Body image in adolescent survivors of childhood cancer: The role of chronic health conditions
Abstract
Background
Cancer and its treatment may impair the body image of childhood cancer survivors during adolescence. We compared the body image between adolescent cancer survivors and their siblings, and determined whether survivors’ body image is associated with socio-demographic characteristics, clinical characteristics and chronic health conditions.
Procedure
As part of the nationwide Swiss Childhood Cancer Survivor Study, we sent questionnaires to adolescents (aged 16–19 years), who survived >5 years after having been diagnosed with childhood cancer between 1989 and 2010. Siblings received the same questionnaire. We assessed the level of agreement with three body image statements referring to body satisfaction and preferences for changes. Chronic health conditions were classified into cardiovascular, pulmonary, endocrine, musculoskeletal, renal/digestive, neurological and hearing or vision impairment. We used ordered logistic regression models to identify determinants of a more negative body image.
Results
Our study included 504 survivors (48% female) with a median age at study of 17.7 years (interquartile range: 16.8–18.6) and 136 siblings. Survivors and siblings reported overall comparable levels of agreement with body image statements (all p > .05). Female survivors (all odds ratio [ORs] ≥1.7), survivors treated with haematopoietic stem cell transplantation (HSCT; all ORs ≥2.2), and survivors with ≥2 chronic health conditions (all ORs ≥1.4) reported a more negative body image. This was particularly pronounced for survivors suffering from musculoskeletal or endocrine conditions.
Conclusion
Female survivors, survivors treated with HSCT or with chronic health conditions are at risk of body image concerns during adolescence. Increased awareness among clinicians and targeted psychosocial support could mitigate such concerns.
Abbreviations
-
- CI
-
- confidence interval
-
- CNS
-
- central nervous system
-
- GvHD
-
- graft-versus-host disease
-
- HSCT
-
- haematopoietic stem cell transplantation
-
- ICCC-3
-
- International Classification of Childhood Cancer - Third Edition
-
- IQR
-
- interquartile range
-
- OR
-
- odds ratio
-
- SCCR
-
- Swiss Childhood Cancer Registry
-
- SCCSS
-
- Swiss Childhood Cancer Survivor Study
1 INTRODUCTION
A cancer diagnosis during childhood and its intensive treatment requiring frequent hospitalizations and invasive procedures raises a broad range of medical and psychosocial challenges for the affected children.1 Despite steadily increasing survival rates, reaching 85% in Switzerland,2 childhood cancer survivors have a lifelong increased risk of chronic health conditions.3, 4 The treatment's immediate impact on the body, visible and invisible late consequences such as scars, amputations or endocrine problems such as growth hormone deficiency may interfere with survivors’ psycho-sexual development and body image.5-7 Growth hormone deficiency is a common consequence of childhood cancer treatment and has been previously shown to impair linear growth, reduce cardiac muscle mass and increase fat mass.8 The cancer experience may further disrupt social interactions with peers, and survivors may perceive their bodies as something that has failed them or as a source of pain.9 A healthy body image is of particular importance during adolescence, which is a critical developmental period characterized by many challenges such as puberty, establishing autonomy, first partner relationships and realization of own sexuality.10-12
Body image is a complex psychological construct including body-related self-perceptions and self-attitudes, such as beliefs, feelings and behaviours.13, 14 Qualitative studies suggest negative effects of childhood cancer on survivors’ body image.15-19 However, a systematic review from 2009 concluded that there is no consistent evidence regarding differences in body image between children and adolescents with cancer and healthy controls.13 This review included seven studies focusing on children (<12 years old), 19 on adolescents (13–19 years old), two on children and adolescents, and three on adolescents and young adults (>20 years old).13 However, evidence from quantitative population-based studies including adolescent childhood cancer survivors and a healthy comparison group is limited and it remains unclear how chronic health conditions affect the body image of long-term survivors. Understanding the impact of childhood cancer on the body image of adolescent survivors is critical to guide adequate support strategies and to mitigate adverse consequences on survivors’ future quality of life and psychosocial well-being.20, 21 In this study, we therefore aimed to compare the body image between adolescent cancer survivors and their siblings and to determine whether survivors’ body image is associated with socio-demographic characteristics, clinical characteristics and chronic health conditions.
