.0691 |
| 1–4 | 3202 | 23.6% | 29,996 | 24.0% | |
| 5–9 | 2760 | 20.3% | 21,794 | 17.4% | |
| 10–14 | 2317 | 17.1% | 25,030 | 20.0% | |
| 15–19 | 4433 | 32.6% | 39,711 | 31.8% | |
| Sex | |||||
| Male | 7318 | 53.9% | 66,885 | 53.5% | 0.3739 |
| Female | 6263 | 46.1% | 58,182 | 46.5% | |
| Race/Ethnicity | |||||
| Non-Hispanic White | 9699 | 71.4% | 58,932 | 47.1% | <0.0001 |
| Non-Hispanic Black | 977 | 7.2% | 13,343 | 10.7% | |
| Hispanic | 2446 | 18.0% | 43,033 | 34.4% | |
| Other | 459 | 3.4% | 9759 | 7.8% | |
| Median Household Income | |||||
| ≤$40,000 | 1119 | 8.2% | 45 | <0.05% | <0.0001 |
| $40,000 to $99,000 | 12,393 | 91.3% | 103,711 | 83.0% | |
| ≥$100,000 | 69 | 0.5% | 21,311 | 17.0% | |
| Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|
| RUCC5 | N | HR (95% CI) | p-value | aHR (95% CI) | p-value |
| Urban: >1 million | 87,867 | Reference | Reference | ||
| Urban: 250,000–1 million | 27,312 | 1.05 (1.00–1.11) | 0.0683 | 1.08 (1.02–1.14) | 0.0058 |
| Urban: <250,000 | 9888 | 1.19 (1.10–1.29) | <0.0001 | 1.25 (1.16–1.36) | <0.0001 |
| Rural adjacent to urban | 8723 | 1.08 (0.99–1.18) | 0.0805 | 1.20 (1.09–1.31) | <0.0001 |
| Rural not adjacent to urban | 4858 | 1.15 (1.02–1.28) | 0.0189 | 1.27 (1.13–1.42) | <0.0001 |
| Race/Ethnicity | N | ||||
|---|---|---|---|---|---|
| Non-Hispanic White | 68,631 | Reference | Reference | ||
| Non-Hispanic Black | 14,320 | 1.69 (1.58–1.80) | <0.0001 | 1.71 (1.50–1.82) | <0.0001 |
| Hispanic | 45,479 | 1.34 (1.28–1.41) | <0.0001 | 1.42 (1.35–1.49) | <0.0001 |
| Non-Hispanic A/PI | 9468 | 1.31 (1.20–1.43) | <0.0001 | 1.39 (1.27–1.52) | <0.0001 |
| Non-Hispanic AI/AN | 750 | 1.31 (0.98–1.74) | 0.0642 | 1.38 (1.04–1.83) | 0.0277 |
- a Variables adjusted for include sex, type of cancer, and race/ethnicity.
A total of 8201 individuals died within 12 months of diagnosis, with rural patients experiencing a higher rate of early death (6.3% vs. 5.9%, p < 0.05). Kaplan–Meier 12-month survival curves were developed for both rurality and race/ethnicity. Kaplan–Meier survival curves indicated that urban counties with fewer than 250,000 residents had the lowest 12-month survival rates, and non-Hispanic Blacks had the lowest overall survival (Figures 1 and 2).
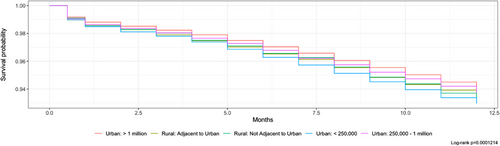
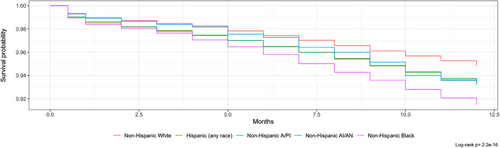
Cox regression models were developed for both unadjusted and adjusted hazard ratios. After completing a DAG, adjustment of race/ethnicity, type of cancer, and sex was necessary to estimate the total effect of rurality and race/ethnicity on early mortality. The adjusted hazard ratio for early mortality compared to urban counties greater than 1 million was significant (p < 0.05) for all degrees of rurality. Pediatric cancer patients in rural counties not adjacent to urban counties were 27% (95% CI: 1.13–1.42) more likely to die within 1 year of diagnosis. Urban counties less than 250,000 had a 25% (95% CI: 1.16–1.36) increased risk of early mortality. When looking at differences between early mortality for race/ethnicity seen in Table S2, all groups show significant increases in early mortality (p < 0.05) compared to non-Hispanic Whites. Non-Hispanic Blacks were 70% (95% CI: 1.60–1.82) more likely to die within 1 year of diagnosis. Table S1 shows unadjusted and adjusted hazard ratios. Hematologic malignancies in non-metropolitan counties saw a 21% (1.08–1.37) higher risk of early mortality within 12 months compared to metropolitan counties (see Table S1).
