The French FRACTURE database: A way to improve knowledge on management of children with very rare tumors
Abstract
Introduction
Very rare pediatric tumors (VRTs), defined by an annual incidence ≤2 per million inhabitants, represent a heterogeneous group of cancers. Due to their extremely low incidence, knowledge on these tumors is scant. Since 2012, the French Very Rare Tumors Committee (FRACTURE) database has recorded clinical data about VRTs in France. This study aims: (a) to describe the tumors registered in the FRACTURE database; and (b) to compare these data with those registered in the French National Registry of Childhood Cancer (RNCE).
Methods
Data recorded in the FRACTURE database between January 1, 2012 and December 31, 2018 were analyzed. In addition, these data were compared with those of the RNCE database between 2012 and 2015 to evaluate the completeness of the documentation and understand any discrepancies.
Results
A total of 477 patients with VRTs were registered in the FRACTURE database, representing 97 histological types. Of the 14 most common tumors registered in the RNCE (772 patients), only 19% were also registered in the FRACTURE database. Total 39% of children and adolescent VRTs registered in the RNCE and/or FRACTURE database (323 of a total of 828 patients) were not treated in or linked to a specialized pediatric oncology unit.
Conclusion
VRTs represent many different heterogenous entities, which nevertheless account for 10% of all pediatric cancers diagnosed each year. Sustainability in the collection of these rare tumor cases is therefore important, and a regular systematic collaboration between the FRACTURE database and the RNCE register helps to provide a more exhaustive picture of these VRTs and allow research completeness for some peculiar groups of patients.
Abbreviations
-
- ACC
-
- adrenocortical carcinoma
-
- ACT
-
- adrenocortical tumors
-
- CDDP
-
- cisplatin
-
- CR
-
- complete remission
-
- CT
-
- chemotherapy
-
- EXPeRT
-
- European Cooperative Study Group for Pediatric Rare Tumors
-
- FRACTURE
-
- French Very Rare Tumors Committee
-
- NET
-
- neuroendocrine tumor
-
- PARTNER
-
- PAediatric Rare Tumours Network - European Registry
-
- PD
-
- progressive disease
-
- PMSI
-
- Information System Medicalisation Programme (programme de médicalisation des systèmes d'information)
-
- PPB
-
- pleuropulmonary blastoma
-
- RNCE
-
- French National Pediatric Tumor Registry (Registre National des Cancers de l'Enfant)
-
- SFCE
-
- French Society of Pediatric Oncology (Société Française de lutte contre les Cancers et les leucémies de l'Enfant et de l'adolescent)
-
- SPPP
-
- solid pseudopapillary neoplasms of the pancreas
-
- STEP
-
- German Pediatric Rare Tumor Group
-
- TREP project
-
- Italian Project on Rare Pediatric Tumors
-
- UCNT
-
- undifferentiated nasopharyngeal carcinoma
-
- VRTs
-
- very rare tumors
About 2200 new cases of child and adolescent cancers are diagnosed every year in France. They are systematically registered in the French National Registry of Childhood Cancer (Registre National des Cancers de l'Enfant - RNCE).1 Overall, 8%–10% of these malignancies are considered “very rare tumors” (VRTs), with an annual incidence of less than two per million inhabitants.2 VRTs in children and adolescents represent a heterogeneous group of cancers. Due to their extremely low incidence, knowledge on these tumors is scant and they are the subject of only very few prospective studies; for example, a nasopharyngeal carcinoma study in Germany.3 Consequently, therapeutic recommendations are difficult to produce, and physicians have to treat VRTs on an individual basis with weak evidence-based medicine support.4 In order to face these difficulties, over the foregoing years, the European Pediatric Oncology Community has developed projects specifically dedicated to VRTs.5 The main types of tumors anticipated were undifferentiated nasopharyngeal carcinoma (UCNT), solid pseudopapillary neoplasms of the pancreas (SPPP), pleuropulmonary blastoma (PPB), carcinoid tumor, pheochromocytoma/paraganglioma, adrenocortical tumors (ACT), thyroid carcinoma, and cutaneous melanoma. Some VRTs such as PPB arise exclusively in pediatric patients, while other tumors develop generally during adulthood but are rarely seen in children and adolescents (i.e., colon cancer and malignant melanoma). These rare malignant tumors are less known to pediatricians and even pediatric oncologists. However, in recent years, the European EXPeRT group (European Cooperative Study Group for Pediatric Rare Tumors) published some specific recommendations to help clinicians in treating patients with these rare entities.5-12 The PARTNER (PAediatric Rare Tumours Network - European Registry) project aims to extend knowledge of theses rare diseases and to build a single European registry that links the existing national registries dedicated to children and adolescents (0–18 years) with VRTs. European countries, where no VRT registry exists, should register their patients directly in this new European registry.5 In France, the Very Rare Tumor Committee (FRACTURE) of the Société Française de lutte contre les Cancers et les leucémies de l'Enfant et de l'adolescent (French Society of Pediatric Oncology - SFCE) was founded in 2007 with several objectives.5 Its main aim was to record as many childhood and adolescent cases of VRTs as possible on a national scale, using the FRACTURE database, a national collection database open since 2012. The purpose of the database was to facilitate analysis of these rare diseases, mainly in order to develop harmonized dedicated guidelines for diagnosis, treatment, and follow-up.13-16 The number and heterogeneity of these pathologies, the variety of their age of onset, and the various sites of care (surgery, oncology, dermatology, ophthalmology, endocrinology, etc.) make it difficult to systematically register these patients. To date, very few countries have reported data from their national database or experience with rare pediatric tumors (Germany, Italy, Brazil). Here, we present the French experience, which represents one of the oldest and largest databases.
