Clinical practice guideline-inconsistent chemotherapy-induced vomiting prophylaxis in pediatric cancer patients in community settings: A Children's Oncology Group study
Abstract
Background
This study aimed to determine the proportion of patients receiving clinical practice guideline (CPG)-inconsistent care related to chemotherapy-induced vomiting (CIV) prophylaxis, and to describe the association between CPG-inconsistent care and site size. The association between delivery of CPG-inconsistent care and patient outcomes (CIV control, admission prolongation, and unplanned healthcare visits) was also described.
Methods
This was a retrospective study conducted at Children's Oncology Group (COG) National Cancer Institute Community Oncology Research Program (NCORP) sites. Eligible patients received highly (HEC) or moderately emetogenic chemotherapy (MEC) as inpatients from January 2014 through December 2015, and were previously enrolled in a COG study. The COG generated a patient list from which patients were randomly selected for chart review by participating sites. A central panel adjudicated CIV prophylaxis received as CPG-consistent or -inconsistent.
Results
Twenty-four sites participated. Over half of patients received CPG-inconsistent CIV prophylaxis (HEC: 59/112, 52.6%; MEC: 119/215, 55.3%). The most common reasons for CPG-inconsistency were shortened duration of antiemetic administration or omission of dexamethasone. Site size was not found to be associated with CPG-inconsistent care delivery (HEC: adjusted odds ratio [OR]: 0.96, 95% confidence interval [CI]: 0.76–1.23; MEC: adjusted OR: 1.07; 95% CI: 0.92–1.24). Additionally, there was no statistically significant association between receipt of CPG-inconsistent care and patient outcomes.
Conclusions
Patients receiving MEC or HEC often received CPG-inconsistent CIV prophylaxis. Site size was not associated with receipt of CPG-inconsistent care. Future studies should evaluate strategies to improve CIV control among pediatric oncology patients including those aimed at improving CPG adherence.
Abbreviations
-
- CIV
-
- chemotherapy-induced vomiting
-
- COG
-
- Children's Oncology Group
-
- CPG
-
- clinical practice guideline
-
- HEC
-
- highly emetogenic chemotherapy
-
- IRB
-
- Institutional Review Board
-
- MEC
-
- moderately emetogenic chemotherapy
-
- NCORP
-
- National Cancer Institute Community Oncology Research Program
1 INTRODUCTION
Provision of evidence-based care is a recognized hallmark of high-quality care.1 Clinical practice guidelines (CPGs) are care recommendations that are grounded by a systematic review of the literature and created by a panel of experts, including patients or their representatives.2 Thus, CPGs are powerful tools for high-quality care delivery, and CPG-consistent care has been associated with improved patient outcomes across a breadth of clinical conditions and contexts, including control of chemotherapy-induced vomiting (CIV).3-10
From the inception of cancer chemotherapy, patients have identified vomiting as a distressing, treatment-related symptom.11 Antiemetic agents with demonstrated efficacy in preventing CIV became available in the 1980s. Since then, several organizations have developed and updated CPGs that provide evidence-based recommendations for CIV prophylaxis.12-14 The Children's Oncology Group (COG) Supportive Care Guideline Task Force first endorsed a CPG on CIV prophylaxis in 2014.
Implementation of CPG-consistent care is challenging and may be especially so in community-based or minority/underserved cancer centers, such as National Cancer Institute Community Oncology Research Program (NCORP) sites. Previously, clinicians at pediatric NCORP sites identified several barriers to CPG-consistent care delivery, including organizational tolerance of inconsistencies, lack of trust in the recommendations on the part of individuals, administrative hurdles, lack of inclusivity during implementation, and knowledge gaps.15 We undertook the present study to determine the prevalence of CPG-inconsistent care delivery relating to CIV prophylaxis at NCORP sites and to describe the association between site size and delivery of CPG-inconsistent care. We also investigated the association between CPG-inconsistent care and patient outcomes including CIV control.