2 METHODS
2.1 Design, study population and research setting
The Swiss Childhood Cancer Survivor Study (SCCSS) is a nationwide follow-up study of all patients registered in the Swiss Childhood Cancer Registry (SCCR), who were diagnosed with cancer between 1976 and 2010 before the age of 21 years, and who survived at least 5 years after diagnosis.22 The SCCR centrally registers all children and adolescents, who are diagnosed with leukaemia, lymphoma, central nervous system (CNS) tumours, malignant solid tumours or Langerhans cell histiocytosis in Switzerland.23, 24 As part of the SCCSS, we sent questionnaires to adolescent survivors of childhood cancer aged 16–19 years at study between 2007 and 2017. The SCCSS questionnaires were developed based on the questionnaires used in North American and British childhood cancer survivor studies to increase international comparability.25, 26 We added questions on socio-economic characteristics adapted to the Swiss context.27, 28 We asked survivors for consent to contact their siblings as comparison group. Adolescent siblings of the same age range (16–19 years) received the same questionnaire between 2009 and 2012 without cancer-related questions. Ethical approval of the SCCR and the SCCSS was granted by the Ethics Committee of the Canton of Bern (166/2014; 2021-01462).
2.2 Outcome measures: body image
We assessed the body image of survivors and siblings with three statements referring to body satisfaction and preferences for changes in the questionnaire: (i) I am satisfied with my body image, (ii) I would like to change a few things regarding my body, and (iii) I would like to change many things regarding my body. These statements have been previously used in healthy adolescents in Switzerland as part of the Swiss multicentre adolescent survey on health in 2002 (SMASH-2002).29 Survivors and siblings were asked to indicate their level of agreement with these statements on a 4-point Likert scale (0 = completely agree; 1 = slightly agree; 2 = slightly disagree; 3 = completely disagree). For analysis purposes, we reverse-coded statements (ii) and (iii) with higher scores indicating a more negative body image.
2.3 Socio-demographic characteristics
For survivors and siblings, we assessed the following socio-demographic characteristics in the questionnaire: age at study (16–17, 18, 19 years), sex, language region in Switzerland (German, French or Italian), migration background, and whether they currently have a boyfriend or girlfriend (no; yes). We considered survivors as having a migration background if they were not living in Switzerland since birth, were not Swiss citizens since birth, or had another nationality than or in addition to the Swiss nationality.
2.4 Clinical characteristics and chronic health conditions
We obtained the following clinical characteristics from the SCCR: age at diagnosis (<5, 5–10, >10 years), cancer diagnosis according to the International Classification of Childhood Cancer - Third edition (ICCC-3),30 treatment, time since diagnosis (<10, 10–15, >15 years), and history of relapse (no; yes). For analyses, cancer diagnoses were categorized into leukaemia (ICCC-3 Group I), lymphoma (II), CNS tumour (III), bone tumour and soft tissue sarcoma (VIII, IX), and other tumours (IV, V, VI, VII, X, XI, XII and Langerhans cell histiocytosis). Treatment modalities were coded hierarchically to account for treatment intensity and multiple treatments: surgery only, chemotherapy (may have had surgery), radiotherapy (may have had surgery or chemotherapy), and haematopoietic stem cell transplantation (HSCT; may have had surgery, chemotherapy or radiotherapy).
In the questionnaire, we collected information on chronic health conditions involving the cardiovascular, pulmonary, and endocrine system, hearing and vision problems, and musculoskeletal, renal or digestive, and neurological conditions. Chronic health conditions were asked using questions from the North American25 and British26 Childhood Cancer Survivor Studies. Survivors had to indicate whether they suffer from symptoms/diseases involving the respective body systems. Survivors were classified as having the chronic health condition if at least one of the corresponding symptoms/diseases was reported. If information on symptoms/diseases was missing, we assumed the condition is not present or at least not serious, as done previously.31 We then created a sum score based on the number of body systems affected by chronic health conditions for each survivor.
2.5 Statistical analysis
We used descriptive statistics to describe the study population and chi-square tests to compare clinical characteristics between participating and non-participating survivors. To increase the comparability between survivors and siblings, we standardized siblings for age at study and sex according to the distribution in survivors as previously done.32-34 We used multivariable logistic regression with being a sibling as outcome to calculate appropriate weights. The weight for survivors was set to 1 and all subsequent analyses were based on weighted siblings.
First, we used chi-square tests to compare the level of agreement with the three body image statements between survivors and weighted siblings. We applied ordered logistic regression to identify potential differences across ordered categories. Among survivors, we then fitted ordered logistic regression models to identify associations between the level of agreement with the three body image statements and socio-demographic characteristics, clinical characteristics, chronic health conditions by body system, and the sum score of body systems affected by chronic health conditions. Separate models were created for each body image statement. We a priori decided to adjust each model for the potential confounding factors age at study and sex based on previous literature.14 In addition, we separately analysed the association between body image and growth hormone deficiency as part of endocrine conditions due to its relatively high prevalence and direct impact on the body.8 All analyses were based on complete cases and performed using Stata version 15.1 (StataCorp. 2017, Stata Statistical Software: Release 15, College Station, TX: StataCorp LLC).