The main objective of this article is to describe the cases of VRTs registered in the FRACTURE database and to perform a detailed analysis for the most common entities, describing their treatments and outcomes. The secondary objective is to compare these data with those registered in the French RNCE to analyze the rate of effective registration in the dedicated VRT database and to explore the reasons of discrepancies.
1 MATERIALS AND METHODS
This retrospective study analyzed all data recorded in the FRACTURE database between 2012 and 2018 and compared them to data available in the RNCE during a 4-year period (2012–2015). The FRACTURE database is a prospective voluntary repository that since 2012 has collected clinical, biological, and therapeutic information on patients from 0 to 18 years old treated in one of the 32 SFCE centers for very rare malignant and borderline tumors. The inclusion criterion was a very rare malignant tumor or a very rare tumor of intermediate malignancy occurring in a person younger than 18 years old. VRTs were described as tumors with an annual incidence of less than two cases per million inhabitants, as defined by Ferrari et al.2 Central nervous system tumors and hematological malignancies were excluded, as were other tumors within the field of another SFCE working group or registered in a specific clinical trial: rhabdoid tumors, hepatoblastoma, retinoblastoma, malignant germ cell tumors, and rare histotypes of soft part sarcomas. The purpose of the FRACTURE database is not to provide extensive epidemiological analysis, as the voluntary nature of the report was not expected to ensure its completeness. The informed consent of the family and/or the patient is collected in each center by the clinician. Data are anonymously collected in a secured electronic database (EnnovClinical software) housed in the Angers University Hospital. Authorization from the National Commission for Data Protection and Liberties (CNIL-France) was obtained in October 2011. At regular points, a clinical update is performed to collect the last follow-up information. The validation of the data is carried out by the clinical research staff of the University Hospital of Angers. For this study, data were extracted on January 31, 2019, with an update on December 31, 2021.
All data were analyzed using descriptive statistical methods. Tumor failure was defined as relapsed disease, progressive disease (PD), or death. Locoregional relapse was defined as recurrence at the primary thoracic site or in directly adjacent thoracic tissues, and metastatic relapse was defined as appearance of tumors at distant sites. Survival time was calculated from the date of diagnosis (initial biopsy/surgery) to the time of last follow-up or event. Overall survival was calculated by the Kaplan–Meyer method. R software (version 3.5.1) and R packages wordcloud2 (version 0.2.1) and ggplot2 (version 3.0.0) were used to create the figures.
The RNCE is a population-based registry that ensures an exhaustive retrospective collection of all cancer cases in children under 18 years of age in mainland France and four overseas departments.17, 18 Based on the previous French RNCE data, the initial annual expected number of VRT cases was between 150 and 200. The main source of collection is active research by trained investigators of pediatric hematology and oncology departments in France. Active registration is supplemented by an annual request for claim data from the Information System Medicalisation Programme (PMSI) of University Hospitals (CHU) and Cancer Centers, which lists all children aged less than 15 years who have been admitted to any department with a diagnosis of cancer or a brain tumor, including a few borderline diagnoses. This source of information enables identification of children with cancer treated in departments other than pediatric oncology departments. Systematically recorded data include child characteristics, information on cancer diagnosis and patients’ pathway from diagnosis to treatment, initial treatment, vital status, and last contact date. The RNCE produces incidence and survival estimates on a national scale.19 The RNCE is evaluated by the Registry Evaluation Committee, which certifies the quality and completeness of the collected data. At the time of the analysis, RNCE data were exhaustive up to 2015, so the selected period for the comparison with the FRACTURE database was 2012–2015. This comparison was made on August 31, 2019. Moreover, as some VRTs are actually nonmalignant tumors, we widened the selection of the RNCE to include borderline tumors that are also recorded in the registry but not included in the incidence figures. Databases were combined using patients’ initials, date of birth, date of diagnosis, and type of tumor. Pediatric VRTs represent a very heterogeneous group of tumors, with difficult classifications for some entities. To simplify the comparative analysis complicated by this great heterogeneity, we decided to consider for analysis only tumors for which more than 15 cases were reported (RNCE alone, FRACTURE alone, or in both) over the period 2012–2015.
