Successful treatment of young childhood standard-risk hepatoblastoma with cisplatin monotherapy using a central review system
Abstract
Background
The JPLT3-S (Japanese Study Group for Pediatric Liver Tumors-3) study, conducted cisplatin (CDDP) monotherapy for young children (<3 years old) with standard-risk hepatoblastoma (HB) using a central review system in Japan. In the previous JPLT2 study, cases with resectable tumors without any annotation factors in the PRETEXT (PRETreatment EXTent of disease) classification (standard-risk HB) showed favorable outcomes with treatment consisting of CDDP and pirarubicin, but showed toxicities and late complications. In the JPLT3-S trial, a less intense regimen consisting of CDDP alone was evaluated.
Methods
Patients who were less than 3 years of age and with PRETEXT I, II, or III HB without any annotation factors (e.g., E1, E1a, E2, E2a, H1, N1, P2, P2a, V3, and V3a) were eligible for inclusion in this study. In this trial, the central radiological and pathological features of all patients were reviewed. The primary outcome was the 3-year progression-free survival (PFS).
Results
A total of 38 patients (23 female) were included. The median patient age was 12 months (range: 2–34). Two patients discontinued treatment because of progressive disease, and five patients discontinued treatment for other reasons. The 3-year PFS rate was 93.9% (95% confidence interval [CI]: 86.4%–100%). All 38 patients survived (follow-up period 38–98 months), and the OS rate was 100% (CI: 100). Eighteen of the 38 patients (47.4%) experienced ototoxicity as a late complication.
Conclusion
CDDP monotherapy regimen is feasible in young patients with localized HB, as classified by a central review.
Abbreviations
-
- AFP
-
- α-fetoprotein
-
- CDDP
-
- cisplatin, cis-diamminedichloroplatinum
-
- CI
-
- confidence interval
-
- CRB
-
- Certified Review Board
-
- ELBWI
-
- extremely low birth weight infant
-
- HB
-
- hepatoblastoma
-
- JPLT
-
- Japanese Study Group for Pediatric Liver Tumor
-
- LBWI
-
- low birth weight infant
-
- OS
-
- overall survival
-
- PD
-
- progressive disease
-
- PFS
-
- progression-free survival
-
- PR
-
- partial remission
-
- PRETEXT
-
- PRETreatment EXTent of disease
-
- RECIST
-
- Response Evaluation Criteria in Solid Tumors
-
- SAE
-
- serious adverse event
-
- SD
-
- stable disease
-
- SIOPEL
-
- Société Internationale d'Oncologie Pédiatrique Epithelial Liver Tumor study group
-
- STS
-
- sodium thiosulfate
-
- THP-ADR
-
- pirarubicin
PRETEXT Annotation factors
C caudate lobe involvement
E extrahepatic abdominal disease
F tumor focality
H tumor rupture or intraperitoneal hemorrhage
M distant metastasis
N lymph node metastasis
P portal vein involvement
V involvement of the inferior vena cava and/or hepatic veins
1 INTRODUCTION
Hepatoblastoma (HB) is a rare hepatic malignant tumor in children that accounts for approximately 1% of pediatric malignant neoplasmas.1 In 1989, the Japanese Study Group for Pediatric Liver Tumor (JPLT) was launched, and clinical trials (JPLT1-4 studies) for pediatric hepatic malignant tumors have been sequentially carried out since that time.2-8 In this report, we present the cure rates and toxicities of CDDP (cisplatin, cis-diamminedichloroplatinum) monotherapy as a less intense therapy for patients under 3 years of age in the standard-risk group (resectable tumors, without any annotation factors in the PRETreatment EXTent of disease [PRETEXT] classification). Patients were evaluated using central radiological and pathological reviews.
In the JPLT2 trial, patients with resectable PRETEXT I, II, and III HB were mainly treated with a combination of CDDP and pirarubicin (CITA regimen), and the cure rate (event-free survival) was satisfactory in these standard-risk HB patients. However, the incidence of late complications remains not a few.3, 4 The JPLT3 study was conducted using a new risk stratification based on the survival analyses of the JPLT2 cohort.