2 METHODS
This retrospective multisite study formed one component of ACCL15N1CD, a cancer care delivery research study developed by COG and conducted within the NCORP, a network of community-based and minority/underserved institutions across the United States.16 The broad objective of ACCL15N1CD was to evaluate supportive care CPG uptake in pediatric NCORP institutions. Of the 37 COG-member NCORP institutions invited to open the study, 26 chose to participate and 24 contributed patients to this component of the study. The National Cancer Institute's Pediatric Central Institutional Review Board (IRB) approved this study, and waived the need for informed consent due to the study's retrospective design as did the IRB at each participating site.
2.1 Patients
Eligibility was assessed among patients with a cancer diagnosis who had been enrolled on any COG trial, including biology and therapeutic studies. Among this group, patients who had received highly emetogenic chemotherapy (HEC) or moderately emetogenic chemotherapy (MEC) as an inpatient after COG trial enrollment at a participating site from January 1, 2014 through December 31, 2015 were identified. As existing CPG recommendations for patients receiving HEC during the study period varied by age, patients receiving HEC were required to be less than 12 years old at the time of the CIV episode, whereas patients receiving MEC were required to be less than 21 years old.
The COG generated a list of all patients at participating sites who met eligibility criteria based on age, receipt of care at participating sites and COG study enrollment, and randomly selected patients from this list for chart review. The number of patients selected per site was proportional to the number of COG registrations per site. Site personnel reviewed the health records of listed patients to determine eligibility including receipt HEC or MEC as inpatients.
Each patient contributed no more than two CIV episodes: one MEC and one HEC. If several eligible episodes were identified within a category (HEC or MEC) during the study period for a patient, the first to occur within the study period was included.
2.2 Definitions
HEC and MEC were defined using the CPG endorsed by COG at the time of the study.17 A chemotherapy block was defined as a series of one or more consecutive days of intravenous (IV) or subcutaneous chemotherapy administration. The acute phase began with administration of the first chemotherapy dose of the chemotherapy block, and continued for 24 hours after administration of the last chemotherapy dose of the chemotherapy block. The number of patient enrollments into therapeutic COG studies was used as a proxy for site size.
2.3 Outcomes
The primary study outcome was the proportion of patients receiving HEC or MEC who received care that was inconsistent with selected strong recommendations of the COG-endorsed CPG18 on CIV prevention. In most circumstances, strong recommendations can be implemented as policy, as the degree of certainty regarding the risks and benefits of the recommended care is very high.19 The recommendations selected for adjudication were: (i) use ondansetron or granisetron plus dexamethasone for children less than 12 years old receiving HEC; (ii) use ondansetron or granisetron plus dexamethasone for children receiving MEC; and (iii) for patients receiving MEC, dose dexamethasone based on body surface area (BSA): BSA ≤0.6 m2: 2 mg/dose IV/orally [PO] every 12 hours and BSA >0.6 m2: 4 mg/dose IV/PO every 12 hours (halve the dexamethasone dose if given concurrently with aprepitant).
For recommendations (i) and (ii), CIV prophylaxis was deemed to be CPG-inconsistent if ondansetron/granisetron and dexamethasone were not administered on a scheduled basis during the entire acute phase. Omission of dexamethasone or either ondansetron or granisetron, if contraindicated or discouraged by a COG treatment protocol or due to patient-specific history of toxicity, was adjudicated as CPG-consistent. Administration of antiemetics in addition to ondansetron/granisetron or dexamethasone was also categorized as CPG-consistent. For example, patients receiving MEC who received aprepitant or olanzapine (over-prophylaxis) in addition to CPG-consistent antiemetics, were adjudicated as CPG-consistent. For recommendation (iii), CIV prophylaxis was deemed to be CPG-inconsistent if dexamethasone was administered at a lower dose, or if was administered less frequently than that recommended. For patients receiving HEC, CPG-inconsistency was based only on recommendation (i). For patients receiving MEC, CPG-inconsistent care was defined as care that was inconsistent with respect to recommendation (ii), (iii), or both.