3 RESULTS
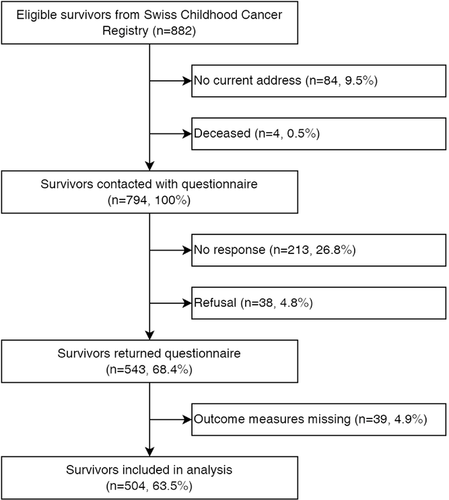
Of 882 eligible survivors aged 16–19 years, 794 received the questionnaire (Figure 1). Of those, 543 completed the questionnaire (response rate 68%). We excluded 39 (5%) survivors with missing information on body image and finally included 504 (64%) in the analysis. The final sibling population consisted of 136 participants. Most survivors and siblings were from a German-speaking region and did not report a migration background (Table 1). Thirty percent of survivors and siblings reported that they currently have a boyfriend or girlfriend. Among survivors, median age at diagnosis was 5.8 years (interquartile range [IQR]: 2.6–9.9), median time since diagnosis 11.6 years (IQR: 8.2–14.8), and median age at study 17.7 years (IQR: 16.8–18.6). The most frequent cancer diagnoses were leukaemia (32%) and CNS tumours (17%). Clinical characteristics were similar between participating and non-participating survivors (Table S1).
| Survivors (n = 504) | Siblings (n = 136)a | ||
|---|---|---|---|
| Socio-demographic characteristics | n | % | %a |
| Age at study | |||
| 16–17 years | 296 | 59 | 57 |
| 18 years | 119 | 24 | 28 |
| 19 years | 89 | 18 | 15 |
| Sex | |||
| Male | 260 | 52 | 52 |
| Female | 244 | 48 | 48 |
| Language region | |||
| German | 350 | 69 | 89 |
| French or Italian | 154 | 31 | 11 |
| Migration background | |||
| No | 348 | 69 | 76 |
| Yes | 156 | 31 | 24 |
| Boyfriend/girlfriend | |||
| No | 343 | 68 | 70 |
| Yes | 153 | 30 | 30 |
| Missing | 8 | 2 | – |
| Survivors (n = 504) | Siblings (n = 136)a | ||
|---|---|---|---|
| Clinical characteristics | n | % | |
| Age at diagnosis | |||
| <5 years | 227 | 45 | |
| 5–10 years | 152 | 30 | |
| >10 years | 125 | 25 | |
| Diagnosis (ICCC-3) | |||
| Leukaemia | 162 | 32 | |
| Lymphoma | 75 | 15 | |
| CNS tumour | 84 | 17 | |
| Bone tumour/soft tissue sarcoma | 46 | 9 | |
| Other solid tumourb | 137 | 27 | |
| Treatmentc | |||
| Surgery | 75 | 15 | |
| Chemotherapy | 263 | 52 | |
| Radiotherapy | 126 | 25 | |
| HSCT | 30 | 6 | |
| Missing | 10 | 2 | |
| Time since diagnosis | |||
| <10 years | 193 | 38 | |
| 10–15 years | 195 | 39 | |
| >15 years | 116 | 23 | |
| History of relapse | |||
| No | 452 | 90 | |
| Yes | 52 | 10 | |
| Number of chronic health conditionsd | |||
| 0 | 150 | 30 | |
| 1 | 141 | 28 | |
| ≥2 | 213 | 42 | |
- Abbreviations: CNS, central nervous system; HSCT, haematopoietic stem cell transplantation; ICCC-3, International Classification of Childhood Cancer - Third Edition; n, number.
- a Calculated on weighted analysis (weights on age at study and sex according to the distribution in survivors).
- b Neuroblastoma (n = 30), retinoblastoma (n = 14), renal tumour (n = 44), hepatic tumour (n = 3), germ cell tumour (n = 15), Langerhans cell histiocytosis (n = 23), other malignant epithelial neoplasms, malignant melanomas and other or unspecified malignant neoplasms (n = 8).
- c Treatment was coded hierarchically into surgery only, chemotherapy (may have had surgery), radiotherapy (may have had surgery or chemotherapy) and haematopoietic stem cell transplantation (may have had surgery, chemotherapy or radiotherapy).
- d Chronic health conditions include conditions involving the cardiovascular, pulmonary, and endocrine system, hearing and vision problems, and musculoskeletal, renal or digestive, and neurological conditions.