In the JPLT3-S trial, we aimed to evaluate the effectiveness of CDDP monotherapy in HB patients under 3 years of age, considering the risk due to age at diagnosis. As a new initiative of the JPLT3, central radiological and pathological reviews were conducted to accurately define eligibility and evaluate the response in all cases. The Société Internationale d'Oncologie Pédiatrique Epithelial Liver Tumor (SIOPEL) group conducted the SIOPEL 3 trial using regimens mainly based on PLADO (CDDP + doxorubicin) and CDDP monotherapy for standard-risk patients, and showed the non-inferiority of CDDP monotherapy for standard-risk HB in comparison to PLADO,9 but 19 of 126 cases treated with CDDP monotherapy had relapse or disease progression, indicating that some additional prognostic factors might be correlated in this cohort. Considering that the age at the diagnosis might be correlated with the malignant grade of HB, which was elucidated later,10, 11 the JPLT3-S study focused on younger patients who were diagnosed under 3 years of age.
Moreover, to ensure diagnostic accuracy, computed tomography (CT)/magnetic resonance imaging (MRI) images and pathological specimens (tumor biopsy specimens) of all patients were centrally diagnosed by the Japan Children's Cancer Group (JCCG) radiological and pathological committees, and the Response Evaluation Criteria in Solid Tumors (RECIST) classification after two and four courses of neoadjuvant chemotherapy were also evaluated based on imaging by central radiologists.
2 METHODS
The trial period for JPLT3 was from 2012 to 2017, followed by a 3-year observation period. This study was approved by the Ethics Committee of Hiroshima University (Ethics Committee No: E-2198) and the Certified Review Board (CRB) for special clinical research (jRCTs061180086). The cases were collected from 116 JPLT participating facilities in Japan and were approved by the institutional ethics committee. Written informed consent was obtained from all parents.
2.1 Inclusion criteria
In this JPLT3-S study, we defined eligibility as follows: patients of age less than 3 years with PRETEXT I, II, or III HB with a serum α-fetoprotein (AFP) level of greater than 100 mg/dL without any annotation factors (e.g., E1, E1a, E2, E2a, H1, N1, P2, P2a, V3, and V3a).12
In the JPLT3 risk stratification, the high-risk group consisted of cases with M1 or N2 PRETEXT annotations, or patients with a serum AFP) level less than 100 ng/dL at the time of diagnosis. Intermediate-risk group included non-high-risk patients with any of the following factors: PRETEXT IV tumor, one or more positive PRETEXT annotation factors (E1, E1s, E2, E2a, H1, N1, P2, P2a, V3, or V3a), multifocal tumor (F), age ≥3 years at diagnosis, or tumor rupture detected at the time of diagnosis. The remaining patients were defined as having standard risk. For JPLT3, we adopted the 2005 version of PRETEXT classification.12
2.2 Exclusion criteria
In this study, the following were excluded: patients with cardiac, renal, liver, or bone marrow dysfunction; those in whom treatment was started more than 15 days after the biopsy or diagnosis; and those with multiple cancers, recurrence, possibility of pregnancy, and extremely poor general condition.
2.3 Central imaging diagnosis, central pathology diagnosis, and surgical review
The CT, MRI, and other images of all enrolled patients were centrally reviewed, and the RECIST criteria were applied after the completion of the second and fourth courses by seven radiologists.6 In principle, a tumor biopsy was performed before any treatment, and the pathology slides were examined by two central pathologists. Furthermore, according to facility requirements, surgical reviews at the end of the second and fourth courses were performed by a board of six pediatric and hepatic surgical experts.7
2.4 Treatment flowchart
A treatment flowchart for JPLT3-S is presented in Figure 1. After performing a biopsy to confirm the diagnosis by central pathological review, four courses of preoperative chemotherapy with CDDP alone were administered, followed by surgery. Two courses of CDDP monotherapy were administered postoperatively. In patients who initially underwent upfront surgery, which was allowed only for those with PRETEXT I disease, four courses of CDDP treatment were administered postoperatively.