A subanalysis of adolescent patients ages 15–19 years was completed. The adjusted hazard ratio for early mortality compared to urban counties greater than 1 million was significant (p < 0.05) for all degrees of rurality except urban counties with a population of 250,000–1 million, which only had a 9% (95% CI: 0.98–1.21) increased risk of early mortality. Adolescent pediatric cancer patients in rural counties not adjacent to urban counties were 54% (95% CI: 1.26–1.90) more likely to die within 1 year of diagnosis. When looking at differences between early mortality for race/ethnicity seen in Table 3, all groups showed significant increases in early mortality (p < 0.05) compared to non-Hispanic Whites. Non-Hispanic American Indian/Alaska Natives saw the highest increases of risk for early mortality of 2.16 times (95% CI: 1.37–3.40), and non-Hispanic Blacks saw the second highest increases of risk of early mortality of 2.13 times (95% CI: 1.88–2.41).
| Unadjusted | Adjusteda | |||
|---|---|---|---|---|
| RUCC5 | HR (95% CI) | p-value | aHR (95% CI) | p-value |
| Urban: >1 million | Reference | Reference | ||
| Urban: 250,000–1 million | 1.04 (0.94–1.16) | 0.4509 | 1.09 (0.98–1.21) | 0.1282 |
| Urban: <250,000 | 1.21(1.04–1.41) | 0.0139 | 1.32 (1.14–1.54) | 0.0003 |
| Rural adjacent to urban | 1.11 (0.94–1.31) | 0.2206 | 1.29 (1.09–1.53) | 0.0027 |
| Rural not adjacent to urban | 1.31 (1.07–1.60) | 0.0098 | 1.54 (1.26–1.90) | <0.0001 |
| Race/Ethnicity | ||||
|---|---|---|---|---|
| Non-Hispanic White | Reference | Reference | ||
| Non-Hispanic Black | 2.18 (1.93–2.47) | <0.0001 | 2.13 (1.88–2.41) | <0.0001 |
| Hispanic | 1.68 (1.53–1.84) | <0.0001 | 1.66 (1.51–1.82) | <0.0001 |
| Non-Hispanic A/PI | 1.51 (1.27–1.78) | <0.0001 | 1.50 (1.27–1.78) | <0.0001 |
| Non-Hispanic AI/AN | 2.11 (1.34–3.32) | 0.0013 | 2.16 (1.37–3.40) | 0.0009 |
- a Variables adjusted for include sex, type of cancer, and race/ethnicity.
4 Discussion
This study is among the first to analyze early mortality in pediatric cancer through the lens of rurality and racial/ethnic disparities. The findings underscore that rurality and race/ethnicity are significant risk factors for early mortality, with the highest risks occurring in the most rural areas. Geographical barriers to cancer care and the underrepresentation of racial/ethnic minorities in clinical trials contribute to these disparities.
Our analysis showed that the risk of early death increases with each additional degree of rurality, pointing to the highest risk in the most rural communities. Rurality as a potential risk factor emphasizes the critical importance of access to cancer diagnostics and treatment in rural areas. Notably, approximately 90% of Commission-on-Cancer-accredited programs are located in metropolitan counties [6]. Commission-on-Cancer accreditation is provided by the American College of Surgeons for meeting and maintaining 34 quality care standards. Moreover, while some individuals may have access to a treatment facility, they might not live near a clinical trial-participating facility, which can especially impact those with late-diagnosed or more aggressive cancers [27]. The geographical distance to care, combined with the financial strain of traveling on top of healthcare bills, poses a significant burden on rural families. Multiple factors contribute to limited access to high-quality cancer care for rural populations.