Extent of acute phase CIV control and incidences of admission prolongation and unplanned healthcare visits due to CIV were secondary study outcomes. CIV control was classified as complete (no vomiting), partial (maximum one or two vomits in any 24-hour period), or failed (three or more vomits in any 24-hour period). Admission prolongation was considered to have occurred when a healthcare professional noted in the health record that discharge was delayed due to uncontrolled CIV. Each patient's health record was reviewed for 1 week following discharge to identify unplanned healthcare visits (admission, emergency department, or clinic).
2.4 Care adjudication
Site personnel uploaded de-identified documents from the health record for each eligible patient into the study database. Using these documents, one study team member (A. Vennettilli, A. Sivananthan, or N. Stesco) created a standardized summary describing the care delivered. A second team member (L. Lee Dupuis) checked each summary against the source documents for accuracy. During virtual video conferences, three panel members (Allison C. Grimes, Aaron J. Sugalski, Lillian Sung) adjudicated the care described in the summaries against the relevant CPG recommendation as either CPG-consistent or CPG-inconsistent. Five percent of all summaries, selected randomly, were re-adjudicated. If the adjudication decisions differed, the panel finalized the episode adjudication through further discussion.
2.5 Statistical analysis
Our primary objective was to determine the extent of CPG-inconsistent care delivery relating to CIV prophylaxis at NCORP sites. Based on available reports of CPG-inconsistent care prevalence,7, 20 we hypothesized that the proportion of patients receiving CPG-inconsistent care was greater than .5, and tested this hypothesis using a modified one-sided, one-sample z-test of proportion with a significance level of .025. With 100 CIV episodes, the study had approximately 90% power to detect a difference of .20 using a within-institution correlation of .2. Standard error for the hypothesis test was estimated using a hierarchical bootstrap approach, using 5000 bootstrap samples, to account for correlation between episodes treated at the same institution.21
Generalized linear mixed effects models were used to estimate the association between CPG-inconsistent care and site size, as well as the association between CPG-inconsistent care and clinical outcomes (CIV control [complete vs. partial or none], prolonged hospital admission, and unplanned healthcare visits). The model for the analysis of the association between delivery of CPG-inconsistent care and site size was adjusted for site type (pediatric vs. mixed facility and minority/underserved vs. other). The model for the association between delivery of CPG-inconsistent care and clinical outcome (CIV control) was adjusted for the following variables: patient age at the start of chemotherapy, sex, race/ethnicity, and area deprivation index (ADI, as a proxy for household socioeconomic disadvantage). Complete case analyses were used for all models, and all analyses were stratified by receipt of HEC or MEC. Random intercepts were used to account for within-site correlation.
The ADI of Brokamp et al. was used as a proxy for socioeconomic status.22 This index is based on the 2015 five-year American Community Survey and was developed for each census tract using principal components analysis. The index was developed using the following measures: the fraction of households with income below poverty level, the median household income in 2015 inflation-adjusted dollars, the fraction of the population 25 years of age and older with at least a high school graduation or General Education Development equivalency, the fraction of population with no health insurance coverage, the fraction of households receiving public assistance income, food stamps, or Supplemental Nutritional Assistance Program, and the fraction of vacant houses. The ADI for each patient's zip code tabulation area was calculated using the mean of all intersecting census tracts. Higher ADI indicates higher levels of disadvantage.
In post hoc analyses, the associations between CPG-inconsistent care receipt and site type (pediatric vs. mixed; minority/underserved vs. other) and patient characteristics (age, sex, race/ethnicity, and ADI) were evaluated using unadjusted generalized linear mixed effects models. All analyses were conducted using R version 4.2.2.
