Unveiling the Impact of Downscale Reactions on P3HT Synthesis: A Comprehensive Exploration of Properties and Photovoltaic Device Performance
Funding: This work was supported by the Brazilian Council for Scientific and Technological Development (CNPq), the Coordination for the Improvement of Higher Education Personnel (CAPES), the Carlos Chagas Filho Foundation for Supporting Research in the State of Rio de Janeiro (FAPERJ), the São Paulo State Research Support Foundation (FAPESP), and Fraunhofer IAP.
ABSTRACT
In photovoltaic polymer synthesis research, it is routine to adapt chemical reactions while respecting the stoichiometric proportions of the reagents. Generally, researchers reduce the amounts of chemical reagents to save materials or adjust the necessary quantity according to the need to avoid waste, as these materials undergo chemical changes and alterations in their properties over time, compromising the performance of their photovoltaic devices. P3HT is considered a reference in the field of solar cells. This study demonstrated that downscaling P3HT synthesis significantly influences the optoelectronic properties and photovoltaic device performance. To further investigate this effect, P3HT devices were fabricated on large-area substrates with varying pixel sizes, allowing for a comprehensive assessment of the impact of laboratory-scale synthesis reductions and their potential implications for market applications. The scale-down synthesis with 50% of the reaction system decreases optoelectronic properties and the performance of a photovoltaic device. The standard synthesis performed in duplicate also demonstrated devices with efficiency similar to the scale-down method. Synthesis using proportions close to 3.5 g of monomer resulted in better optoelectronic properties, molar mass, and devices with efficiencies of up to 3.04% ± 0.11% for a 0.16 cm2 pixel and 2.23% ± 0.14% for a large area pixel of 1 cm2.
1 Introduction
In recent years, the field of photovoltaic polymer synthesis has gained significant attention due to its potential to revolutionize solar energy conversion [1]. Photovoltaic polymers, with their tunable optoelectronic properties, offer a promising alternative to traditional silicon-based solar cells [2]. Simple structures composed solely of combinations of different monosubstituted thiophenes have already reached power conversion efficiencies above 17% [3, 4]. Among these materials, poly(3-hexylthiophene) (P3HT) has emerged as a reference material, widely recognized for its excellent photovoltaic performance and better stability [4, 5]. Understanding the synthesis and optimization of P3HT is crucial for advancing the efficiency and applicability of polymer-based solar cells.
P3HT is prominent in the photovoltaic community due to its balanced combination of electrical conductivity, thermal stability, and ease of processing [6]. In the active layer of OPV devices, this donor already achieves energy conversion efficiencies of up to 10% depending on the choice of electron acceptor, layer combination, pixel size (typically fabricated with small pixel sizes, often < 0.04 cm2, in laboratory-scale research), thermal or solvent treatments, solvent additives, or substrate geometry [7]. The combination of P3HT:PCxBM ([6,6]-phenyl-Cx-butyric acid methyl ester) is widely studied due to its stability, favorable cost–benefit ratio, and ease of device preparation [7, 8]. However, the synthesis of P3HT and similar polymers often involves meticulous adjustments to the chemical reactions to ensure the desired stoichiometric proportions of reagents [9]. Researchers typically reduce the amounts of chemical reagents to conserve materials and adjust the quantities as needed to avoid waste. These practices are not only economically beneficial but also critical in maintaining the integrity of the materials, as they undergo chemical changes and property alterations over time that can compromise device performance [8, 10-12]. Although this practice is very common in laboratories, it is rarely reported in the literature, and there are few, if any, studies focusing specifically on this issue [9].
It is already established in the literature that changes in synthesis can significantly affect the molar mass, crystallinity, and overall efficiency of the polymer [12-19]. The synthesis of P3HT by the Grignard metathesis (GRIM method) using Bannock's methodology [20] is interesting because it does not require subzero temperatures and still obtains a P3HT with high molar mass and regioregularity. One of the primary challenges in photovoltaic polymer synthesis is maintaining the material's optoelectronic properties while scaling down or scaling up the synthesis process. In this study, we investigate the impact of reducing the synthesis scale of P3HT on its optoelectronic properties and the performance of the resulting photovoltaic devices.
2 Experimental
2.1 Synthesis of Poly(3-Hexylthiophene) (P3HT)
- P3HT(2×): two different flasks containing each one 4 g (12 mmol) of monomer and 9.1 mL (11.8 mmol) of iPrMgCl in 40 mL of THF, and then after 30 min 10 mg (0.0184 mmol) of Ni(dppp)Cl2 in 16 mL of THF. The final product of the two flasks was a mixture.
- P3HT(2 g): 2 g (6 mmol) of monomer and 4.55 mL (5.9 mmol) of iPrMgCl in 20 mL of THF, and then after 30 min, 5 mg (0.0092 mmol) of Ni(dppp)Cl2 in 8 mL of THF.
- P3HT(3 g): 3 g (9 mmol) of monomer and 6.83 mL (8.58 mmol) of iPrMgCl in 30 mL of THF, and then after 30 min, 7.5 mg (0.0138 mmol) of Ni(dppp)Cl2 in 12 mL of THF.
- P3HT(3.5 g): 3.5 g (10.5 mmol) of monomer and 7.99 mL (10.47 mmol) of iPrMgCl in 35 mL of THF, and then after 30 min, 8.75 mg (0.0162 mmol) of Ni(dppp)Cl2 in 14 mL of THF.
For consistency in the analysis, the material tested in operation was from the same batch. Multiple syntheses were conducted and combined to ensure the analyzed product matched the one tested in operation. Specifically, the P3HT(2×) sample involved synthesizing two parallel reactions and mixing the products. To avoid material waste and degradation over time, syntheses were proportionally reduced while preserving stoichiometry.
The synthesized samples were prepared based on the following requirements.
P3HT(2×): two independent syntheses were conducted, as described above. Following polymer precipitation, the resulting products were amalgamated to assess system reproducibility when employing duplicates and combining products.
P3HT(2 g): the reactional system was reduced by half, using 2 g of monomer with recalculated reagents to maintain stoichiometry. This evaluated reproducibility in cases of proportional reduction, a common laboratory practice.
P3HT(3 g) and P3HT(3.5 g): similar to P3HT(2 g), these samples used 3 g and 3.5 g of monomer, respectively, to assess the impact of different monomer quantities on synthesis outcomes.
This approach allowed for a thorough examination of how reducing the synthesis scale affects the optoelectronic properties and performance of P3HT in photovoltaic devices. This question may seem simple, as it is routine practice in laboratories. However, its influence on the reproducibility of results in different proportions is often overlooked. In other words, conventional P3HT reactions were conducted at 50%, 75%, and 87.5% of the standard reaction scale. P3HT is an excellent candidate for this type of evaluation due to its well-established role as a reference semiconductor polymer in the field of OPV solar cells.