Our results also provided evidence of racial/ethnic disparities in early mortality, consistent with previous studies examining overall survival and early death by race/ethnicity. For example, Zhao et al., using National Cancer Database data from 2004 to 2015, observed that non-Hispanic Blacks and Hispanics had significantly poorer overall survival across all cancers compared to non-Hispanic Whites, even after adjusting for health insurance and socioeconomic status [28]. Similarly, an analysis of SEER data (1992–2011) demonstrated that Black race and Hispanic ethnicity were associated with early death in pediatric hematologic, CNS, and solid tumor malignancies [10]. A possible explanation for this increased early mortality among ethnic minorities could be the higher rate of late-stage diagnoses compared to non-Hispanic Whites [29]. Limited access to treatment facilities with high standards of care likely exacerbates this issue. Additionally, the lack of health insurance and general financial hardship—common social determinants of health—may further increase the risk of early mortality among non-Hispanic Black and Hispanic pediatric patients [30]. It is crucial for providers to recognize the role race and ethnicity play in early survival outcomes for pediatric cancer patients.
The subanalysis of adolescent pediatric cancer patients ages 15–19 years provided insight on the unique population of adolescents within oncology. Similar patterns to the whole cohort were seen; however, risk of early mortality increased significantly compared to non-Hispanic Whites when analyzing racial/ethnic differences. Race/ethnicity may be a stronger risk factor for adolescent patients specifically than all pediatric cancer patients. Further research on how early-mortality risks differ between pediatric cancer patients and adolescent pediatric cancer patients is needed, especially as adolescents tend to be included with young adults in current literature, but potential reasons for these differences could include the location of care, whether the adolescent was treated on childhood versus adult protocols, and disparities in access to survivorship care during transition periods among adolescents [21, 22].
Several potential solutions have been proposed to address these disparities in early mortality related to both rurality and race/ethnicity. Financial navigation services could help alleviate the travel costs for rural pediatric patients and reduce out-of-pocket expenses for minority patients who may be uninsured or of lower socioeconomic status [27]. A small case study involving four hospital financial navigation services demonstrated a reduction in patient costs and financial losses for hospitals offering these services [31]. Establishing rural outreach centers in partnership with urban medical centers may further mitigate early-mortality risks in rural pediatric cancer patients. Shalowitz et al. proposed an ethical framework to guide rural cancer outreach efforts, aiming to improve outcomes [32]. While some hospitals have initiated rural cancer collaborations, Arens and Early found that increased education on cancer vulnerability and symptom awareness is necessary based on the results of seven cancer outreach events in the Midwest [33]. In addition to physical outreach centers, the rise of telehealth presents an opportunity to bridge rural and urban healthcare gaps. Kermani et al. conducted a scoping review of telehealth in pediatric oncology, finding that it may reduce both costs and mortality rates [34]. Free health clinics, while successful in screening for adult cancers in uninsured patients, offer limited options for pediatric cancer screenings, as these are typically recommended only for children with predisposition syndromes [35]. The lack of generalized pediatric cancer screening may contribute to delayed diagnoses. Increased awareness and education on cancer symptoms are essential in underserved and rural populations. As pediatric cancer treatments continue to advance, so does the need for treatment centers to reduce the disparities in care for rural and racial/ethnic minority patients.
Despite the many advantages of using SEER data, there are several limitations to consider. Incomplete individual data can be a challenge, though this was mitigated by excluding any incomplete case listing from the final cohort. Another limitation is the gaps in treatment and follow-up data when patients move in and out of SEER and non-SEER regions or between different RUCC codes [36]. If relocation and outcomes are linked, such as when a patient moves from a rural to an urban area with better access to care, the reduced risk of early mortality could introduce bias. Additionally, SEER data do not allow for adjustments based on health insurance or individual-level socioeconomic status, potentially decreasing the effect size of the findings. These limitations underscore the need for continued research and the expansion of the SEER database.
Our study showed that both rurality and race/ethnicity are associated with early mortality for pediatric cancer. Further research is necessary to evaluate the effectiveness of financial navigation services, rural–urban collaboration in cancer care, the use of telehealth, and the role of urban underserved clinics in improving pediatric cancer outcomes and addressing early mortality disparities. In addition, as adolescent medicine continues to change, further research comparing pediatric patients ages 0–14 years and adolescent patients 15–19 years in terms of early mortality will be necessary.
Acknowledgments
We extend our gratitude to the individuals, institution, and University of Nebraska Medical Center's Medical Student Summer Research Program whose support has allowed the completion of this work.
Conflicts of Interest
The authors declare no conflicts of interest.