3 RESULTS
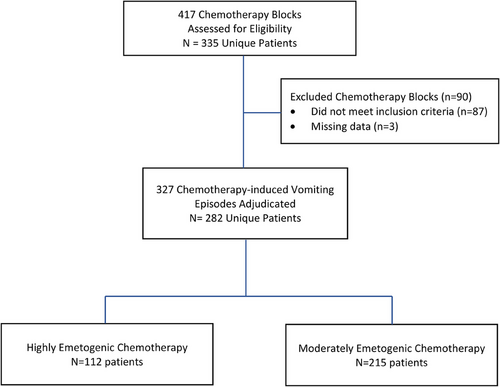
Figure 1 shows the flow diagram of patient identification and care adjudication at 24 sites. The care of 112 patients who received HEC at 22 sites, and 215 patients who received MEC at 23 sites, was adjudicated. Forty-five patients contributed two care episodes each: one involving MEC and another involving HEC. Tables 1 and 2 present patient and site characteristics, respectively. The antiemetic agents administered on a scheduled basis to included patients are presented in Table S1.
| Highly emetic chemotherapy | Moderately emetic chemotherapy | |||||
|---|---|---|---|---|---|---|
| Characteristic |
All episodes (n = 112) |
CPG-consistent (n = 53) |
CPG-inconsistent (n = 59) |
All episodes (n = 215) |
CPG-consistent (n = 96) |
CPG-inconsistent (n = 119) |
| Median age in years (Q1, Q3) | 5.6 (3.5, 7.8) | 5.6 (3.9, 8.1) | 5.6 (3.3, 7.6) | 8.6 (4.1, 15.2) | 8.5 (3.6, 14.9) | 8.6 (4.7, 15.4) |
| Female sex, n (%) | 55 (49.1) | 24 (45.3) | 31 (52.5) | 95 (44.2) | 42 (44.5) | 53 (43.8) |
| Cancer diagnosis, n (%) | ||||||
| Acute lymphoblastic leukemia | 58 (51.8) | 29 (54.7) | 29 (49.2) | 127 (59.1) | 60 (62.5) | 67 (56.3) |
| Acute myeloid leukemia | 9 (8.0) | 5 (9.4) | 4 (6.8) | 45 (20.9) | 21 (21.9) | 24 (20.2) |
| Central nervous system tumor | 6 (5.4) | 5 (9.4) | 1 (1.7) | 3 (1.4) | 1 (1.0) | 2 (1.7) |
| Ewing sarcoma | 13 (11.6) | 3 (5.7) | 10 (16.9) | 7 (3.3) | 0 (0.0) | 7 (5.9) |
| Germ cell tumor | 5 (4.5) | 3 (5.7) | 2 (3.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Non-Hodgkin lymphoma | 6 (5.4) | 4 (7.5) | 2 (3.4) | 19 (8.8) | 10 (10.4) | 9 (7.6) |
| Othera | 15 (13.3) | 4 (7.5) | 11 (18.6) | 14 (6.5) | 4 (4.1) | 10 (8.4) |
| Race/Ethnicity, n (%) | ||||||
| Hispanic/Latino | 24 (21.4) | 11 (20.8) | 13 (22.0) | 68 (31.6) | 27 (28.6) | 41 (34.5) |
| Non-Hispanic Asian | 6 (5.4) | 5 (9.4) | 1 (1.7) | 13 (6.0) | 8 (8.3) | 5 (4.2) |
| Non-Hispanic Black | 10 (8.9) | 4 (7.5) | 6 (10.2) | 17 (7.9) | 8 (8.3) | 9 (7.6) |
| Non-Hispanic-Native Hawaiian/Pacific Islander | 1 (0.9) | 1 (1.9) | 0 (0.0) | 1 (0.5) | 0 (0.0) | 1 (0.8) |
| Non-Hispanic White | 67 (59.8) | 29 (54.7) | 38 (64.4) | 105 (48.8) | 45 (46.9) | 60 (50.4) |
| Other/Unknown | 4 (3.6) | 3 (5.7) | 1 (1.7) | 11 (5.2) | 8 (8.3) | 3 (2.5) |
| Median income by zip code, US$ | ||||||
| Minimum | 23,311.4 | 31,415.5 | 23,311.4 | 22,625.8 | 26,003.7 | 22,625.8 |
| 20th Percentile | 42,656.0 | 45,036.1 | 39,722.0 | 40,939.90 | 42,999.4 | 39,251.8 |