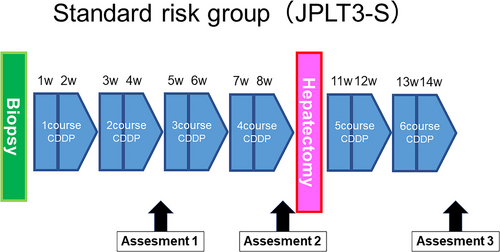
The dose of CDDP in each course was 80 mg/m2 for those weighing more than 10 kg, 2.7 mg/kg for those weighing 5−10 kg, and 1.8 mg/kg for those weighing less than 5 kg, over 24 hours.
2.5 Measurement of serum AFP
Serum AFP levels were measured at diagnosis and within 5 days before starting each treatment (1–6), before and after surgery; 30 days after the end of treatment; and 1, 2, and 3 years after the start of treatment.
2.6 Endpoints
The primary endpoint was the 3-year progression-free survival (PFS). As secondary endpoints, the protocol completion rate, relationship between RECIST criteria and AFP trends evaluated as log decline, complete surgical resection rate and complications, serious adverse events (SAEs), overall survival (OS), and late complications were investigated. SAEs were evaluated using the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. To evaluate late complications, hearing tests, renal function tests, and echocardiography were performed within 14 days before inclusion, during treatment, and 30 days after the end of treatment. Hearing tests were conducted using age-appropriate tests (e.g., pure audiometry and auditory brain response).
2.7 Participants
The number of patients in this trial was statistically calculated from the results of JPLT2, and was designed to be a number that could prove that the treatment was similarly promising. The primary endpoint was 3-year PFS, which was expected to be 90%, and a threshold 3-year PFS rate of 80% was estimated.3 A sample size of 65 was estimated based on the assumption of a 3-year follow-up period, two-sided α = .1, and β = .2. An interim analysis was conducted when the number of registered cases 3 years after the start of treatment reached 20. The 3-year PFS of approximately half of the patients was known at that time, and it was expected that the threshold of 80% would be sufficiently exceeded, even if the number of patients was less than 65. Therefore, we conducted an analysis of 43 patients.
2.8 Statistical analyses
The PFS and OS were examined using a Kaplan–Meier analysis. The log-rank test was used for comparisons. The degree of decrease in AFP during treatment was evaluated using the natural logarithm, and Student's t-test was used to compare the relationship between the RECIST criteria and the AFP decline ratio. Statistical significance was set at p < .05.
3 RESULTS
A total of 43 patients were enrolled in this study between 2012 and 2017. Of the 43 patients enrolled, five were deemed ineligible for the following reasons: outside the target age, registered before CRB approval, late registration, misdiagnosis (adrenocortical tumor) by central pathological review, or staging changed after the central imaging review (the decision was made as PRETEXT annotation factor N1(+)). Finally, 38 patients were eligible for analysis in JPLT3-S (Figure 2).
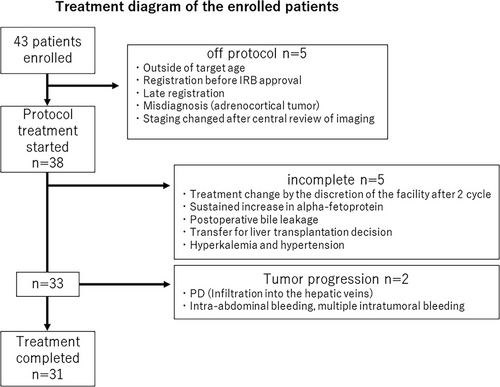
Among these 38 patients, two were diagnosed with progressive disease (PD) during preoperative chemotherapy. In one of these cases, the tumor was progressive and invaded three hepatic veins, leading to a change in treatment. Liver transplantation was performed and disease-free survival was achieved. Early emergent surgery was performed in one patient because of intratumoral bleeding after one course of chemotherapy. Five other cases deviated from the protocol at the facility's discretion. In two cases, the tumor showed stable disease (SD) on imaging, but the attending physician determined that the treatment was ineffective and changed to second-line treatment. Treatment (sixth course of CDDP) was delayed in one patient due to postoperative bile leakage. In one case, the institutional surgeon determined that consultation with a transplant facility was necessary after four cycles, and deviated from the protocol. In one case, treatment was discontinued and early surgery was performed because of hyperkalemia and hypertension. Thirty-one patients (81.6%) completed the study.