Chronic health conditions were reported by 354 (70%) survivors, with 141 (28%) reporting one condition and 213 (42%) more than two conditions. Neurological (33%), pulmonary (24%), renal and digestive (23%) and musculoskeletal conditions (23%) were most frequently reported (Table 2). The most commonly reported symptoms within these conditions were balance disorders (15%), asthma (12%), chronic constipation or diarrhoea (9%) and prolonged pain in bones or joints (9%). Growth hormone deficiency was reported by 49 (10%) survivors.
| Chronic health conditiona | Symptoms/diseases | n (%)b |
|---|---|---|
| Cardiovascular | Arrhythmiac | 20 (4) |
| Cardiomyopathy | 11 (2) | |
| Heart attack | 1 (<1) | |
| Valvular heart disease | 6 (1) | |
| Total cardiovascular | 33 (7) | |
| Pulmonary | Asthma | 61 (12) |
| Chronic coughd | 23 (5) | |
| Pneumonia | 49 (10) | |
| Lung fibrosis | 4 (<1) | |
| Chest wall abnormalities | 11 (2) | |
| Total pulmonary | 119 (24) | |
| Endocrine | Diabetes mellituse | 3 (<1) |
| Hypo- or hyperthyroidism | 58 (12) | |
| Goiter or thyroid enlargement | 4 (<1) | |
| Thyroid nodules or tumour | 17 (3) | |
| Growth hormone deficiency | 49 (10) | |
| Total endocrine | 92 (18) | |
| Hearing | Mild, moderate, severe hearing loss | 55 (11) |
| Tinnitus | 14 (3) | |
| Total hearing | 62 (12) | |
| Vision | Severe visual impairment or blindnessf | 42 (8) |
| Cataract | 16 (3) | |
| Glaucoma | 5 (1) | |
| Retinal disorders | 6 (1) | |
| Dry eye syndrome | 29 (6) | |
| Eye movement disorders (including strabismus) | 19 (4) | |
| Total vision | 80 (16) | |
| Musculoskeletal | Shortened extremities | 25 (5) |
| Reduced flexibility of joints | 41 (8) | |
| Prolonged pain in bones or joints | 47 (9) | |
| Scoliosis | 48 (10) | |
| Total musculoskeletal | 118 (23) | |
| Renal and digestive | Repeated cystitis or nephritis | 32 (6) |
| Gallstone | 5 (1) | |
| Chronic constipation or diarrhoea | 43 (9) | |
| Gastro-oesophageal reflux disease | 36 (7) | |
| Problems with oesophagus | 13 (3) | |
| Chronic or acute pancreatitis | 2 (<1) | |
| Frequent nauseag | 25 (5) | |
| Total renal and digestive | 114 (23) | |
| Neurological | Weakness or inability to move arms or legs | 54 (11) |
| Hypoesthesia | 40 (8) | |
| Balance disorders | 76 (15) | |
| Dysphagia or chewing difficulties | 29 (6) | |
| Anosmia or ageusia | 25 (5) | |
| Speech disordersh | 41 (8) | |
| Epilepsyi | 38 (8) | |
| Total neurological | 168 (33) |
- a Survivors were classified as having a chronic health condition if at least one of the respective symptoms/diseases were reported.
- b Missings were coded as absence of the respective condition.
- c Requiring follow-up by a physician.
- d For more than 3 months.
- e Controlled with diet or medication.
- f Unilateral or bilateral.
- g Without a clear cause.
- h Including stammering or stuttering.
- i Including convulsions and blackouts.
3.1 Body image in adolescent childhood cancer survivors and their siblings
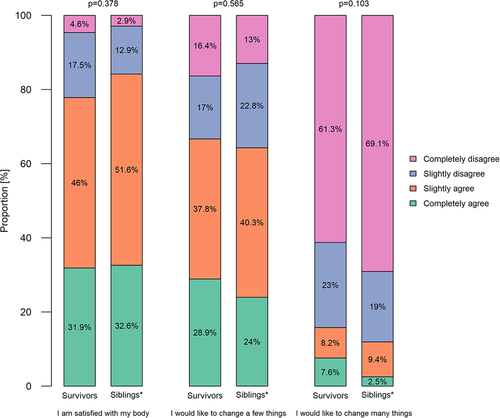
Survivors and siblings reported overall comparable body image (Figure 2; all p-values from chi-square tests and ordered logistic regression >.05). Few survivors (4.6%) and siblings (2.9%) completely disagreed with the statement I am satisfied with my body image. Slightly more survivors than siblings completely agreed with the statements I would like to change a few things regarding my body (28.9% vs. 24.0%) and I would like to change many things regarding my body (7.6% vs. 2.5%).