For the classification of patient treatment sites, the term “specialized pediatric oncology unit” corresponds to pediatric oncology units in university hospitals and pediatric oncology units in cancer centers.
2 RESULTS
2.1 Description of the FRACTURE database
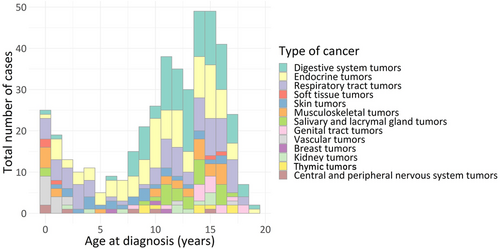
Overall, 477 patients fulfilling the inclusion criteria were included in the FRACTURE database, accounting for 97 different tumor histological types (Figure 1, Table 1) over a period of 7 years (January 1, 2012 to December 31, 2018). Total 258 patients were female (58%) and 185 were male (42%) (unknown: 34 cases). The median age of patients at diagnosis was 12 years (range: 0.0–18.9). Many different entities were included, regardless of the age of the patient. Two peaks of occurrence are present: before the age of 3, and after the age of 10 years (Figure 1).
| Histotypes | Number of cases |
|---|---|
|
Neuroendocrine tumors: Appendiceal Lung Pancreas Liver |
66 54 9 2 1 |
|
Thyroid carcinoma Papillary thyroid carcinoma Follicular thyroid carcinoma Medullary thyroid carcinoma |
52 39 9 4 |
|
Nasopharyngeal carcinoma UCNT Nasopharyngeal papillary carcinoma |
44 43 1 |
|
Pancreatic tumors Solid pseudopapillary neoplasm Acinar cell carcinoma Pancreatoblastoma Undifferentiated carcinoma |
38 35 1 1 1 |
|
Pulmonary and pleural tumors Pleuropulmonary blastoma Mucoepidermoid carcinoma NUT carcinoma Adenocarcinoma Epidermoid carcinoma Mesothelioma Pulmonary chondroid hamartoma |
35 25 3 3 1 1 1 1 |
|
Pheochromocytoma/paraganglioma Pheochromocytoma Paraganglioma |
34 21 13 |
|
Cutaneous tumors Melanoma Basal cell carcinoma Solitary cutaneous mastocytoma |
28 26 1 1 |
| Adrenocortical tumor | 27 |
|
Salivary glands tumor Mucoepidermoid carcinoma Acinar cell carcinomas Undifferentiated carcinoma Mammary analog secretory carcinoma Salivary gland analog tumor Adenoid cystic carcinoma Sialoblastoma |
27 14 7 2 1 1 1 1 |
| Hemangioendothelioma | 14 |
|
Ovarian non-germ cell tumor Mucinous cystadenoma Carcinoma Adenocarcinoma Serous cystadenoma Sclerosing stromal tumor Steroid cells tumor |
13 5 2 2 2 1 1 |
| Gastrointestinal carcinoma | 12 |
| Kidney tumors | 10 |
| Giant cells tumors | 9 |
| Thymic tumors | 8 |
| Gastrointestinal stromal tumor | 5 |
| Others rare tumors | 55 |
| Total | 477 |
- Abbreviations: FRACTURE, French Very Rare Tumors Committee; UCNT, undifferentiated nasopharyngeal carcinoma.