| 40th Percentile | 50,350.0 | 51,886.8 | 48,904.3 | 48,874.3 | 48,003.7 | 49,637.2 |
| 60th Percentile | 59,145.1 | 60,548.2 | 57,909.7 | 56,923.1 | 55,073.8 | 57,043.0 |
| 80th Percentile | 70,289.1 | 70,121.1 | 71,406.2 | 68,231.8 | 68,503.8 | 67,817.4 |
| Maximum | 209,785.4 | 209,785.4 | 163,151.5 | 209,785.4 | 110,006.0 | 209,785.4 |
| Median proportion with assisted income (Q1, Q3) | 0.13 (0.08, 0.20) | 0.13 (0.08, 0.19) | 0.13 (0.07, 0.20) | 0.14 (0.09, 0.20) | 0.14 (0.09, 0.20) | 0.14 (0.09, 0.22) |
| Median proportion with a high school education (Q1, Q3) | 0.89 (0.82, 0.93) | 0.89 (0.82, 0.93) | 0.90 (0.83, 0.94) | 0.88 (0.81, 0.92) | 0.88 (0.81, 0.92) | 0.88 (0.82, 0.92) |
| Median proportion with no health insurance (Q1, Q3) | 0.12 (0.09, 0.19) | 0.12 (0.08, 0.15) | 0.14 (0.10, 0.21) | 0.14 (0.09, 0.20) | 0.13 (0.09, 0.18) | 0.14 (0.09, 0.21) |
| Median proportion in poverty (Q1, Q3) | 0.14 (0.09, 0.20) | 0.13 (0.09, 0.18) | 0.15 (0.09, 0.21) | 0.15 (0.10, 0.21) | 0.15 (0.10, 0.21) | 0.15 (0.10, 0.21) |
| Median proportion in vacant housing (Q1, Q3) | 0.12 (0.07, 0.16) | 0.11 (0.07, 0.15) | 0.13 (0.07, 0.19) | 0.11 (0.08, 0.16) | 0.12 (0.08, 0.18) | 0.11 (0.07, 0.15) |
| Median deprivation index of location of residence (Q1, Q3) | 0.37 (0.30, 0.44) | 0.37 (0.30, 0.42) | 0.39 (0.30, 0.45) | 0.38 (0.32, 0.46) | 0.38 (0.32, 0.45) | 0.38 (0.32, 0.46) |
- Abbreviations: CPG, clinical practice guideline; n, number; Q1, first quartile; Q3, third quartile.
- a Other: Hodgkin lymphoma, neuroblastoma, osteosarcoma, rhabdomyosarcoma, solid tumor—other, leukemia—other.
| Site characteristic |
Highly emetogenic chemotherapy (n = 22) |
Moderately emetogenic chemotherapy (n = 23) |
|---|---|---|
| Minority/Underserved NCORP sites (vs. other), n (%) | 8 (36.4) | 10 (43.5) |
| Pediatric sites (vs. mixed adult and pediatric), n (%) | 16 (72.7) | 15 (65.2) |
| Location, n (%) | ||
| Western US | 2 (9.1) | 2 (8.7) |
| Southwestern US | 4 (18.2) | 5 (21.7) |
| Northeastern US | 1 (4.5) | 1 (4.3) |
| Southeastern US | 9 (40.1) | 10 (43.5) |
| Midwestern US | 6 (27.3) | 5 (21.7) |
| Median number of patients registered on COG treatment studies during study perioda (Q1, Q3) | 39.5 (27.5, 52.5) | 39.0 (26.5, 51.0) |
- Abbreviations: n, number; NCORP, National Cancer Institute Community Oncology Research Program; Q1, first quartile; Q3, third quartile; US, the United States.
- a January 1, 2014 to December 31, 2015.