3.2 Determinants of a more negative body image in survivors
In terms of socio-demographic determinants, we found that female survivors were more likely to have a negative body image compared to male survivors (Table 3). This was evident for all three body image statements (all odds ratios [ORs] ≥1.7). After adjustment for age at study and sex, we further found that survivors from French- or Italian-speaking Switzerland (OR = 1.6, 95% confidence interval [CI]: 1.1–2.3) and those with a migration background (OR = 1.5, 95% CI: 1.0–2.1) more often would like to change many things regarding their bodies. We identified cancer treatment as the most important clinical determinant. Compared to surgery only, survivors that received HSCT were more likely to have a negative body image. This was observed for all three body image statements (all ORs ≥2.2). Similar findings were observed including cancer treatments as binary variables (Table S2). CNS tumour survivors were less often satisfied with their body image compared to leukaemia survivors (OR = 1.6, 95% CI: 1.0–2.7).
| I am satisfied with my body (n = 496) | I would like to change a few things (n = 495)a | I would like to change many things (n = 475)a | ||||
|---|---|---|---|---|---|---|
| Crude OR (95% CI)b | Adjustedc OR (95% CI)b | Crude OR (95% CI)b | Adjustedc OR (95% CI)b | Crude OR (95% CI)b | Adjustedc OR (95% CI)b | |
| Socio-demographic characteristics | ||||||
| Age at study | ||||||
| 16–17 years | ref | – | ref | – | ref | – |
| 18 years | 1.3 (0.9–2.0) | 1.3 (0.9–1.9) | 1.1 (0.7–1.6) | |||
| 19 years | 1.8 (1.1–2.8) | 1.1 (0.7–1.7) | 1.1 (0.7–1.9) | |||
| Sex | ||||||
| Male | ref | – | ref | – | ref | – |
| Female | 1.9 (1.3–2.6) | 1.7 (1.2–2.3) | 1.9 (1.3–2.7) | |||
| Language region | ||||||
| German | ref | ref | ref | ref | ref | ref |
| French or Italian | 1.1 (0.8–1.6) | 1.2 (0.8–1.7) | 0.9 (0.7–1.3) | 1.0 (0.7–1.4) | 1.5 (1.0–2.2) | 1.6 (1.1–2.3) |
| Migration background | ||||||
| No | ref | ref | ref | ref | ref | ref |
| Yes | 1.1 (0.8–1.5) | 1.1 (0.8–1.6) | 1.3 (0.9–1.9) | 1.4 (1.0–2.0) | 1.4 (0.9–2.0) | 1.5 (1.0–2.1) |
| Boyfriend/girlfriend | ||||||
| No | ref | ref | ref | ref | ref | ref |
| Yes | 1.0 (0.7–1.4) | 0.8 (0.6–1.2) | 1.0 (0.7–1.4) | 0.9 (0.6–1.3) | 1.0 (0.7–1.5) | 0.9 (0.6–1.3) |
| Clinical characteristics | ||||||
| Age at diagnosis | ||||||
| <5 years | ref | ref | ref | ref | ref | ref |
| 5–10 years | 0.8 (0.5–1.1) | 0.8 (0.5–1.2) | 0.8 (0.5–1.2) | 0.8 (0.6–1.2) | 0.7 (0.5–1.1) | 0.8 (0.5–1.2) |
| >10 years | 1.1 (0.7–1.7) | 1.0 (0.6–1.5) | 1.2 (0.8–1.8) | 1.2 (0.8–1.9) | 1.1 (0.7–1.7) | 1.1 (0.7–1.8) |
| Diagnosis (ICCC-3) | ||||||
| Leukaemia | ref | ref | ref | ref | ref | ref |
| Lymphoma | 1.3 (0.8–2.2) | 1.3 (0.8–2.2) | 1.0 (0.6–1.7) | 1.1 (0.6–1.8) | 0.8 (0.5–1.5) | 0.9 (0.5–1.6) |
| CNS tumour | 1.5 (0.9–2.5) | 1.6 (1.0–2.7) | 1.2 (0.8–2.0) | 1.3 (0.8–2.2) | 1.1 (0.6–1.9) | 1.2 (0.7–2.1) |
| Bone tumour/soft tissue sarcoma | 1.1 (0.6–2.1) | 1.1 (0.6–2.0) | 1.2 (0.7–2.2) | 1.2 (0.6–2.2) | 0.8 (0.4–1.6) | 0.8 (0.4–1.6) |
| Other tumour | 1.1 (0.7–1.8) | 1.1 (0.7–1.8) | 1.0 (0.7–1.5) | 1.0 (0.7–1.5) | 1.0 (0.6–1.6) | 1.0 (0.7–1.7) |
| Treatment | ||||||
| Surgery | ref | ref | ref | ref | ref | ref |
| Chemotherapy | 1.4 (0.8–2.2) | 1.3 (0.8–2.1) | 1.0 (0.6–1.6) | 1.0 (0.6–1.6) | 1.1 (0.6–1.9) | 1.0 (0.6–1.8) |
| Radiotherapy | 1.6 (0.9–2.7) | 1.5 (0.8–2.6) | 1.2 (0.7–2.0) | 1.1 (0.7–1.9) | 1.5 (0.8–2.7) | 1.3 (0.7–2.4) |
| HSCT | 2.7 (1.2–6.1) | 2.5 (1.1–5.7) | 2.3 (1.0–5.1) | 2.2 (1.0–4.9) | 3.2 (1.4–7.5) | 2.9 (1.3–6.8) |
| Time since diagnosis | ||||||
| <10 years | ref | ref | ref | ref | ref | ref |
| 10–15 years | 0.8 (0.5–1.1) | 0.8 (0.6–1.2) | 0.8 (0.6–1.2) | 0.8 (0.6–1.2) | 0.9 (0.6–1.4) | 1.0 (0.6–1.5) |
| >15 years | 1.1 (0.7–1.6) | 1.0 (0.7–1.6) | 0.9 (0.6–1.3) | 0.8 (0.5–1.3) | 0.9 (0.6–1.5) | 0.9 (0.6–1.5) |
| History of relapse | ||||||
| No | ref | ref | ref | ref | ref | ref |
| Yes | 1.2 (0.7–2.0) | 1.2 (0.7–2.2) | 1.0 (0.6–1.7) | 1.0 (0.6–1.8) | 1.1 (0.6–2.0) | 1.2 (0.7–2.2) |
- Note: Statistically significant associations at p < .05 are highlighted in bold.