2.2 Clinical characteristics of the most frequent tumors registered in the FRACTURE database
2.2.1 Neuroendocrine tumors (n = 66)
The appendix was the most common location of pediatric neuroendocrine tumors (NET) (54 cases) (Table 2). All tumors were diagnosed at a localized stage, and 39 patients had a tumor of less than 20 mm. Treatment consisted of tumor resection alone in 40 patients, which was always macroscopically complete (unknown: 11 cases). A second surgery was necessary for three patients after initial incomplete surgery. One of them was in complete response after a right hemicolectomy, performed following analysis of histological criteria. Overall, 37 patients were in complete remission at the end of treatment (unknown: 17 cases). After a median follow-up of 8 months (range: 0–141), all patients were alive without any recurrence. Overall survival at 5 years was 100% (unknown: 12 cases).
| NET of appendix | SPPP | PPB | Pheochromocytoma | Paraganglioma | Papillary thyroid carcinoma | ACC | UCNT | Cutaneous melanoma | |
|---|---|---|---|---|---|---|---|---|---|
| Number of cases | |||||||||
| 54 | 35 | 25 | 21 | 13 | 39 | 25 | 43 | 26 | |
| Median age (years) | |||||||||
|
12.5 [6.4; 17.6] |
12.4 [7.0; 17.2] |
2.2 [0.2; 4.2] |
10.3 [6.8; 15.1] |
14.1 [8.6; 18.0] |
12.8 [4.2; 17.5] |
4.7 [0.4; 15.4] |
13.7 [8.5; 18.2] |
8.9 [1.1; 15.5] |
|
| Staging | |||||||||
| Locoregional | Localized: 54 |
N0: 27 N1: 1 NS: 7 |
NS |
N0: 20 N1: 1 Bilateral: 1 |
N0: 9 NS: 4 |
N0: 6 N1: 16 NS:17 |
NS |
8th TNM staging: Stage II: 1 Stage III: 7 Stage IVa: 12 NS: 12 |
N1: 7 |
| Metastasis | 0 |
M0: 31 NS: 4 |
M0: 17; M1: 3, NS: 5 |
M0: 19 M1: 2 |
N0: 10 NS: 3 |
M0: 21 M1: 12 (8 in lung) NS: 6 |
M0: 20 M1: 4 NS: 1 |
Stage IVb: 11 | M1: 4 |
| Treatment | |||||||||
| Surgical resection | 43 (NS 11) | 31 (NS 4) | 18 (NS 4) | 20 | 8 (NS 3) | 29 (NS 4) | 19 (NS 2) | 0 (NS 5) | 24 (NS 2) |
| Chemotherapy | 0 | 0 | 18 (NS 6) | 0 | 0 | 0 | 8 | 38 (NS 5) | 3 (NS 2) |
| Radiotherapy | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 35 (NS 8) | 0 |
| Other treatment | 0 | 0 | 0 | MIBG therapy: 1 | 0 | Iodine-131: 19 | 0 | Interferon: 25 (NS 10) | Immunotherapy: 2 (NS 2) |
| Median FU (months) | |||||||||
| 8.0 | 18.8 | 51.0 | 42.3 | 56.5 | 25.0 | 18.8 | 48.2 | 45.0 | |
| Status at last visit | |||||||||
| Alive | 54 | 31 | 23 | 18 | 9 | 31 | 17 | 34 | 19 |
| Dead | 0 | 0 | 2 | 0 | 0 | 0 | 6 | 1 | 6 |
| NS | 0 | 5 | 0 | 3 | 4 | 8 | 2 | 8 | 1 |
- Abbreviations: ACC, adrenocortical carcinoma; FRACTURE, French Very Rare Tumors Committee; FU, follow-up; M0, no metastatic involvement; M1, metastatic involvement; N0, no lymph node involvement; N1, lymph node involvement; NET, neuroendocrine tumor; NS, not specified; PPB, pleuropulmonary blastoma; SPPP, solid pseudopapillary neoplasms of the pancreas; UCNT, undifferentiated nasopharyngeal carcinoma.
NETs involved another site for 12 patients: nine in lung (median follow-up 60 months, range: 9.9–87.9, eight in first complete remission, one alive with disease), two in pancreas (one patient died of disease 24 months of evolution; unknown: one case), and one in the liver (alive without disease after 59 months of follow-up).
2.2.2 Solid pseudopapillary neoplasms of the pancreas (n = 35)
Among the 35 patients, almost all were diagnosed at a localized stage (and one patient had regional nodal involvement N1). Treatment was exclusive surgery for all patients, with complete remission for 31 patients (four unknown). After a median follow-up of 19 months (range: 0.5–129), all patients were alive without disease and one had a PD (unknown: five cases). Overall survival at 5 years was 100% (unknown: five cases).