2.2 Calculation of Yield of Synthesized P3HT
In the synthesis of P3HT, 1 mol of 2,5-dibromo-3-hexylthiophene (326.09 g) undergoes debromination, leaving 166.28 g available for product formation (50.99% theoretical yield). For a 4 g reaction, this corresponds to 2.04 g. Considering the 4:1 regioisomer ratio in the Grignard reagent, only 80% contributes to polymerization, adjusting the expected yield to 1.63 g at 100% efficiency. Final rounding was applied to minimize propagation errors. In the synthesis of P3HT(2 g), the theoretical 100% yield is 0.816 g, a value applicable to other samples as well. In this study, the reaction yield is determined by the amount of polymer extracted in chloroform. Yield calculations based solely on the precipitated product often lead to overestimation, as the extraction process also removes catalyst residues, monomers, oligomers, and insoluble byproducts, inflating the measured mass.
2.3 Materials Characterization
2.3.1 1H-Nuclear Magnetic Resonance (NMR)
The analysis was carried out on Bruker equipment, with a frequency of 400 MHz, at IQ-UFRJ. The samples (10 mg of polymer) were solubilized in deuterated chloroform and transferred to a 5 mm tube, and trimethylsilane (TMS) was added as an internal reference. Analysis was performed in duplicate.
2.3.2 Fourier-Transform Infrared Spectroscopy (FT-IR)
FT-IR analysis was performed to characterize the chemical structure of each sample by identifying the functional groups present. The FT-IR spectrum was carried out on KBr pellets in a range between 4000 and 600 cm−1, with a resolution of 4 cm−1.
2.3.3 Gel Permeation Chromatography (GPC)
Characterizing the molar mass of synthesized polymers is very important since the properties of these materials are directly related to the size of the chains. High molar mass values contribute to a greater extension of π conjugations and, consequently, better charge transport. However, very high molar masses can hinder the solubility and processing of device layers. Therefore, molar mass control becomes extremely essential for this research. Through GPC, it is possible to acquire parameters related to molar mass, such as the number-average (Mn) and the weight average (Mw). The dispersity (Ð) is obtained through the Mw/Mn ratio. The equipment used was the Waters 150 CV gel permeation chromatograph, in which the calibration curve will be obtained with polystyrene standards of known molar mass, determined using an absolute method. The samples were prepared using 2–5 mg of the polymer dissolved in chloroform. Conventional and high-temperature GPC were also carried out in an Agilent1260 Infinity II High-Temperature GPC System coupled to a refractive index detector, with Agilent PL gel 10 μm Mixed-B 300 × 7.5 mm column (PN: PL1110-6100), the flow rate of 1.0 mL/min, temperature 160°C. Analysis was performed in duplicate.
2.3.4 Thermogravimetric Analysis (TGA)
The thermogravimetry was performed at a heating rate of 10°C min−1, under nitrogen flow at 50 mL/min. From the curves obtained, the onset temperature of the thermal events (Tonset), the temperature at which mass loss occurs at the maximum rate (Tmax), and the temperature at which 5% and 10% mass loss occur (T5% and T10%) were determined. Furthermore, the moisture content was calculated by measuring mass loss within the temperature range of 25°C–125°C. The residual percentage of the sample was determined to be at 700°C. Analysis was performed in duplicate.
2.3.5 Differential Scanning Calorimetry (DSC)
2.3.6 X-Ray Diffraction (XRD)
2.3.7 Ultraviolet–Visible Spectroscopy (UV–Vis)
In the present work, the wavelength value determined by the tangent of the absorbance peak (λonset) was used, representing the lowest energy value necessary to cause an electronic transition. Analysis was performed in duplicate.
2.3.8 Cyclic Voltammetry (CV)
Cyclic voltammetry analysis consists of the evaluation of an electrochemical system composed of an electrolyte (solution of tetrabutylammonium hexafluorophosphate (Bu4NPF6)) in 0.1 M acetonitrile, reference electrode (Ag/AgCl), counter-electrode (platinum wire), and working electrode (platinum disc). The polymer in solution at a 5 mg/mL concentration was deposited on the working electrode, forming a thin film. The electrochemical experiments were performed using a Metrohm Autolab potentiostat/galvanostat equipped with a high-power booster. Measurements were conducted over a potential range from −1.5 V to either 1.5 or 2.0 V, with the scan initiated at 0 V. The system operated with a bandwidth of 1 MHz and a potential sweep rate of 50 mV/s. The maximum positive voltage applied was chosen based on the stability of the samples and the need to ensure the reproducibility of the analyses. Color changes observed in the P3HT(2 g) and P3HT(3 g) samples indicate a compromise in material stability when voltages exceeding +1.5 V were applied. This technique allows the oxidation and reduction potentials of the films to be obtained from the onset point of the curves. Using these values, the HOMO and LUMO energy levels were estimated, along with the difference between them, determining the electrochemical bandgap (EgEC).
2.4 Manufacturing of Photovoltaic Devices
The devices were manufactured in a clean room. The photovoltaic devices were prepared in conventional architecture (Glass/ITO/PEDOT:PSS/active layer/Ca/Ag).
- 2% Helmanex II solution at 70°C for 15 min, then rinsed with deionized water.
- Sonicated in deionized water at 80°C for 5 min.
- Sonicated in acetone for 5 min (room temperature).
- Sonicated in isopropanol at 50°C for 5 min.
- Sonicated in deionized water at 80°C for 5 min.
The cleaned substrates were dried under a nitrogen stream and immediately transferred to the plasma treatment chamber. Plasma treatment was performed for both cleaning and hydrophilization. Initially, the substrates were placed under vacuum inside the chamber for 5 min, followed by exposure to an ozone atmosphere for another 5 min. Finally, they underwent plasma treatment for 200 s.
- 10 s at 500 rpm, depositing the solution.
- Acceleration ramp from 500 rpm/s to 2000 rpm and maintained for 60 s. Immediately afterward, the contacts are cleaned using a cotton swab.
Then, the substrate is stored on a hot plate at 100°C. When all substrates were deposited, annealing was carried out at 150°C for 15 min. After this period, the substrate was transferred directly to the glove box and heated at 150°C for 10 min to remove residual surface moisture. It is important to mention that although PEDOT:PSS is generally stable, it can absorb atmospheric moisture, leading to solvent vapor annealing. This process induces the formation of an insulating PSS layer on the surface, altering morphology and reducing conductivity. The HTL had a thickness of approximately 40 nm, as confirmed by profilometry.
The 20 mg/mL P3HT:PC61BM (1:1 m\m) solution was prepared using a solvent mixture (CHCl3:CB, 4:1 v\v). P3HT and PC61BM were weighed outside the glove box, and an antechamber vacuum was used to remove moisture. The solution was prepared inside the glove box using an anhydrous solvent, and the vial was then sealed. After that, the solutions were heated to 60°C overnight and then ultrasonicated for 30 min at 60°C. The solution was cooled to room temperature and was deposited via spin coating in dynamic mode at 500 rpm for 25 s using 180 μL in a 1 mL micropipette inside a glove box followed by solvent vapor annealing treatment (CHCl3:CB, 4:1) for 5 min and thermal annealing at 140°C for 10 min. Under identical spin-coating conditions, the active layers consistently exhibited a thickness of approximately 200 nm. Finally, contacts are cleaned with a cotton swab with solvent. After that, the substrates are taken to the thermal evaporation chamber. Calcium (Ca, 20 nm) was deposited by thermal evaporation in a high vacuum at 10−6 Torr. Deposition rate: 0.1 Å/s up to 2 nm, then 0.5 Å/s up to 20 nm. The 100 nm-thickness silver metal contact was deposited by thermal evaporation in a high vacuum of 10−6 Torr. The deposition rate was 0.1 Å/s up to 2 nm, 0.5 Å/s up to 20 nm, and 1.0 Å/s up to 100 nm.