3.1 Patient background (n = 38), Table 1
Among the 38 patients (male, n = 15; female, n = 23) who were included in the present study, 19 (50%) were between 1 and 11 months of age at diagnosis. The median age at diagnosis was 12 months (range: 2–34 months).
| Characteristics | n | % |
|---|---|---|
| Number eligible | 38 | – |
| Age | ||
| 1–11 months | 19 | 50 |
| 1–2 years | 19 | 50 |
| Median (months) | 12 (2–34) | |
| Sex | ||
| male | 15 | 39.5 |
| female | 23 | 60.5 |
| PRETEXT | ||
| I | 8 | 21.1 |
| II | 18 | 47.4 |
| III | 12 | 31.6 |
| Comorbidities | ||
| FAP | 1 | |
| Beckwith–Wiedemann syndrome | 2 | |
| Sotos syndrome | 1 | |
| 18 Trisomy | 1 | |
| Neurofibromatosis | 1 | |
| LBWI | 4 | |
| ELBWI | 2 |
- Abbreviations: ELBWI, extremely low-birth-weight infant; FAP, familial adenomatous polyposis; LBWI, low-birth-weight infant.
3.2 Radiological risk stratification
The PRETEXT classification was reviewed using central imaging. The PRETEXT classification was as follows: I, n = 8; II, n = 18; and III, n = 12. The annotation factors were C(+) in one case and V(+), P(+), and C(+) in one case. Comorbidities included two cases of Beckwith–Wiedemann syndrome and one case each of familial adenomatous polyposis (FAP), Sotos syndrome, trisomy 18, and neurofibromatosis. Four patients were low birth weight infants (LBWIs), and two patients were extremely low birth weight infants (ELBWIs). The median AFP level at the time of diagnosis was 379,651 (583.4–2,180,000).
3.3 Pathology review (n = 31), Table 2
A central pathology review of the tumor biopsy specimens was performed in 31 patients using specimens obtained before treatment. Twenty out of 31 cases had combined fetal and embryonal types (epithelial mixed type according to the WHO Pediatric Tumors Classification, 5th edition). There were six mixed epithelial and mesenchymal type cases, four fetal type cases, and one embryonal type case.
| Fetal type | Well-differentiated | 1 |
|---|---|---|
| Mitotically active | 0 | |
| Not clear | 3 | |
| Combined fetal and embryonal typea | 20 | |
| Embryonal type | 1 | |
| Macrotrabecular type | 0 | |
| Small cell undifferentiated type | 0 | |
| Mixed epithelial and mesenchymal type | Simpleb | 5 |
| Teratoid | 0 | |
| Not clear | 1 |
- a Epithelial mixed type according to the WHO Pediatric Tumors Classification, 5th edition.
- b Mixed epithelial and mesenchymal without teratoid features according to the WHO Pediatric Tumors Classification, 5th edition.
3.4 Primary endpoint
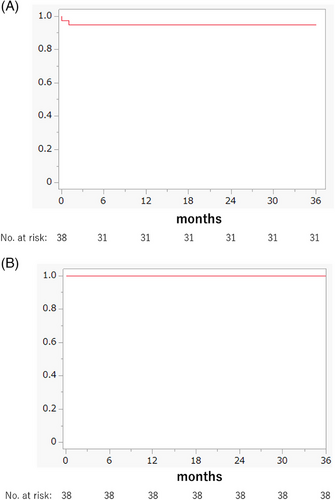
The 3-year PFS rate, which was the primary endpoint of this study, is shown in Figure 3A. Among the 38 patients, two had PD, and there were five deviated cases. The 3-year PFS rate was 93.9% (95% confidence interval [CI]: 86.4%–100%).