- Abbreviations: CI, confidence interval; CNS, central nervous system; HSCT, haematopoietic stem cell transplantation; ICCC-3, International Classification of Childhood Cancer - Third Edition; OR, odds ratio; ref, reference group.
- a Level of agreement reverse coded.
- b Odds ratio from ordered logistic regression models: OR >1 indicate a higher likelihood of a negative body image. OR <1 indicate a lower likelihood of a negative body image.
- c Adjusted for age at study and sex.
Survivors with more than two chronic health conditions were more likely to have a negative body image compared to survivors without chronic health conditions (Table 4). This was observed for all three body image statements (all ORs ≥1.5). Survivors suffering from musculoskeletal and endocrine conditions consistently reported a more negative body image compared to survivors without such conditions. Among endocrine conditions, we found particularly pronounced associations for growth hormone deficiency. Survivors with growth hormone deficiency were less often satisfied with their body image (OR = 3.4, 95% CI: 1.9–6.0) and more often would like to change few (OR = 2.2, 95% CI: 1.3–3.9) or many things regarding their bodies (OR = 2.3, 95% CI: 1.3–4.1). Survivors with renal and digestive conditions more often would like to change few things regarding their bodies (OR = 1.6, 95% CI: 1.1–2.4). Survivors with neurological conditions were less often satisfied with their body image (OR = 1.4, 95% CI: 1.0–2.0). These associations remained virtually unchanged after adjustment for age at study and sex.
| I am satisfied with my body (n = 496) | I would like to change a few things (n = 495)a | I would like to change many things (n = 475)a | ||||
|---|---|---|---|---|---|---|
| Crude OR (95% CI)b | Adjustedc OR (95% CI)b | Crude OR (95% CI)b | Adjustedc OR (95% CI)b | Crude OR (95% CI)b | Adjustedc OR (95% CI)b | |
| Chronic health conditions | ||||||
| Number of chronic health conditions | ||||||
| 0 | ref | ref | ref | ref | ref | ref |
| 1 | 1.2 (0.8–1.8) | 1.1 (0.7–1.7) | 1.1 (0.8–1.7) | 1.1 (0.7–1.7) | 0.8 (0.5–1.3) | 0.8 (0.5–1.2) |
| ≥2 | 1.9 (1.3–2.8) | 1.6 (1.1–2.4) | 1.6 (1.1–2.4) | 1.5 (1.0–2.2) | 1.5 (1.0–2.4) | 1.4 (0.9–2.1) |
| Cardiovascular conditions | ||||||
| No | ref | ref | ref | ref | ref | ref |
| Yes | 1.5 (0.8–2.9) | 1.6 (0.8–3.0) | 0.9 (0.5–1.6) | 0.9 (0.5–1.6) | 1.1 (0.6–2.2) | 1.1 (0.6–2.2) |
| Pulmonary conditions | ||||||
| No | ref | ref | ref | ref | ref | ref |
| Yes | 1.0 (0.7–1.5) | 1.0 (0.7–1.4) | 1.0 (0.7–1.4) | 1.0 (0.7–1.4) | 1.0 (0.6–1.5) | 0.9 (0.6–1.4) |
| Endocrine conditions | ||||||
| No | ref | ref | ref | ref | ref | ref |
| Yes | 1.7 (1.1–2.7) | 1.7 (1.1–2.6) | 1.7 (1.1–2.6) | 1.7 (1.1–2.6) | 1.5 (1.0–2.4) | 1.5 (0.9–2.4) |
| Growth hormone deficiency | ||||||
| No | ref | ref | ref | ref | ref | ref |
| Yes | 3.4 (1.9–6.0) | 3.6 (2.0–6.4) | 2.2 (1.3–3.9) | 2.4 (1.4–4.1) | 2.3 (1.3–4.1) | 2.4 (1.3–4.3) |
| Hearing impairment | ||||||
| No | ref | ref | ref | ref | ref | ref |
| Yes | 1.2 (0.8–2.0) | 1.2 (0.7–2.0) | 1.1 (0.7–1.9) | 1.1 (0.7–1.9) | 1.6 (0.9–2.7) | 1.6 (0.9–2.7) |
| Vision impairment | ||||||
| No | ref | ref | ref | ref | ref | ref |
| Yes | 1.2 (0.8–1.9) | 1.2 (0.8–1.9) | 1.2 (0.8–1.9) | 1.1 (0.7–1.8) | 1.9 (1.2–3.0) | 1.9 (1.2–3.0) |
| Musculoskeletal conditions | ||||||
| No | ref | ref | ref | ref | ref | ref |
| Yes | 2.1 (1.4–3.2) | 2.0 (1.3–3.0) | 1.7 (1.2–2.6) | 1.6 (1.1–2.4) | 2.3 (1.5–3.4) | 2.1 (1.4–3.2) |
| Renal and digestive conditions | ||||||