2.2.3 Pleuropulmonary blastoma (n = 25)
Among the 25 patients with PPB, seven had pleural involvement (unknown: one case). Metastatic disease was present in three patients (bone in two cases, contralateral lung in one case). Almost all patients for whom the data were available had an initial surgery (18 R0 [complete histologic] resection, three biopsy, unknown: four cases) and received chemotherapy (18 chemotherapy; unknown: six cases). Complete remission was achieved for 24 patients (one PD). After a median follow-up of 51 months (range: 3–110), two patients died of disease and 23 were alive. Overall survival at 5 years was 89% (unknown: four cases).
2.2.4 Pheochromocytoma (n = 21) and paraganglioma (n = 13)
Among the 21 patients with pheochromocytoma, one had a bilateral disease, one a lymph node involvement, and two had metastasis (lung and bone metastasis: one each). Twenty patients underwent an initial surgical resection (one biopsy alone), resulting in microscopic complete resection for 17 patients (85%). No patients received chemotherapy or radiotherapy. One patient (with bone metastases) received MIBG therapy.
At the end of the treatment, complete remission was achieved for 20 patients (one partial remission). All patients were alive at the last follow-up (median follow-up: 42 months; range: 1–107) including one patient in third complete remission and another alive with disease (unknown: three cases). Overall survival at 5 years was 100% (unknown: three cases).
Among the 13 patients with paraganglioma, none had lymph node involvement (unknown: three cases) or metastatic disease at diagnosis (unknown: two cases). Nine patients underwent complete resection surgery at diagnosis, one a biopsy followed by complete resection, and one a delayed surgery. One patient did not undergo any surgical procedure (unknown: one case). One patient had multiple paragangliomas; he underwent complete resection of three lesions (one coelio-mesenteric lesion and two carotid lesions), and irradiation with proton therapy of a tympano-jugular lesion not accessible to surgery. No other patient received radiotherapy (unknown: one case) or chemotherapy (unknown: one case). All patients were in complete remission at the last follow-up (median follow-up: 34 months, range: 1–54) (unknown: two cases). Overall survival at 5 years was 100% (unknown: two cases).
2.2.5 Papillary thyroid carcinoma (n = 39)
Among the 39 patients with a papillary thyroid carcinoma, 16 had regional nodal extension (unknown: 17 cases) and 12 metastatic diseases mostly located in the lung (eight patients; unknown: six cases). Carcinoma diagnosis was made after thyroid biopsy for six patients or surgical resection for 29 patients (unknown: four cases). Treatment with iodine-131 was undertaken in 30 patients. No patients received external radiotherapy. After a median follow-up of 25 months (range: 3–124), all patients are alive with six patients having residual disease and one in second complete remission (unknown: eight cases). Overall survival at 5 years was 100% (unknown: eight cases).
2.2.6 Adrenocortical carcinoma (n = 25)
Among the 25 patients with adrenocortical carcinoma (ACC), four patients had metastasis (two in lung and two in peritoneum due to a tumor rupture). Postsurgical treatment comprised chemotherapy (etoposide–cisplatin–doxorubicin) and mitotane for seven patients with stage III and IV ACC. One patient with stage I carcinoma received only mitotane, and one patient received only chemotherapy. Two patients presented an early death, one by toxicity and one during surgery. After a median follow-up of 19 months (range: 0–192), six patients died, two during initial treatment and four by disease after relapse or progression and 17 were alive (unknown: two cases). Overall survival at 5 years was 65% (unknown: three cases).
2.2.7 Undifferentiated nasopharyngeal carcinoma (n = 43)
Among the 43 patients with UCNT included in the FRACTURE database, only one patient had lung metastasis at diagnosis. All patients received chemotherapy (unknown: five cases), 34 with 5-fluorouracile (5-FU)-based regimens, including 27 with 5-FU and cisplatin (CDDP). All patients received radiotherapy (unknown: eight cases) and 25 received maintenance β-interferon (unknown: 10 cases). After a median follow-up of 48 months (5–98), 33 patients were alive in first complete remission, one patient died after PD, and one was alive with disease (unknown: eight cases). Overall survival at 5 years was 96% (unknown: eight cases).
2.2.8 Cutaneous melanoma (n = 26)
Among the 26 patients with melanoma, six melanomas were classified as spitzoid melanomas. The main tumor sites were head and neck (nine patients) and extremities (nine patients; nonspecified site: three cases). A lymphatic involvement was present in seven patients. Distant metastases were present in four patients (15%) at diagnosis (central nervous system involvement: two cases; distant lymph node: one case; lung: one case). Among them, one died of disease despite chemotherapy and immunotherapy and another with intracranial melanoma died despite treatment with pembrolizumab. One patient with lung metastasis was still alive with stable disease, after ipilimumab treatment. The patient with the distant lymph node involvement is currently alive in complete remission (CR)-2 after a local relapse. After a median follow-up of 45 months (range: 5–69), 16 patients were alive in first CR, two in second CR, one was alive with stable disease, and two were alive with PD. Four patients died of PD (unknown: one case). Overall survival at 5 years was 69% (unknown: one case).