3.5 Secondary endpoints
3.5.1 Overall survival
All 38 patients survived (median follow-up period: 63 months; range: 38–98 months), and the OS rate was 100% (CI: 100) (Figure 3B).
3.5.2 Treatment and response
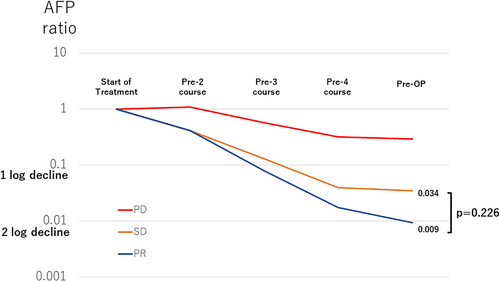
Preoperative AFP levels after four courses of CDDP were obtained from 33 patients, except for five cases, who underwent surgery at different times. The median preoperative AFP level was 59,142 (22.0–116,743) ng/mL. In 32 cases, except for one in which imaging was unavailable, the responses according to the RECIST criteria based on central imaging were as follows: PD, n = 1; SD, n = 11; and partial remission (PR), n = 20. The relationship between the RECIST criteria and median AFP decline ratio is shown in Figure 3. A preoperative AFP decrease was observed in the PR group (median preoperative AFP/AFP ratio at diagnosis = 0.009), and in the SD group (median preoperative AFP/AFP ratio at diagnosis = 0.034), but the difference was not significant (p = .226). Of the 32 patients, 25 had at least a 1-log decline in AFP at the completion of four courses of CDDP. The PD patient showed less than a 1-log decrease in AFP levels (Figure 4).
3.5.3 Surgery (n = 34)
Detailed surgical records were available for 34 of the 38 patients. Except for one liver transplantation, 16 patients underwent right or left hemihepatectomy, five underwent right or left trisegmentectomy, two underwent lateral segmentectomy, and 10 underwent other methods, including sectorectomy and partial resection. Initial upfront surgery (partial resection) was performed in one patient. There were no cases with positive microscopic margins, and complete resection was achieved in all cases, including the PD case (complete surgical resection rate: 100%).
3.5.4 Serious adverse events (n = 38)
One case of intraoperative cardiac arrest due to massive intraoperative bleeding during partial resection of segments 4 and 5 recovered without sequelae (grade 4). In one patient with postoperative bile leakage, Cycle 6 chemotherapy was delayed (grade 3). Regarding SAEs other than surgical complications, one case of asymptomatic hypomagnesemia and one case of hypercholesterolemia were observed during the sixth course of CDDP (grade 4). One patient required emergency surgery because of intratumoral hemorrhage after one course of treatment (grade 3). One patient required 6 days of glucose-insulin therapy because of hyperkalemia after one course of treatment (grade 3).
3.5.5 Late complications
Regarding late complications, high-frequency hearing loss was detected in 18 of the 38 patients (47.4%). The diagnosis of hearing loss was made according to the institutional criteria. Two patients had mild renal dysfunctions. One patient with ELBWI had short stature, and one patient with trisomy 18 had epilepsy. There were no cases of cardiac toxicity, and none of the patients developed secondary malignant neoplasms.
4 DISCUSSION
In this JPLT3-S study, the 3-year PFS was 93.9% (CI: 86.4%–100%) and OS was 38/38 (100%), which was the expected result. The primary endpoint, the 3-year PFS, was significantly higher than the 80% threshold. From this result, we can conclude that CDDP monotherapy is a suitable first-line treatment for standard-risk group HB cases diagnosed at less than 3 years old.
In the JPLT2 trial, performed from 1999 to 2012 in Japan, patients with a standard risk of PRETEXT I, II, and III were mainly treated with CITA and/or low-CITA therapy.3 Of the 212 patients, 163 were retrospectively re-categorized into the same standard-risk group according to JPLT3-S risk stratification. The 3-year OS of these 163 patients was 93.4% (CI: 40%–100%). The current JPLT3-S results showed that the OS was 100% with a median follow-up period of 63 months (range: 38–98 months), which was not significantly different from that of the previous JPLT 2 trial (p = .74). Therefore, CDDP monotherapy is an effective regimen for standard-risk JPLT3 patients.