2.5 Device Characterization
2.5.1 JV Curves
Current density-voltage (JV) data were measured under nitrogen using a Keithley 2400 source meter with 100 mW/cm2 of simulated solar irradiation (AM 1.5G, K.H Steuernagel light source). A silicon reference diode with a KG3 filter calibrated was used to adjust the light intensity before the measurements. Based on the curves under 1 Sun illumination (100 mW/cm2, standard AM1.5G solar spectrum), the photovoltaic parameters—fill factor (FF), open-circuit voltage (Voc), short-circuit current density (Jsc), and power conversion efficiency (PCE)—were calculated to evaluate the device performance. It is worth noting that the measurements were conducted 1 day after the complete fabrication of the devices. The same substrate contained two different pixel sizes (0.16 and 1.00 cm2), and a shadow mask was applied during the measurements.
2.5.2 Profilometry
Profilometry was employed to measure and control the thickness of the layers used in device fabrication. Thickness measurements were carried out using the Veeco Dektak 150 profilometer. The measurements were taken based on a step created with a scratch using a scalpel blade. At least three measurements in four regions (close to each pixel area) were taken to ensure correct thickness.
3 Results and Discussion
The synthesized P3HT samples were analyzed to evaluate the success of the synthesis and its key properties relevant to photovoltaic device fabrication. Characterization techniques were employed to verify the chemical structure formed, regioregularity, polymer chain size, stability, thermal resistance, crystallography, absorption in the ultraviolet spectrum region, and energy levels of the molecular orbitals. Subsequently, devices were fabricated to correlate these characteristics with the performance of the devices obtained. This section of the study aimed to investigate the primary issues concerning the reproducibility of laboratory syntheses. Table 1 shows the reaction yield for each synthesized P3HT. The synthesis yield decreased with scale reduction, likely due to differences in reaction kinetics and mass transfer. The chloroform-soluble fraction (desired polymer) was highest for P3HT(3.5 g) (66%) and lowest for P3HT(2 g) (34%), indicating that smaller-scale reactions may lead to premature chain termination or side reactions. The insoluble fraction was highest in P3HT(3.5 g) (310 mg) and lowest in P3HT(2 g) (120 mg), suggesting that larger-scale reactions favor higher molecular weight polymers. These variations impact photovoltaic applications, as molecular weight and solubility affect film morphology and charge transport. The results highlight the importance of optimizing synthesis conditions to maintain polymer quality across different scales.
| Sample | Extraction concerning the mass of the reactive monomer | Insoluble after extraction | |||
|---|---|---|---|---|---|
| Hexane | Chloroform | Chlorobenzene | — | ||
| (%) | (%) | (%) | (%) | (mg) | |
| P3HT(2×) | 1.4 | 61 | 0.6 | 24.4 | 795 |
| P3HT(2 g) | 1.7 | 34 | 1.7 | 7.4 | 120 |
| P3HT(3 g) | 1.8 | 56 | 0.5 | 16.5 | 269 |
| P3HT(3.5 g) | 5.6 | 66 | 5.1 | 19.0 | 310 |
3.1 1H Nuclear Magnetic Resonance (NMR) of Synthesized P3HT
1H-NMR was utilized to probe the structure of the polymer formed, particularly focusing on regioregularity (rr). Figure 1 presents the spectra obtained for each sample, highlighting the hydrogen signals at different molecular positions. All materials demonstrated a high degree of purity, as evidenced by well-resolved peaks. The unidentified weak oscillating signals around 7.40, 6.75, 5.75, 5.30, and 3.50 ppm are likely attributed to the possible chain ends, exhibiting low intensity and lack of clear definition.
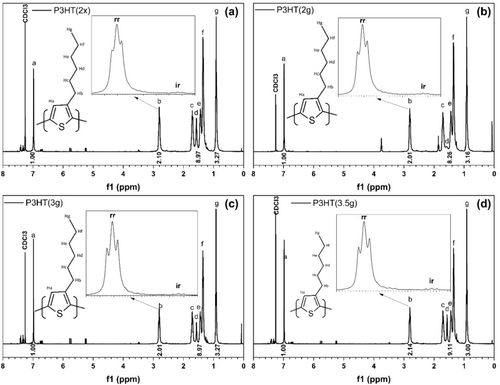
All reactions were quenched by precipitation in ice-cold methanol. Methanol typically undergoes nucleophilic substitution (SN2) with the bromine at the chain end. Consequently, the signal at 7.40 ppm corresponds to the hydrogen of the thiophene ring with bromine at the unreplaced end (Ha). The signal at 3.50 ppm suggests the possible presence of a hydroxyl group at the chain end. The signals at 6.75, 5.75, and 5.30 ppm are associated with the carbonic end hydrogens resulting from the Grignard reagent isopropyl magnesium chloride. These signals related to the chain terminals were appropriately identified based on the work of Li et al. (2014) [27].
Regioregularity refers to the arrangement of a polymer's head-to-tail (HT) linkages during polymerization. Polymers with an HTHT diad configuration generally exhibit greater coplanarity. HH or TT linkages in the polymer chain lead to the spatial proximity of side groups, potentially influencing molecular packing and polymer properties. This steric hindrance leads to distortions in the three-dimensional conformation of the polymer, which act as defects that impede charge transport and photon collection [28]. A high regioregularity favors crystallization processes and optical and electronic properties. In the 1H-NMR spectrum, the HT chain generates peaks with chemical shifts of the methylene protons closest to the thiophene ring between 2.70 and 2.90 ppm, with the most intense central peak value at 2.80 ppm. The HH or TT chain, also known as irregular regioisomer (ir), exhibits chemical shifts of methylene protons between 2.50 and 2.65 ppm, with a peak centered at 2.60 ppm. The ratio of the area relative to the HT chain concerning the sum of the total area of these isomers can be obtained by the percentage of regioregularity of P3HT. The integration of c-f peaks is generally slightly higher than theoretical, as observed in other literature. The integration values were compatible with the samples from Li et al. (2014) [27].
Table 2 displays the regioregularity values of the synthesized materials, all of which exhibit high degrees of regioregularity.
| Sample | rr (%) |
|---|---|
| P3HT(2×) | 97.3 |
| P3HT(2 g) | 96.8 |
| P3HT(3 g) | 97.8 |
| P3HT(3.5 g) | 97.2 |
This suggests their potential suitability for applications in high-performance photovoltaic devices. Notably, P3HT(3 g) demonstrated the highest regioregularity at 97.8%. Although the polymer synthesized using the reduced reaction system experienced a slight 1% decrease, it remains noteworthy that regioregularity values exceeding 95% are still considered excellent for high-performance photovoltaic devices. Enhanced organization and packaging of P3HT chains promote charge transport through π–π stacking, thereby enhancing device efficiency in energy transfer and dissipation [29].