2.2.9 Other tumors
Other groups of borderline or malignant tumors were frequently present in the FRACTURE database. The group of salivary gland tumors represented 27 cases, with 14 mucoepidermoid and seven acinar cell carcinomas as the most frequent histologies.
The group of rare pulmonary and pleural tumors (excluding PPB and carcinoid tumor) also represented a significant group of tumors (10 cases). Three of these tumors were mucoepidermoid carcinomas, three NUT carcinoma, one adenocarcinoma, one epidermoid carcinoma, one mesothelioma, and one pulmonary chondroid hamartoma.
Concerning aggressive vascular tumors, 14 cases of kaposiform hemangioendotheliomas were collected in the database. Nine cases of giant cell bone tumors were also described.
Finally, ovarian non-germ-cell tumors were present in a group of 13 patients: five cases of mucinous cystadenoma, two ovarian adenocarcinoma, two ovarian carcinoma, and two serous cystadenoma.
2.3 Cross-match between the French National Registry of Childhood Cancers and the FRACTURE database
Between 2012 and 2015, 877 cases of VRTs were registered in the RNCE, while during the same period, 250 patients were included in the FRACTURE database.
Taking the 14 most frequent tumor types (Table 3), 828 VRTs were registered between 2012 and 2015 in the two databases. Among these, 156 (19%) were in the two databases, 616 (74%) only in the RNCE, and 56 (7%) only in FRACTURE.
| Histotypes | RNCE | FRACTURE database | Concordant cases | Discordant cases: RNCE only | Discordant cases: FRACTURE database only |
|---|---|---|---|---|---|
| Thyroid cancer | 263 | 26 | 16 | 247 | 10 |
| Melanoma | 119 | 16 | 15 | 104 | 1 |
| Neuroendocrine tumor of the appendix | 110 | 29 | 14 | 96 | 15 |
| Adrenocortical carcinoma | 39 | 17 | 17 | 22 | - |
| UCNT | 38 | 27 | 27 | 11 | - |
| Aggressive vascular tumor | 32 | 9 | 3 | 29 | 6 |
| Rare ovarian cancer | 29 | 5 | 5 | 24 | - |
| Salivary glands tumor | 28 | 16 | 12 | 16 | 4 |
| SPPP | 25 | 19 | 12 | 13 | 7 |
| Pulmonary carcinoma | 23 | 9 | 9 | 14 | - |
| Giant cell tumor | 23 | 6 | 5 | 18 | 1 |
| Pleuropulmonary blastoma | 19 | 17 | 17 | 2 | - |
| Chondrosarcoma | 15 | 1 | 1 | 14 | - |
| Pheochromocytoma | 9 | 15 | 3 | 6 | 12 |
| Total | 772 | 212 | 156 | 616 | 56 |
| Total number of cases | 828 | ||||
- Abbreviations: FRACTURE, French Very Rare Tumors Committee; RNCE: National Registry of Childhood Cancers; SPPP: solid pseudopapillary neoplasms of the pancreas; UCNT: undifferentiated nasopharyngeal carcinoma.
According to these results, VRTs represent a total of approximately 200 new cases per year in France. During the 2012–2015 period covered by our study, the RNCE registered approximately 1920 new tumor cases per year (including hematologic malignancies, Langherhans cells histiocytosis, and low-grade brain tumors). Without guarantee of completeness, about 240 borderline tumors are also registered in the RNCE each year, within an annual total of about 2160 tumors. VRTs thus represent about 10% of all new cases of malignant and intermediate tumors registered each year in children and adolescents in France.
Tumor types registered only in the RNCE were mainly thyroid cancers, melanomas, and NETs of the appendix.