| No | ref | ref | ref | ref | ref | ref |
| Yes | 1.5 (1.0–2.3) | 1.3 (0.9–2.0) | 1.6 (1.1–2.4) | 1.5 (1.0–2.2) | 1.3 (0.8–1.9) | 1.1 (0.7–1.7) |
| Neurological conditions | ||||||
| No | ref | ref | ref | ref | ref | ref |
| Yes | 1.4 (1.0–2.0) | 1.3 (1.0–1.9) | 1.2 (0.9–1.8) | 1.2 (0.9–1.7) | 1.2 (0.8–1.8) | 1.2 (0.8–1.7) |
- Note: Statistically significant associations at p < .05 are highlighted in bold.
- Abbreviations: CI, confidence interval; OR, odds ratio; ref, reference group.
- a Level of agreement reverse coded.
- b Odds ratio from ordered logistic regression models: OR >1 indicate a higher likelihood of a negative body image. OR <1 indicate a lower likelihood of a negative body image.
- c Adjusted for age at study and sex.
4 DISCUSSION
This nationwide population-based study showed that the body image of adolescent survivors of childhood cancer was overall comparable to that of their healthy siblings. Female survivors, survivors treated with HSCT and those with a higher burden of chronic health conditions had a more negative body image. This was particularly pronounced for survivors suffering from musculoskeletal or endocrine conditions.
This is one of the few population-based studies investigating the body image of adolescent survivors of childhood cancer. Although cancer and treatment-related changes in appearance and the high prevalence of physical late consequences after successful treatment would be expected to adversely interfere with survivors’ body image,4 our study showed that the body image of adolescent survivors was overall comparable to that of their healthy siblings. This is in line with a study from the Netherlands including adult survivors9 and a previous systematic review including quantitative and qualitative studies and patients on active treatment.13
Similar to a recent study from Sweden including adult childhood cancer survivors35 and findings in healthy adolescents in the general population of Switzerland,29 we found that female survivors were more likely to have a more negative body image than male survivors. This confirms the extensive literature on sex differences in body image, indicating that females are more likely to negatively self-evaluate their appearance and report higher levels of body image dissatisfaction than men.14 Societal pressures and attractive body image expectations may be particularly pronounced in Western cultures.21 Our findings indicated that survivors from French- or Italian-speaking Switzerland were more likely to report a negative body image than survivors from German-speaking parts. This may be explained by the subjective nature of body image perceptions and satisfaction that are influenced by societal or cultural norms and environmental experiences.14
We further found survivors treated with HSCT were more likely to have a negative body image. HSCT is often used for high-risk disease or as second-line treatment.36 Physical side effects and toxicity may occur at various stages of HSCT treatment from intensive pretransplantation therapy, conditioning regimens, chronic immunosuppression, and acute or chronic graft-versus-host disease (GvHD).37 Transplanted survivors may also experience unique late consequences, such as delayed immune reconstitution leading to recurrent infections and chronic dermatologic conditions as a result of chronic GvHD of the skin that may adversely interfere with their body image in the long-term.37 However, our study included only 30 survivors treated with HSCT, and more research is needed to elucidate the underlying mechanisms.