3.1 CPG-inconsistent care
CPG-inconsistent care was delivered to 59 (52.6%) patients receiving HEC and 119 (55.3%) patients receiving MEC. The proportion of patients receiving CPG-inconsistent care was not statistically significantly different from .5 (HEC: p-value = .38; MEC: p-value = .18). The reasons for CPG-inconsistent care are presented in Table 3. The most common sources of CPG-inconsistent care for HEC were duration of dexamethasone too short (6/12, 50.0%), dexamethasone not given (15/36, 41.7%), or duration of ondansetron or granisetron too short (44/109, 40.4%). The most common sources of CPG-inconsistent care for MEC were dexamethasone dose inconsistent (5/6, 83.3%), dexamethasone not given (11/17, 64.7%), and dexamethasone dose or frequency too low (3/6, 50.0%).
| Highly emetogenic chemotherapy | Moderately emetogenic chemotherapy | |||
|---|---|---|---|---|
| CPG-inconsistent care delivered | Number of evaluable patients | Number of evaluable patients receiving CPG-inconsistent care (%) | Number of evaluable patients | Number of evaluable patients receiving CPG-inconsistent care (%) |
| CPG-inconsistent care: global assessment | 112 | 59 (52.6) | 215 | 119 (55.3) |
| Specific CPG-inconsistent care delivered: | ||||
| Ondansetron/granisetron not given | 112 | 3 (2.7) | 215 | 13 (6.1) |
| Duration of ondansetron/granisetron too short | 109 | 44 (40.4) | 202 | 93 (46.0) |
| Dexamethasone not givena | 36 | 15 (41.7) | 17 | 11 (64.7) |
| Duration of dexamethasone too shortb | 12 | 6 (50.0) | 6 | 3 (50.0) |
| Dexamethasone dose CPG-inconsistentb | NA | NA | 6 | 5 (83.3) |
- Abbreviations: CPG, clinical practice guideline; NA, not applicable—reported only for patients receiving moderately emetic chemotherapy.
- a Adjudicated only in patients without barriers to dexamethasone use, e.g., cancer treatment protocol or history of adverse effects.
- b Adjudicated only in patients who received dexamethasone as an antiemetic.
Few patients received dexamethasone specifically for CIV prophylaxis (HEC: 12/112, 10.7%; MEC: 6/215, 2.8%). However, some patients received dexamethasone as part of their cancer treatment (HEC: 3/112, 2.7%; MEC: 31/215, 14.4%). The most common reason for omission of dexamethasone was treatment protocol contraindication or discouragement (HEC: 76/91, 83.5%; MEC: 131/148, 88.5%). No patient had a history of adverse reaction or intolerance to dexamethasone noted as the reason for dexamethasone omission.
3.2 CPG-inconsistent care: Site size
Table 4 shows the association between receipt of CPG-inconsistent care and site size in unadjusted and adjusted analyses. After adjustment for the a priori selected potential confounders, the association was not statistically significant (HEC: adjusted odds ratio [OR]: 0.96, 95% confidence interval [CI]: 0.76–1.23; MEC: adjusted OR: 1.07; 95% CI: 0.92–1.24). The coefficient estimates for the factors used in the adjusted model are presented in Table S2.
| CPG-inconsistent care | ||
|---|---|---|
| Unadjusted odds ratio (95% CI) | Adjusted odds ratio (95% CI) | |
| Highly emetogenic chemotherapy | ||
| Site size per 5 patients enrolled | 0.94 (0.75–1.19) | 0.96 (0.76–1.23) |
| Moderately emetogenic chemotherapy | ||
| Site size per 5 patients enrolled | 1.03 (0.89–1.20) | 1.07 (0.92–1.24) |
- Note: Adjusted models included site type (pediatric vs. mixed and minority/underserved vs. other). Odds ratio <1 indicates that the odds of receiving CPG-inconsistent care are lower among larger sites.
- Abbreviations: CI, confidence interval; CPG, clinical practice guideline.