All patients included in the FRACTURE database were treated or managed in specialized pediatric oncology units, and reporting was performed by pediatric oncologists. Concerning the site of care (type of hospital and medical unit) for patients with the 14 main VRT groups exclusively registered in the RNCE, only 48% were treated in a specialized pediatric oncology unit (Table 4). The majority was not treated in specialized pediatric oncology units, but in medical non-oncology units or adult oncology units in university hospitals (133 patients: 22%), in surgical units of university hospitals (129 patients: 21%), or in non-university hospital centers (public or private, excluding cancer centers; 61 patients: 10%). The main discrepancies arise mostly in relation to thyroid cancers, melanoma, and NETs of the appendix, which tend not to be directly treated in pediatric oncologic units.
| Categories of hospital centers concerning VRT exclusively registered in RNCE | |||||
|---|---|---|---|---|---|
| Histotypes | 1 | 2 | 3 | 4 | Total |
| Thyroid cancer |
104 (42.1 %) |
77 (31.2 %) |
56 (22.7 %) |
10 (4.0 %) |
247 |
| Cutaneous melanoma |
46 (44.2 %) |
34 (32.8 %) |
12 (11.5 %) |
12 (11.5 %) |
104 |
| NET of the appendix |
35 (36.5 %) |
7 (7.3 %) |
17 (17.7%) |
37 (38.5%) |
96 |
| Aggressive vascular tumors |
20 (69.0 %) |
1 (3.4 %) |
8 (27.6 %) |
0 | 29 |
| Rare ovarian cancers |
12 (50.0 %) |
0 |
11 (45.8 %) |
1 (4.2 %) |
24 |
| Adrenocortical carcinoma |
16 (72.7 %) |
4 (18.2 %) |
2 (9.1 %) |
0 | 22 |
| Giant cell tumor |
9 (50.0 %) |
1 (5.6 %) |
8 (44.4 %) |
0 | 18 |
| Salivary glands tumor |
10 (62.5 %) |
0 |
5 (31.2 %) |
1 (6.3 %) |
16 |
| Chondrosarcoma |
11 (78.6 %) |
0 |
3 (21.4 %) |
0 | 14 |
| Pulmonary carcinoma |
8 (57.1 %) |
2 (14.3 %) |
4 (28.6 %) |
0 | 14 |
| SPPP |
10 (77.0 %) |
2 (15.4 %) |
1 (7.7 %) |
0 | 13 |
| UCNT |
8 (72.7 %) |
3 (2.3 %) |
0 | 0 | 11 |
| Pheochromocytoma |
2 (33.3 %) |
2 (33.3 %) |
2 (33.0 %) |
0 | 6 |
| Pleuropulmonary blastoma |
2 (100.0 %) |
0 | 0 | 0 | 2 |
| Total |
293 (48.0%) |
133 (22.0 %) |
129 (21.0 %) |
61 (10.0 %) |
616 |
- Note: Categories of hospital centers: 1: specialized pediatric oncology unit; 2: medical non-oncology unit or adult oncology unit in university hospital; 3: surgical unit in university hospital; 4: non-university hospital centers (public or private).
- Abbreviations: NET, neuroendocrine tumor; RNCE, National Registry of Childhood Cancers; SPPP, solid pseudopapillary neoplasms of the pancreas; UCNT, undifferentiated nasopharyngeal carcinoma; VRT, very rare tumors.
Concerning patients registered in the RNCE and/or in the FRACTURE database (828 patients), 505 patients (60.9%) were treated in a specialized pediatric oncology unit. The other 323 patients (39.1%) were treated in a medical non-oncology unit in a university hospital (133 patients: 16.1%), in a surgical unit in university hospital (129 patients: 15.6%), or in a non-university/non-cancer center hospital (61 patients: 7.4%). Of these 505 patients, only 212 patients (41.9%) were registered in the FRACTURE database.
3 DISCUSSION
In recent years, a number of countries have set up their own registries for very rare pediatric tumors in order to better understand these diseases.20-23 The Italian TREP project (Project on Rare Pediatric Tumors) was the first of these when it was launched in 2000.21 Overall, 297 patients were registered in this database between 2000 and 2005. Our French experience shows a similar distribution of the different tumors with a median age among those patients registered of about 12 years. The German Pediatric Rare Tumor Group (STEP) was founded in 2006.23 Between 2008 and 2018, 623 patients with VRTs were registered in STEP registry.22 The inclusion criteria and the type of tumors registered are very similar to those of the FRACTURE database.