3.2 Fourier-Transform Infrared Spectroscopy (FT-IR) of Synthesized P3HT
FT-IR analysis was conducted on the synthesized P3HT in a complementary manner to NMR to confirm the presence of the main chemical groups in the sample. A sample of commercial polymer, P3HT(C), (Ossila grade M1010: rr = 97.3%, Mn = 35,240, PDI = 2.1) was also analyzed for comparison. Figure 2 delineates the principal bands corresponding to the chemical groups. The spectra exhibit three primary regions: 3100–2800, 1550–1300, and 1000–800 cm−1.
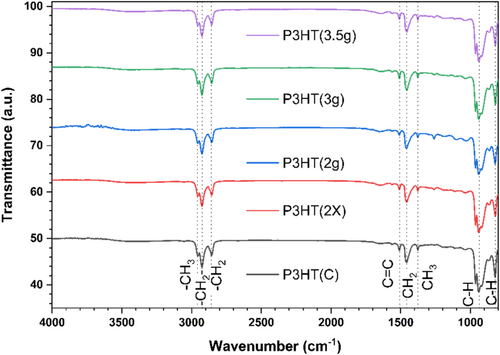
Firstly, at 3056 cm−1, the CH stretching of the thiophene ring is observed [30]. At 2955 cm−1, the CH3 asymmetric stretching; at 2926 cm−1, the CH2 asymmetric stretching; and at 2856 cm−1, the CH2 symmetric stretching relative to the aliphatic branching are present [31]. Concerning the second region, Ramani et al. (2010) attribute the band at 1510 cm−1 to the CC stretching of the thiophene ring [32]. At 1457 and 1377 cm−1, CH2 bending (scissoring mode) and CH3 symmetric bending are present, respectively [32, 33].
At 823 cm−1, a strong band can be observed due to out of plane CH bending, which relates to coplanarity exclusive to high regioregularity P3HT [34]. This result is in line with the high regioregularity values observed in 1H-NMR. The band at 941 cm−1 is less cited, but Ansari et al. (2018) and Hernández-Martínez et al. (2020) state that it is the CH2 rock that is shifted from 1060 cm−1 [35, 36]. The authors associate such vibration not only with the chemical shift, but also with the shape of the band accompanied by weaker vibrations at 920 and 860 cm−1.
3.3 Gel Permeation Chromatography (GPC) of Synthesized P3HT
Gel permeation chromatography (GPC) plays a crucial role in characterizing polymers by providing information on the material's molar mass. This technique enables the inference of modifications in synthesis concerning chain length. Moreover, molar mass directly influences polymer properties, impacting processing and film coating and even delving into molecular orbitals' energetic levels, electrical or thermal conductivity, charge mobility, ultraviolet absorption spectrum profile, and various mechanical, thermal, electrical, and optoelectronic properties [37].
The present study showcases results obtained from weight-average molar mass (Mw), number-average molar mass (Mn), and dispersity (Ð) acquired through conventional GPC and high-temperature GPC (HT-GPC). HT-GPC allows for the measurement of molar mass parameters at elevated temperatures, improving accuracy for materials with limited solubility due to high molar mass or strong aggregation tendencies. Although P3HT is generally considered highly soluble, comparative studies between conventional GPC and HT-GPC remain scarce in the literature.
Table 3 presents the results obtained by conventional and high-temperature GPC analysis. It is observed that reducing the reagent quantities by half, while maintaining the system's stoichiometry, tends to decrease the polymer molar mass. In laboratories, it is common practice to proportionally reduce reagent quantities in synthesis to optimize resource usage and minimize waste. However, in the P3HT(2 g) sample, the average molar mass decreased by nearly half compared to the samples synthesized with 3 and 3.5 g of monomer. The variation in number-average molar mass was approximately 8000–10,000 g/mol compared to the other samples. The highest molar mass was obtained by the sample synthesized with 3 g of monomer, reaching 28,700 and 68,500 g/mol for Mn and Mw, respectively. Conducting two syntheses without altering the stoichiometric ratio and then mixing the products obtained, as in sample P3HT(2×), is another strategy for obtaining larger samples, but it can increase the dispersity.
| Sample | Conventional gel permeation chromatography (GPC) | High-temperature gel permeation chromatography (HT-GPC) | ||||
|---|---|---|---|---|---|---|
| Mn (103 g/mol) | Mw (103 g/mol) | Ð | Mn (103 g/mol) | Mw (103 g/mol) | Ð | |
| P3HT(C) | 35.2a | 74.0 | 2.1 | 28.2 ± 1.1 | 67.4 ± 3.4 | 2.4 ± 0.03 |
| P3HT(2×) | 25.5 ± 1.7 | 61.4 ± 4.4 | 2.3 ± 0.3 | 16.2 ± 0.1 | 42.2 ± 0.7 | 2.6 ± 0.03 |
| P3HT(2 g) | 17.6b | 37.4 | 2.1 | 14.3 ± 0.2 | 30.6 ± 0.1 | 2.1 ± 0.04 |
| P3HT(3 g) | 28.7 | 68.5 | 2.4 | 25.1 ± 3.0 | 50.2 ± 1.7 | 2.0 ± 0.17 |
| P3HT(3.5 g) | 24.8 | 56.4 | 2.3 | 22.5 ± 1.7 | 43.8 ± 1.3 | 2.0 ± 0.21 |
- a Value reported by Ossila Ltd.
- b Analysis performed only once.
All HT-GPC results showed a slight reduction in molar mass, even when compared to the value informed by Ossila company (grade M1010) for its commercial P3HT(C), which has an Mw of 74,000. These differences in results are expected, as increasing the temperature enhances polymer solubility. As a result, the polymer chains disperse more effectively in the solvent, leading to a reduction in hydrodynamic volume. Consequently, as the samples pass through the chromatography column, they behave like smaller molecules due to their reduced hydrodynamic volume. This results in a longer retention time and, consequently, a lower apparent molar mass measurement. Furthermore, based on the results of P3HT synthesized with monomer quantities of 2, 3, and 3.5 g, it can be observed that increasing the amount of monomer tends to reduce the dispersity (Ð). Performing two syntheses and mixing the products tends to increase Ð. This outcome is expected, as mixing two samples with slight variations in molar mass results in a broader chain size distribution curve, indicated by a higher Ð value. A higher Ð value demonstrates greater heterogeneity in polymer chain sizes.
Furthermore, based on the results of P3HT synthesized with varying monomer amounts, it is observed that increasing the monomer quantity tends to reduce Ð. Performing two syntheses and mixing the products tends to increase Ð. It is believed that using a smaller amount of monomer increases the error related to weighing and measuring the volume of reagents, resulting in slight variations in the system. This assumption also holds for mixing products from two syntheses, which generate polymer chains with slightly different sizes due to minor variations. It is important to highlight that even P3HT(2×) presented good molar mass and Ð values for manufacturing high-quality photovoltaic devices.
Even though the concentration of all reactants—monomer, solvent, catalyst, and Grignard reagent—was kept constant across different reaction scales, the molecular weight of P3HT does not increase linearly with the amount of monomer due to intrinsic factors of the polymerization process.