3.3 CPG-inconsistent care: Patient outcomes
Patient outcome findings are presented in Table S3. Complete acute phase CIV control was experienced by 65.1% and 64.2% of patients receiving HEC and MEC, respectively. Table 5 shows the association between receipt of CPG-inconsistent care and complete CIV control in adjusted and unadjusted analyses. The association between receipt of CPG-consistent and complete CIV control was not statistically significant. Receipt of CPG-inconsistent CIV prophylaxis among patients receiving HEC or MEC was not associated with unplanned healthcare visits within 7 days of discharge in unadjusted models (HEC: estimated OR: 0.45, 95% CI: 0.04–5.17; MEC: estimated OR: 1.12; 95% CI: 0.24–5.11). Because very few patients had unplanned hospital visits, we were not able to fit adjusted models. As only one patient was noted to have their hospital admission prolonged due to CIV, we were unable to estimate the impact of CPG-inconsistent care.
| Number of evaluable episodes | Number of events | Adjusted odds ratio (95% CI) | |
|---|---|---|---|
| Highly emetogenic chemotherapy | |||
| Complete CIV control | 106 | 69 | 0.45 (0.17–1.21)a |
| Moderately emetogenic chemotherapy | |||
| Complete CIV control | 204 | 131 | 1.69 (0.89–3.20)b |
- Note: Odds ratio <1 indicates that the odds of complete CIV control are lower in patients receiving CPG-inconsistent care compared to patients receiving CPG-consistent care.
- Abbreviations: CI, confidence interval; CIV, chemotherapy-induced vomiting; CPG, clinical practice guideline.
- a 4 Patients excluded due to missing race and 1 due to missing deprivation index.
- b 10 Patients excluded due to missing race and 11 due to missing outcome.
3.4 CPG-inconsistent care: Site type and patient characteristics
The associations between CPG-inconsistent care and site type (pediatric vs. mixed, minority/underserved vs. other) and patient characteristics (sex, age, race/ethnicity, and ADI) were assessed in post hoc analyses (Table S4). No association examined was statistically significant.
4 DISCUSSION
Among pediatric cancer patients receiving HEC and MEC at US community sites, we found that administration of CPG-inconsistent CIV prophylaxis was common. Site size was not statistically significantly associated with CPG-inconsistent CIV prophylaxis, and CPG-inconsistent prophylaxis was not statistically significantly associated with CIV control or unplanned hospital visits.
A 2014 survey of 69 COG sites to determine CIV prophylaxis practices reported a high level of awareness of CIV CPGs among respondents, but poor implementation.23 When responding to clinical scenarios, a high proportion of respondents selected CPG-inconsistent antiemetic regimens among patients less than 12 years old receiving HEC (59%), and all respondents chose CPG-inconsistent antiemetic regimens for patients who were unable to receive corticosteroids as antiemetics. In the present study, we also found that CPG-inconsistent CIV prophylaxis was common, with early discontinuation of ondansetron, granisetron or dexamethasone, and/or omission of dexamethasone being the most prevalent. Similar to the survey results, we found that when dexamethasone was given as an antiemetic to pediatric patients receiving MEC, it was most often given at a dose other than that recommended by the CPG. Early discontinuation of ondansetron or granisetron may reflect an effort by institutions to minimize costs. The antiemetic use of dexamethasone is often avoided due to concerns such as risk of hyperglycemia, fungal infection, avascular osteonecrosis, and restriction of chemotherapy distribution through the blood–brain barrier.24-28 Ideally these risks are balanced against the benefit of improved CIV control for each patient. When a decision is made to avoid use of dexamethasone as an antiemetic for a pediatric patient, recommendations from current CPGs on the management of CINV should be followed and CPG-consistent plans for managing breakthrough CINV should be in place.29
Despite their lack of statistical significance, the point estimates of the ORs for several analyses reveal associations that may merit evaluation in future studies. For example, we observed a negative association between receipt of CPG-inconsistent care and complete CIV control among patients who received HEC but not among patients who received MEC. Among patients receiving MEC, the impact of CPG-inconsistent CIV prophylaxis on CIV control was uncertain, as evidenced by a CI that ranged from 0.89 to 3.20. MEC is defined as entailing a vomiting risk of 30%–90% when antiemetics are not given. It may be that a high number of included patients received chemotherapy regimens with an actual emetic risk at the lower end of this range. Further, several patients in our cohort who received MEC, received HEC-style antiemetic regimens29 that included aprepitant and olanzapine. These patients were considered to have received CPG-consistent CIV prophylaxis as long as they received ondansetron or granisetron for the entire acute phase. Thus, the underlying risk of CIV in our cohort of patients receiving MEC may have been lower than anticipated. Studies aiming to refine the MEC classification may permit more finely tuned recommendations for antiemetic use.