Chronic health conditions were an important determinant of a more negative body image in our study. Even though we included mild chronic conditions also, we found that the more conditions adolescent survivors experienced, the higher was their risk to report a negative body image. This was particularly pronounced for survivors suffering from musculoskeletal or endocrine conditions. Musculoskeletal conditions such as prolonged pain in bones or joints, scoliosis, or reduced flexibility of joints are visible disabilities and likely to interfere with survivors’ everyday life compared to non-visible conditions such as cardiovascular or pulmonary diseases. Indeed, qualitative research indicated that childhood cancer survivors felt negatively about their bodies because of the visible effects of the treatment such as hair loss, weight gain, scarring or amputations.9, 15-19 However, in terms of long-term psychosocial adjustment, Thompson and Wiener argue that adolescents’ subjective perception may be more important than their actual appearance.14 Further quantitative and longitudinal research using consistent and validated body image measures is needed to further elaborate on this.
The most common endocrine condition in our study included growth hormone deficiency that may cause short stature, and hypo- and hyperthyroidism leading to hormonal imbalance.38 Such conditions may directly interfere with survivors’ body image and psycho-sexual development, particularly during adolescence. Indeed, we found a strong association between growth hormone deficiency and a more negative body image. Treatment with recombinant human growth hormones significantly improves height in children with growth hormone deficiency; however, affected children may still not achieve their genetic potential.38 Endocrine problems and hormonal imbalances may further interfere with pubertal development. Reduced breast development may to some extent also explain the more negative body image of female survivors compared to male survivors.
Collectively, our findings highlight that healthcare professionals should be aware of the risk of body image concerns in survivors with a high burden of chronic health conditions and therefore address this problem during follow-up care.39 Additional support and counselling by an interdisciplinary team involving psychologists may help affected survivors to reduce body image concerns. This is of particular importance, as an adverse body image has been previously shown to be associated with psychological distress21 and sexual dysfunction20 in childhood cancer survivors. In turn, this may affect survivors’ involvement in intimate relationship and family planning and their quality of life in the long-term.9 While somatic health conditions after childhood cancer are usually well cared for during long-term follow-up care, this may be less standardized for aspects related to mental health such as body image concerns.40 In the literature, most body image interventions, such as cognitive behavioural therapy, education-based intervention, strength training, and physical exercise, have been implemented among survivors of breast cancer and it remains unclear whether such approaches would be efficacious in the childhood cancer survivor population.21 A promising approach is the recently established Fex-Can Childhood project that includes an interventional approach to advance knowledge in the areas of sexual function and fertility-related distress after childhood cancer including body image as a secondary outcome.41 If proven efficacious and successfully implemented in survivorship care, such an approach could be particularly beneficial for adolescent survivors with body image concerns and may contribute to mitigate adverse long-term consequences.
A limitation of our study is the relatively small number of siblings. Sibling comparisons are valuable as they offer a possibility to control for possible confounders such as socio-economic background,32 and we further maximized comparability by standardizing for age at study and sex. However, our study may have lacked the statistical power to detect small differences between groups. Another limitation may be reporting bias due to social desirability.42 Survivors may have reported more favourable outcomes and our study therefore may have underestimated the implications of the cancer diagnosis and related chronic health conditions on survivors’ body image. However, this may to some extent also apply to sibling comparisons. Finally, agreement with the statement I would like to change a few things regarding my body should not necessarily be interpreted as an adverse outcome but should be seen in light of the cultural and societal context. A strength of our study is the nationwide and population-based sampling approach and the high response rate that supports the representativeness of our study population. We have previously shown that non-response bias may only play a minor role in Swiss childhood cancer survivor studies.43 In our study, we covered all childhood cancer types and treatment periods from 1976 to 2010. Another strength refers to the use of high-quality clinical information based on medical records from the SCCR and the assessment of chronic health conditions based on standardized questions used in other childhood cancer survivor studies.25, 26
In conclusion, it is encouraging that the body image of adolescent childhood cancer survivors was comparable to healthy siblings. However, female survivors, survivors treated with HSCT or a high burden of chronic health conditions are at risk of body image concerns during adolescence. Increased awareness among clinicians, discussing body image aspects during regular follow-up care, and offering targeted psychosocial support could mitigate such concerns.
ACKNOWLEDGEMENTS
We thank all survivors and siblings for participating in our survey, the study team of the Swiss Childhood Cancer Survivor Study, the data managers of the Swiss Paediatric Oncology Group and the team of the Swiss Childhood Cancer Registry. This study was supported by the Swiss Cancer League and Swiss Cancer Research (KLS/KFS-4825-01-2019, KFS-4722-02-2019, KFS-5027-02-2020), Kinderkrebshilfe Schweiz, Childhood Cancer Switzerland, and the University of Basel Research Fund for Excellent Junior Researchers.
Open Access Funding provided by Universitat Bern
[Correction added on 25 November 2022, after first online publication: CSAL funding statement has been added.]
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the information of this manuscript were accessed on secured servers of the Institute of Social and Preventive Medicine at the University of Bern. Individual-level sensitive data can only be made available for researchers who fulfil the respective legal requirements. All data requests should be communicated to the corresponding author.