One key factor is the influence of chain termination and transfer events, which can become more pronounced at larger reaction scales. Even with stoichiometry and concentration preserved, slight variations in mixing efficiency, temperature distribution and its gradient, and catalyst reactivity at different reaction scales may lead to differences in polymer growth. Additionally, in scaled-up reactions, localized effects such as viscosity changes and polymer aggregation can subtly influence the reactivity of active sites and the termination of growing chains.
Another point that can be raised is the increased probability of side reactions at larger scales. In the case of P3HT(3.5 g), although the yield is higher, the molecular weight is slightly lower than that of P3HT(3 g). This suggests that side reactions—such as chain transfer to the solvent or impurities (e.g., bromide byproducts or non-active Grignard intermediates)—may become more significant, leading to a reduction in the final molecular weight. Furthermore, differences in reagent diffusion and polymer chain interactions in solution can also contribute to variations in molecular weight, even when overall reaction conditions remain consistent. These results highlight that polymerization outcomes are influenced not only by concentration but also by reaction scale and dynamic factors, reinforcing the complexity of achieving perfectly predictable molecular weight trends in polymer synthesis.
Spoltore et al. (2015) reported that increasing Mn slightly increases open-circuit voltage (Voc). However, Jsc tends to decrease significantly. Thus, the most efficient devices (~3.4%) were manufactured using P3HT:PC61BM (1:1) with Mn ranging between 11 and 45 kg/mol [38]. The GPC method employed was in THF at a controlled 40°C. Additionally, Khan and Ashraf et al. (2019) reported that in polymer:NFA devices of P3HT:O-IDTBR (1:1), the highest efficiency values reached 6.6% and 5.7% using P3HT with Mw of 34 and 64 kg/mol, respectively [39]. Both higher and lower Mw values lead to a reduction in FF and short-circuit current density (Jsc), ultimately decreasing overall device performance. The authors explain that optimal molar mass values are associated with adequate domain sizes, leading to better charge generation efficiencies, as demonstrated in IQE. Therefore, all samples in the present work exhibited excellent molar mass values for OPV devices. Based on the GPC results, both the synthesized and commercial P3HT are expected to yield high-performance devices consistent with literature expectations of achieving between 2%–3% power conversion efficiency (PCE).
3.4 Thermogravimetric Analysis (TGA) of Synthesized P3HT
Thermogravimetric (TG) analysis was used to evaluate the resistance and thermal stability of the synthesized P3HT. Figure 3 illustrates the TG curves and their respective derivatives. The degradation of P3HT occurs in a single step. P3HT(2 g) exhibited degradation at lower temperatures, whereas P3HT(2×) displayed similar behavior but commenced degradation at higher temperatures. Conversely, P3HT synthesized with 3 and 3.5 g of monomer showed gravimetric curves with initial degradation at significantly higher temperatures. While the commercial polymer exhibited an initial degradation profile akin to P3HT(2×), the event with a higher mass loss ratio occurred at higher temperatures. In this context, samples P3HT(3 g) and P3HT(3.5 g) demonstrated superior thermal resistance. These samples also displayed thermal degradation events at well-defined temperatures, with narrower DTG curves and peak temperatures at higher values. This suggests that these materials exhibit greater thermal stability compared to commercial P3HT. Notably, all P3HT variations, including P3HT(2 g) and P3HT(2×), exhibited an onset degradation temperature above 200°C, indicating their suitability for photovoltaic applications.
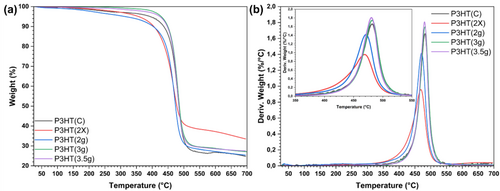
The TG curves of P3HT(2 g), P3HT(2×), and P3HT(C) closely resemble those presented by Milanovich et al. (2020) [40]. Conversely, P3HT(3 g) and P3HT(3.5 g) exhibit similarities to the materials with the highest thermal stability reported by Inque et al. (2019) [41]. In the same study, the authors suggest that mass loss in this lower temperature range is attributed to propagation and chain transfer processes. In a system with reduced scale, unwanted oxidation may occur, leading to decreased resistance and thermal stability. This issue is further exacerbated in large-scale production, where increased solvent usage promotes chain transfer processes.
From the TG and DTG curves, it was possible to quantify some data to clarify the issues of stability and thermal resistance of the materials (Table 4). The moisture content retained in the material is associated with the mass loss observed between room temperature and 125°C. The residue corresponds to the mass remaining after the temperature sweep up to 700°C. The temperature difference between Tmax and T5% provides insight into thermal stability, as it indicates the extent of the material's thermal degradation event. A greater temperature difference suggests lower stability of the material, as it exhibits mass loss over a broader temperature range.
| Sample | Moisture (%) | T5% (°C) | T10% (°C) | Tonset (°C) | Tmax (°C) | Tmax − T5% (°C) | Residue (%) |
|---|---|---|---|---|---|---|---|
| P3HT(C) | 0.5 ± 0.2 | 393 ± 3 | 433 ± 3 | 456 ± 5 | 482 ± 2 | 111 ± 3 | 24.8 ± 0.5 |
| P3HT(2×) | 0.4 ± 0.2 | 355 ± 2 | 405 ± 2 | 429 ± 4 | 469 ± 2 | 114 ± 2 | 33.5 ± 1.0 |
| P3HT(2 g) | 1.0 ± 0.3 | 311 ± 3 | 403 ± 3 | 444 ± 5 | 471 ± 2 | 160 ± 3 | 25.4 ± 0.7 |
| P3HT(3 g) | 0.1 ± 0.2 | 428 ± 3 | 449 ± 2 | 458 ± 5 | 482 ± 3 | 54 ± 3 | 27.1 ± 0.4 |
| P3HT(3.5 g) | 0.3 ± 0.2 | 423 ± 3 | 448 ± 3 | 458 ± 5 | 481 ± 2 | 58 ± 3 | 27.5 ± 0.8 |
Sample P3HT(2 g) exhibits the highest percentage of retained moisture and, simultaneously, the lowest T5%, indicating lower thermal resistance. However, it is noteworthy that the T10% value for P3HT(2 g) is very similar to that of the P3HT(2×) sample. Materials with higher thermal resistance typically exhibit higher temperatures at which degradation begins, as indicated by T5%. Therefore, P3HT synthesized with 3 and 3.5 g of monomer demonstrates superior results compared to the commercial material or the standard synthesis carried out in duplicate. It is speculated that conducting two syntheses and subsequently combining the products results in greater dispersion in the chain sizes with varying thermal resistances, thus leading to increased heterogeneity. This observation also highlights the reproducibility of the synthesis process, as companies often conduct polymerizations in multiple batches to meet customer demands. This hypothesis is commonly accepted in the market and is supported by the data obtained for the commercial P3HT(C) sample, which exhibited values more akin to those of the P3HT(2×) samples.