This study has several strengths. It was conducted in a large number of diverse NCORP sites and evaluated the care delivered concerning strong recommendations of a CPG endorsed by COG. The care delivered was adjudicated using rigorous methods. However, due to its retrospective design, this study was limited by the extent and accuracy of the documentation in the health record. We were unable to include an assessment of chemotherapy-induced nausea as it was not recorded in the health record using a validated measure. Similarly, we were unable to assess the care delivered on an outpatient basis. As we included patients who were not chemotherapy-naïve, we may not have evaluated the impact of CPG-consistent care fairly as CPGs for acute-phase CIV prevention are designed primarily for chemotherapy-naïve patients. Accuracy and precision of estimates of the associations between CPG-inconsistent care delivery and patient outcomes were limited by the low number of outcome events that occurred. Lastly, our findings reflect care delivered in 2014–2015 at NCORP sites. Nevertheless, delivery of CPG-inconsistent CIV prophylaxis to pediatric oncology patients continues to be common internationally.30-33 Thus, the need to vigorously promote CPG-consistent CIV prophylaxis with hope of optimizing patient outcomes continues to be relevant today.
Patients receiving chemotherapy at NCORP sites received CPG-inconsistent CIV prophylaxis in at least half of the cases. Short antiemetic duration and avoidance of dexamethasone were common CPG-inconsistent approaches. Site size was not significantly associated with receipt of CPG-inconsistent CIV prophylaxis. Receipt of CPG-consistent CIV prophylaxis was also not significantly associated with complete CIV control. Complete CIV control should be the goal for all pediatric oncology patients. It may be tempting to interpret the lack of association between CPG-inconsistent CIV prophylaxis and CIV control as a signal that CIV CPGs are ineffective. More relevantly, our findings signal the ongoing need to strengthen future CPGs by identifying new strategies to control CIV in children and adolescents. Individualizing CIV prophylaxis based on patient-level factors coupled with aggressive interventions to implement CPG-consistent CIV management may be one strategy to explore.
ACKNOWLEDGMENTS
We acknowledge C. Bergheimer, E. Ha, and D. Ross for their work in organizing study activities and the clinical research staff at participating sites for identifying care episodes and preparing the required documents. We also appreciate A. Vennettilli, A. Sivananthan, and N. Stesco for preparing the patient summaries for adjudication, and N. Kaur for administrative assistance. We thank Dr. B. Fisher for guidance regarding study design and Dr. H. Dang for assistance with statistical methods. Lillian Sung is supported by the Canada Research Chair in Pediatric Oncology Supportive Care. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under NCORP Grant UG1CA189955 to the Children's Oncology Group. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
CONFLICT OF INTEREST STATEMENT
L. Lee Dupuis received salary support from the Children's Oncology Group; funds were received by her institution. Aaron J. Sugalski received salary support from the Children's Oncology Group; funds were received by his institution. Allison C. Grimes received grant funding to their institution from the National Institutes of Health, Canadian Institute of Health Research, American Cancer Society, and Cancer Prevention Research Institution of Texas. She also received payment from Servier Pharmaceuticals for services as a content expert, the Clark Hill Law Firm for expert testimony. Allison C. Grimes also received salary support from the Children's Oncology Group; funds were received by their institution. Michelle M. Nuño received salary support to their institution from the National Cancer Institute and grant funding to their institution from the National Cancer Institute, Department of Defense, and St Baldrick's Foundation. Susan K. Parsons has received consulting fees from Seagen unrelated to the work described in this manuscript and is an external member of the Dana-Farber/Harvard Cancer Center Data Safety Monitoring Board. No conflicts of interest: Melissa P. Beauchemin, Paula D. Robinson, Nancy Santesso, Lolie C. Yu, Andrea R. Wrightson, and Alexandra M. Walsh.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the Children's Oncology Group upon reasonable request. The data are not publicly available due to privacy or ethical restrictions.