Perilongo et al.9 reported the results of SIOPEL3, a randomized controlled trial that compared CDDP monotherapy and PLADO therapy. This study demonstrated the non-inferiority of CDDP monotherapy in the standard group. In their study, 126 patients received CDDP monotherapy. Table S1 shows a comparison of the results of SIOPEL3 and JPLT3-S. In that report, the 3-year event-free survical (EFS) rate was 83% (CI: 77%–90%) and the 3-year OS rate was 95% (CI: 91%–99%).9 Compared to the results of SIOPEL3, in JPLT3-S results, the 3-year PFS rate was 93.9% and the 3-year OS rate was 100%, which might be more satisfactory, and CDDP monotherapy is considered to be more feasible in these young localized HB cases (JPLT3 standard-risk patients).
In the JPLT3 trial, we conducted a central review of CT images to accurately diagnose and precisely judge the response according to the RECIST criteria. As Miyazaki et al.6 reported, a central review was useful not only in the JPLT-3 standard-risk group but also in the intermediate- and high-risk groups. In fact, the diagnosis changed in only two cases in the standard-risk group after the central review, but we believe that the proper risk classification of such cases contributed to the favorable PFS in this study.
The AFP decline ratios before surgery were compared according to RECIST criteria. Several reports have shown that AFP decline ratios are correlated with the prognosis of HB.13-15 In this report, the average AFP decline in the PR group was greater than 2 before surgery; however, there was no significant difference between the PR and SD groups (p = .226) (Figure 4). Response to chemotherapy may be evaluated by a combination of imaging findings and AFP decline.
In one case, the local surgeon discussed the necessity of liver transplantation after the four scheduled preoperative courses of chemotherapy had been completed. The patient was subsequently referred to a transplant center, but as the referral process was time-consuming, another course of chemotherapy was administered preoperatively before the patient was transferred; thus, this case deviated from the protocol. In the JPLT3 study, we introduced a surgical review system wherein experts from the pediatric liver surgery and liver transplantation fields formed an expert panel to provide surgical suggestions based on imaging studies upon institutional requests.7 The abovementioned case never had the chance to be evaluated by the panel. It is challenging for some low-volume local centers to accurately determine the potential need for liver transplantation, and professional advice is warranted in such cases to facilitate early referral to transplant centers. In the current JPLT4 study, all cases underwent real-time central review by an expert surgical panel periodically during treatment after the diagnosis.
In the CDDP monotherapy protocol, no long-term treatment-related late complications were observed, except high-frequency hearing loss. Sodium thiosulfate (STS) was not used in any of the patients enrolled in this study, because STS was unavailable in Japan at the time of the study. To reduce ototoxicity, it is necessary to consider reducing the amount of CDDP or the combined use of STS in the future.16-18 Although 26/38 patients (68%) in this study were classified as PRETEXT I (n = 8) or II (n = 18), upfront surgery was performed in only one patient. Upfront surgery may decrease the exposure to cisplatin and the long-term risk of ototoxicity.
In this study, none of the patients had cardiac toxicity or secondary primary malignancies due to chemotherapy. In contrast, of the 163 patients in the standard-risk group in JPLT2, secondary malignancies developed in five cases, three of which occurred within 3 years,9 and cardiac toxicity was observed in two cases. CDDP monotherapy may have contributed to the reduction in secondary malignancies and cardiotoxicities due to the omission of anthracycline.
AUTHOR CONTRIBUTIONS
All authors have contributed significantly to the study and have reviewed and agreed upon the manuscript content.
ACKNOWLEDGMENTS
The authors thank all the institutions participating in the study and the JCCG Liver Tumor Committee and current members who organized and operated on the clinical trials. This work was supported by the Japan Agency for Medical Research and Development (AMED) (grant numbers: JP17ck0106332, JP20ck0106609, JP20lk0201066), and the Japan Society for the Promotion of Science (JSPS KAKENHI) (grant number: JP16H02778).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