Calado et al. (2008) reported that P3HT can exhibit up to two thermal mass loss events [42]. The first event is associated with the breakdown of organic side groups stemming from the aliphatic chain of P3HT, while the second event is linked to the rupture of the main chain's CC bonds between the thiophene rings. In certain instances, these processes can manifest as two peaks in the derivative thermogravimetry (DTG). However, in the present study, only a single stage of degradation was observed in DTG. It is hypothesized that, similar to the GPC results, P3HT(2 g) and P3HT(2×) exhibited lower Mn values. Generally, larger chain sizes are associated with better thermal resistance. Likewise, P3HT(2×) may exhibit greater heterogeneity due to the presence of chains with varying lengths, leading to a higher dispersity (Ð) of 2.60 and intermediate thermal properties.
P3HT(3 g) demonstrated the highest thermal stability, as indicated by very narrow values of Tmax − T5%. Analogously, P3HT(3.5 g) showed excellent thermal stability with similar properties. Samples P3HT(C) and P3HT(2×) displayed identical stability. In contrast, the synthesis with the reaction system reduced by half exhibited a degradation range three times broader than the most stable samples and approximately 50% wider than the commercial material. The residue at 700°C was very similar for all samples except for P3HT(2×), which showed a slight increase compared to the others. This suggests that P3HT(2×) produced a higher char residue in the pyrolysis process. During degradation under an inert atmosphere, these materials do not fully decompose but instead form carbon-rich char [41].
3.5 Differential Scanning Calorimetry (DSC) of Synthesized P3HT
DSC was used to determine the thermal transition temperatures and crystallinity of the material. The melting temperature (Tm) was identified by the value of the endothermic peak in the calorimetric curves during the second heating. The glass transition temperature (Tg) was determined using the onset point method of heat flux plateau variation, and it was verified using the first and second derivatives of heat flux as a function of temperature. The crystallization temperature (Tc) was identified by the exothermic peak in the calorimetric curves during the second cooling. The calorimetric curves obtained from the DSC analysis of the synthesized P3HT are shown in Figure 4.
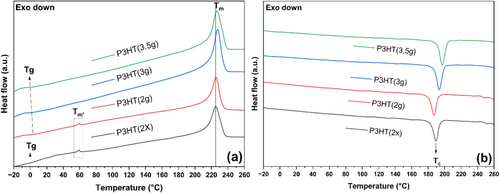
Dattani et al. (2014) obtained calorimetric curves during heating and cooling within this study. In the research, variations in Tg from −9°C to 9°C were reported due to changes in the molar mass of P3HT [43]. The Tc and Tm values were approximately 185°C and 220°C, respectively. Therefore, the data are very similar to those already published in the literature. This pseudo-Tm′ at temperatures of 50°C–60°C is also reported by [20]. This is attributed to the presence of small polymer chains that melt their crystals at much lower temperatures. The cooling calorimetric curves in this study are akin to those demonstrated by Pathiranage et al. (2021), where Tc can fall within ranges of 180°C–200°C, influenced by factors such as crystallinity, end cap, or even the reaction mechanism and reagents used [44].
Table 5 presents data on transition temperatures and the degree of crystallinity of the synthesized P3HT. Mota et al. (2021) obtained P3HT with molecular weights (Mw) between 41 and 58 kg/mol and demonstrated that Tc could reach up to 204°C as Mw increased [45]. This aligns with the results of the present work, as P3HT(3.5 g) exhibited the highest Mw and highest Tc. In this study, materials with molar masses similar to P3HT(2×) and P3HT(2 g) also showed Xc values between 36% and 43%. Performing the synthesis with half the conventional reagent amount (2 g of monomer) resulted in a product exhibiting a reduced degree of crystallinity, lower Tc, and higher Tg. This demonstrates the direct effect of smaller polymer chains, which generate smaller and less dense crystals that require less energy to melt and gain mobility.
| Sample | Tg (°C) | Tc (°C) | Tm (°C) | Xc (%) |
|---|---|---|---|---|
| P3HT(2×) | 0.2 ± 5.0 | 189 ± 2 | 225 ± 2 | 43 ± 3 |
| P3HT(2 g) | 3.0 ± 2.0 | 187 ± 2 | 225 ± 2 | 40 ± 3 |
| P3HT(3 g) | 1.2 ± 2.0 | 193 ± 2 | 228 ± 2 | 52 ± 3 |
| P3HT(3.5 g) | −0.8 ± 2.0 | 197 ± 2 | 227 ± 2 | 61 ± 5 |
Furthermore, the lower Tc suggests a slower crystallization process. For samples P3HT(3 g) and P3HT(3.5 g), a high degree of crystallinity was achieved, accompanied by a slight increase in the melting temperature (Tm). Lee et al. (2021) reported that an increase in regioregularity tends to yield P3HT with a crystallinity of over 50% [28]. In their study, Pascui et al. (2010) reported that the appropriate combination of molar mass, dispersity, and thermal stability can generate P3HT with an Xc of 64% crystallinity [28, 46]. This behavior is attributed to enhanced organization between the crystallites, resulting in the formation of ordered regions at their interfaces. These regions exhibit distinct thermal responses in DSC, reflecting the balance between enthalpic and surface energies that preserves overall stability.
Tg is detected as a stepwise endothermic change in the DSC heat flow or heat capacity. However, for the P3HT(2×) sample, this endothermic transition was very subtle, leading to significant uncertainty in Tg determination, even when using various methods. Furthermore, an inflection of around 18°C was observed for this sample, which, according to Mota et al. (2021), can also indicate a glass transition. In contrast, the other samples exhibited well-defined Tg values that decreased with increasing molar mass [45].
3.6 X-Ray Diffraction (XRD) Analysis of Synthesized P3HT
X-ray diffraction (XRD) was employed to assess the crystallography of the synthesized materials and their CI. Figure 5 illustrates the diffractograms of the P3HT samples. There is a discrepancy in the literature regarding the Bravais lattice that describes the P3HT unit cell, with arguments for both orthorhombic and monoclinic structures. The proposed networks exhibit distinct values for parameters a, b, and c, which in turn lead to discrepancies between them. The orthorhombic structure has all angles equal to 90°, while the monoclinic unit cell has two angles of 90°, and the third angle parameter differs from the right angle. Alberga et al. (2015) utilized computational modeling to ascertain the most stable morphology [47]. In their study, the authors concluded that the monoclinic structure would present an angulation of 85.9°, almost 90°. Orthorhombic structures could also be identified, or even a combination of both. However, due to the presence of the side chain of P3HT, it is more likely that small distortions will occur in the network, generating slight angulations due to the spatial impediment of the alkyl chain during compaction.