Registration in the FRACTURE and the RNCE databases is mainly carried out in pediatric oncology departments. Even though both databases register a significant number of cases each year, there is a significant lack of comprehensiveness in the FRACTURE database (53 cases per year) when compared to the RNCE (193 cases per year), with 74% of patients exclusively registered in RNCE, 7% in FRACTURE, and 19% in both databases. There are several possible explanations for this difference, including the fact that registration in the FRACTURE database is self-reported by a physician with no specific funding for these inclusions, whereas the RNCE provides funding for time spent in the centers. A dedicated team of clinical research associates proactively collect and cross-check data from many sources, such as medical procedure coding systems and pathology laboratories. Finally, registering patients and providing regular follow-up is a time-consuming process for clinicians receiving no financial support for this extra effort. However, and despite all these difficulties, nearly 500 patients were registered in a 7-year period with a large amount of medical data, thus enabling retrospective studies to improve medical knowledge on such rare entities. However, the objectives of these two databases are different. The RNCE is more exhaustive, as it is a systematic publicly funded collection of data, the completeness of which is checked by using the Information System Medicalization Programme.17 It provides essentially epidemiological data. In contrast, the FRACTURE database provides clinical data available for clinical studies and patient treatment. Several retrospective studies have already been performed with these medical data, improving knowledge of some rare entities.11, 13, 24-26 A number of these retrospective studies were performed with the collaboration of other European VRT groups that registered their own patients and within the framework of EXPeRT group.9, 27 Finally, all of these studies allowed the EXPeRT group to build and publish care recommendations for the treatment of such rare diseases.5, 8
One specific aim of the national registry is to improve knowledge about cancer epidemiology. Our analysis confirms that VRTs represent a total of nearly 200 new cases per year in France, that is, about 10% of all new cases of tumors registered each year in children and adolescents. This constitutes a significant proportion of cases, therefore justifying the existence of specialized working groups and committees to treat and follow-up these patients as best as possible.
To increase the number of inclusions in the FRACTURE database, more complete information should be provided to pediatric oncologists and surgeons should be better informed. Multidisciplinary consultation meetings also serve as a reminder of the existence of the database and raise awareness of the inclusions.28 However, our study confirms that patient registration in the FRACTURE database is mostly performed by pediatric oncologists, probably because this database was initially developed within the framework of the SFCE. It appears more difficult to regularly remind all physicians treating these rare tumors, particularly in units that take care of types of cancer typically seen in adolescents or adults (like thyroid carcinoma or melanoma), especially in non-university hospitals.
This study also highlighted that many pediatric patients treated for VRTs are not directly managed by pediatric hematologists/oncologists. Therefore, patients with pediatric thyroid cancers are generally treated by dedicated endocrinologists, patients with cutaneous melanomas by adult dermatologists, those with NETs by surgeons, and so forth. In these situations, pediatricians may not be involved in the treatment of such patients, which is a matter of concern for several reasons. First, in some cases, the same pathology may require different managements between adults and children; for example, hemicolectomies are not indicated in the treatment of NETs of the appendix in childhood,29 or radiotherapy dosages might sometimes be reduced for teenage and young adult patients with UCNT after a favorable tumor response to initial chemotherapy.8, 14 Second, pathologies can differ in their biology (e.g., childhood melanomas25, 30) or etiology (e.g., thyroid carcinoma and DICER1 syndrome31, 32) in pediatric cases and therefore need to be considered specifically in the light of the patient's age. Pediatric oncologists seem to be solicited mostly to manage patients with the most complex cases, as suggested by the proportion of metastatic forms of melanoma, which is three times higher in the FRACTURE database than in the literature (15% vs. 5%).30 Previous studies have shown that children and adolescents with metastatic melanomas are more often managed in pediatric oncology departments than other patients with more localized melanomas.33
4 CONCLUSIONS
This study confirmed the great diversity of VRTs in children, which nevertheless represents 10% of all pediatric cancers diagnosed each year. After 10 years of operation, the main goal of the FRACTURE database has been achieved. Enough patients have been registered to enable the database to contribute to all collaborative studies undertaken with other European countries and for researchers to perform collaborative retrospective studies. As a result, several collaborative national and international studies have already been finalized using these data, and have even led to recommendations on the diagnosis and treatment of some VRTs, such as pancreatoblastoma or ACC.7-9, 27, 30, 34 The sustainability of this collection is therefore important, and a regular systematic collaboration between the FRACTURE database and the RNCE register helps to provide a more exhaustive picture of these VRTs and allows research completeness for specific groups of patients.
Overall, 39.1% of children and adolescents with these VRTs were not treated in or linked to a specialized pediatric oncology unit. Communication on these rare pathologies and their specificities according to the age of onset must be a major objective of the working groups on VRTs in children and adolescents.
ACKNOWLEDGMENTS
The authors would like to thank Mr Jean-Marie Chrétien, Head of the Data Science Department at CHU Angers, for his involvement in setting up and maintaining the FRACTURE database. The French FRACTURE database is supported by the “Enfant, cancers et santé” association, which did not take part in any of the analyses in this manuscript.
CONFLICT OF INTEREST
The authors declare they have no conflicts of interest.
FUNDING INFORMATIONS
French FRACTURE database supported by “Enfant, cancers et santé”