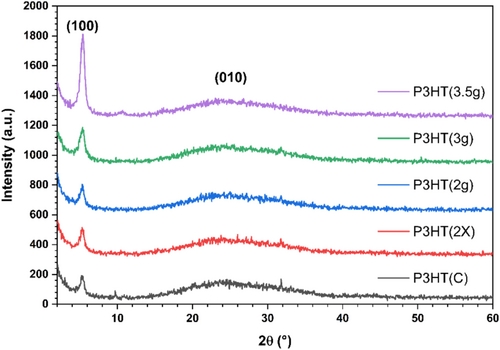
This polymer can adopt three main orientations in relation to the substrate: edge-on, end-on, and face-on. Generally, the most favorable direction is edge-on, followed by face-on. In the analysis conducted with conventional XRD, the diffractograms reveal the (100) and (010) planes out of the plane's edge-on structure. As observed in the diffractogram, the peak around 5.20° is the most intense and corresponds to the π–π stacking of the alkyl chains. The slightly intense plane in (010) corresponds to the π–π stacking between the thiophene rings. The increase in the intensity of these stacks indicates better material organization, bringing the alkyl chains closer together, thereby forming crystals. This favors the transport of intermolecular charges in the direction of stacking the layers of photovoltaic devices, leading to greater photocurrent collection [48]. Other planes that could be identified include (200) and (300) at around 10° and 17°, respectively [49]. However, such weak signals can only be observed in the P3HT(3.5 g) and P3HT(C) samples.
Table 6 presents the data obtained from the deconvolution of the diffractograms of the samples, where 2θ represents the central angle of the peak approximated by the Lorenzian, FWHM stands for full width at half maximum, d(100) and Lc(100) denote the interplanar distance and crystallite size of the (100) plane calculated using Bragg's law and the Scherrer equation, respectively. Additionally, the CI was calculated based on the ratio between the area of the Lorenzian curves of the crystalline peaks and the amorphous halo.
| Sample | 2θ(100) (°) | FWHM(100) (°) | d(100) (nm) | Lc(100) (nm) | CI (%) |
|---|---|---|---|---|---|
| P3HT(C) | 5.25 | 0.8371 | 1.68 | 9.51 | 32.0 |
| P3HT(2×) | 5.25 | 0.8270 | 1.68 | 9.62 | 38.1 |
| P3HT(2 g) | 5.26 | 0.7836 | 1.68 | 10.15 | 40.6 |
| P3HT(3 g) | 5.26 | 0.8593 | 1.68 | 9.25 | 51.9 |
| P3HT(3.5) | 5.33 | 0.6685 | 1.66 | 11.89 | 57.2 |
It can be observed that the materials did not present significant variations concerning the central diffraction angle of the (100) plane, thereby preserving the interplanar distance. The sample P3HT(3.5 g) exhibited a slightly higher interplanar distance, possibly due to the greater molar mass. On the other hand, the width of the peak relative to the (100) plane shows small variations, which directly reflect a larger crystallite size compared to P3HT(C). The increased crystallite size indicates enhanced stacking of the alkyl chains, which results in a more ordered structure and facilitates improved charge mobility toward the electrode. The P3HT(3.5 g) sample displayed a larger crystallite size and a correspondingly higher CI, reaching high values of 57.2% CI. The CI results for the synthesized polymers are consistent with the Xc values obtained by DSC.
Balderrama et al. (2013) report that increasing the intensity of the (100) plane is directly related to improving Jsc in P3HT devices [50]. The increase in packaging toward the electrode tends to improve charge transport, making the device more efficient. Furthermore, the insertion of PCBM into the system also tends to slightly increase d(100). Therefore, it can be suggested that P3HT(3 g) and P3HT(3.5 g) should be the materials among the analyzed samples that could generate devices with slightly higher performance.
3.7 Ultraviolet–Visible Spectroscopy (UV–Vis) of Synthesized P3HT
UV–Vis spectroscopy was employed to investigate the behavior of P3HT concerning its absorption profile in the wavelength range between 350 and 800 nm. The samples were examined as films deposited on a glass substrate. Figure 6a–e shows the individual spectra with normalized UV–Vis intensity of P3HT and then compiled Figure 6f, highlighting the peaks of maximum absorption at 520, 550, and 603 nm.
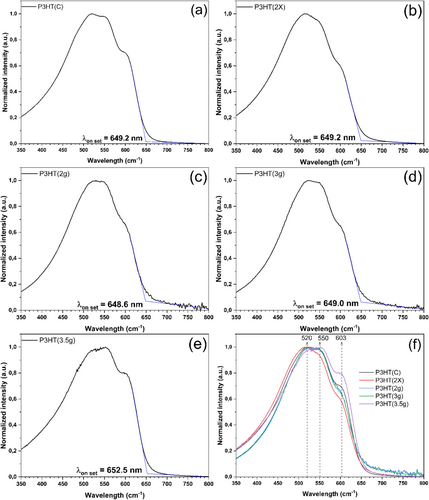
Firstly, all spectra exhibited absorption in the range of approximately 350–700 nm. The spectra of the films closely resemble those presented by Fei et al. (2015) [51]. Additionally, the authors note that UV–Vis analysis conducted in different solvents and at varying temperatures may result in slightly shifted spectra toward shorter wavelengths. Furthermore, the shoulders observed in the peaks at 550/520/603 nm are exclusive to film analysis, providing additional insights into the microcrystalline organization of P3HT, which is characteristic of high regioregularity.
Na et al. (2015) report that the shoulder around 520 nm is related to the intrachain π–π* transition of P3HT [52], while the shoulders at 550 and 603 nm are attributed to interchain π–π stacking interactions. The authors also note that both the color of the polymer and the absorption responsible for the electronic transition of HOMO and LUMO of lower energy are linked to the onset point of the spectrum at longer wavelengths, which corresponds to the smaller energy difference between such energy levels (representing EgOPT). Furthermore, the shoulder observed at 603 nm, attributed to the lamellar organization of the P3HT domains, can be understood in terms of an H-aggregate (face-to-face as observed in XRD) type chromophore with weak interchain coupling interactions. Consequently, this model suggests that the intensity of the first transition (0–0) in the absorption spectrum (λ = 603 nm) corroborates the increasing exciton coupling. The increase in exciton coupling signifies the ease of delocalizing the exciton over a different material, thereby favoring its more efficient dissociation. The peak at 550 nm is related to the vibrational transition (0–1), and the peak at 520 nm to the transition (0–2). In this context, the P3HT(3.5 g) sample would exhibit an improvement in this property, which tends to enhance the device's performance. This sample also showed high crystallinity, higher Lc(100) values, and longer chain lengths compared to the other synthesized P3HT.
Table 7 displays the wavelength onset point and the optical bandgap (EgOPT) obtained through Planck's equation. All samples presented similar onset values and, consequently, the same bandgap. The values obtained were compatible with those in the literature of around 1.85–2.00 eV [51, 53-55].
| Sample | λonset (nm) | EgOPT (eV) |
|---|---|---|
| P3HT(C) | 649.2 | 1.91 |
| P3HT(2×) | 649.2 | 1.91 |
| P3HT(2 g) | 648.6 | 1.91 |
| P3HT(3 g) | 649.0 | 1.91 |
| P3HT(3.5) | 652.5 | 1.90 |
3.8 Cyclic Voltammetry (CV) of Synthesized P3HT
Cyclic voltammetry was employed to determine the energy levels of the molecular materials and to calculate the electrochemical bandgap (EgEC). From the voltammograms, the onset oxidation and reduction potentials are extracted to determine the HOMO and LUMO, respectively. Figure 7a shows the cyclic voltammetry curves of ferrocene, and the half-wave potential value used was the average of 0.50552 eV.
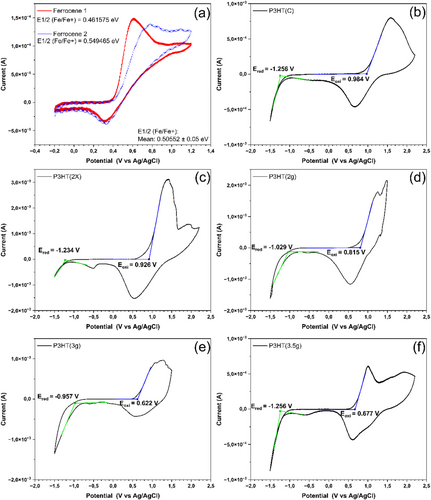
Figure 7b–f presents the voltammograms of P3HT. It can be observed that the samples generally exhibit different profiles and numbers of peaks during oxidation. Curves with varying shapes and numbers of P3HT oxidation/reduction peaks are widely reported in the literature and are related to different oxidation stages of the molecule, internal charge transfer mechanisms, capacitance (non-Faradaic behavior), and film conductivity [35, 56, 57]. Typically, the onset point is chosen to calculate the HOMO value because this region is less influenced by these variables. In other words, at the beginning of oxidation, the molecule predominantly exhibits its intrinsic characteristics. Thus, using the same analysis conditions, it is possible to make accurate comparisons regarding the HOMO energy levels obtained by cyclic voltammetry.
Dingler, Dirnberger, and Ludwigs (2018) reported that P3HT can exhibit three peaks during oxidation [57]. Sometimes, additional peaks appear due to charge transport mechanisms, or fewer peaks may be observed due to such phenomena occurring at very close voltages. These signals can be further analyzed through the deconvolution of the cyclic voltammetry curves. Generally, the three sub-signals can be attributed to the oxidation to the radical cation in ordered, quasi-ordered, and disordered domains in P3HT films. In the same work, the authors mention that these observations are already well-established. Alpatova et al. (2004) reported that thiophene rings can have ionic oxidation states that contribute to charge transport or compatibility between phases in a mixture of materials [58]. This distinction is evident in cyclic voltammetry due to the various peaks observed. Regarding reduction, the reverse scans typically show remarkable similarity across the samples, featuring negative peaks around −0.5 and 0.5 V.
Table 8 presents results from cyclic voltammetry and compares them with those obtained by UV–Vis. Additionally, the energy level of LUMO(EC/OPT) is calculated in this table relative to the combination of the HOMO obtained from cyclic voltammetry and the optical bandgap. This is done because electrons are transferred to LUMO during oxidation. Therefore, LUMOEC is expected to be higher than the actual one since the material has undergone chemical changes due to oxidation and may contain more electrons than the ground state of P3HT. Although other techniques, such as ultraviolet photoelectron spectroscopy (UPS), can determine energy levels directly and are considered more reliable, ultraviolet absorption spectroscopy represents the physical phenomenon that a photovoltaic device is subjected to during operation. Thus, EgOPT is a very representative approach and, when combined with cyclic voltammetry, can accurately predict the distribution diagram of the energy levels of materials. This combination is widely used in literature, with a significantly higher proportion of works employing it compared to those using more sophisticated techniques like UPS.
| Sample | Cyclic voltammetry | UV–Vis | |||||
|---|---|---|---|---|---|---|---|
| Eox (eV) | EHOMO (eV) | Ered (eV) | ELUMOEC (eV) | EgEC (eV) | EgOPT (eV) | ELUMOEC/OPT (eV)a | |
| P3HT(C) | 0.984 | −5.28 | −1.256 | −3.04 | 2.24 | 1.91 | −3.34 |
| P3HT(2×) | 0.926 | −5.22 | −1.234 | −3.06 | 2.16 | 1.91 | −3.31 |
| P3HT(2 g) | 0.815 | −5.11 | −1.029 | −3.27 | 1.84 | 1.91 | −3.20 |
| P3HT(3 g) | 0.622 | −4.92 | −0.957 | −3.34 | 1.58 | 1.91 | −3.01 |
| P3HT(3.5) | 0.677 | −4.97 | −1.211 | −3.08 | 1.89 | 1.90 | −3.07 |
- Abbreviations: EC = electrochemical; OPT = optical.
- a ELUMOEC/OPT = EHOMO + EgOPT.
Firstly, it is worth highlighting that the value obtained for P3HT in cyclic voltammetry is very similar to that reported by Ossila (HOMO = −5.2 eV; LUMO = −3.2 eV). The technique for obtaining LUMO was not reported. This way, the results obtained are very realistic. The analysis error is generally considered to be 0.1 eV, as reported in the literature [35, 56, 57]. HOMO values are reported for P3HT between −4.70 and −5.35 eV and for LUMO levels between −2.70 and −3.35 eV [22-26, 59]. Therefore, all values found are plausible for P3HT with different characteristics.
When the reaction system is reduced by half, yielding the P3HT(2 g) sample, the HOMO energy level tends to shift to more negative values. The system was carried out in duplicate, and the mixed products showed HOMO and LUMOEC values very similar to the commercial ones. Dingler, Dirnberger, and Ludwigs (2018) reported that the degree of organization of the materials' microstructure significantly changes the levels of the HOMO orbitals [57]. In this context, P3HT with a higher degree of crystallinity exhibited lower HOMO levels, at 4.92 and 4.97 eV, for the P3HT(3 g) and P3HT(3.5 g) samples, respectively.
Samples P3HT(2 g) and P3HT(3.5 g) presented similar optical and electrochemical bandgaps, demonstrating the system's stability when electrons are excited by light and electrochemical oxidation. Consequently, the LUMO energy levels identified by both methods are consistent with each other. The P3HT(3 g) sample exhibited an electrochemical bandgap of 0.33 eV, which is smaller than the optical bandgap, an uncommon occurrence. Based on its voltammogram profile, its oxidation was believed to be partial, resulting in a slightly lower reduction set. This is a common issue since, after oxidation, samples undergo chemical transformations that can alter the intrinsic characteristics of the material. Some researchers report that the difference between electrochemical and optical bandgaps can provide information about the exciton binding energy [60, 61]. This is because the electrochemical LUMO is closer to the fundamental LUMO.
The optical bandgap represents the lowest energy involved in the excitation of an electron. In this context, the electron and the hole remain electrostatically linked without the additional energy required for complete electron dissociation from the hole, which is known as the exciton binding energy. Although cyclic voltammetry does not directly measure the fundamental LUMO, the oxidation process can ionize the structure to some extent, resulting in a greater similarity to the fundamental LUMO, which describes the complete separation of the electron from the hole. Thus, the difference between the bandgaps provides interesting comparative information that can be associated with the exciton binding energy. In this context, the P3HT(3.5 g) sample exhibited the highest similarity between the bandgaps, indicating that it likely has the lowest exciton binding energy.
In Figure 8, the variation of HOMO concerning the syntheses carried out can be seen more clearly, as well as the similarity of the results between voltammetry and the combined UV–Vis spectroscopy.
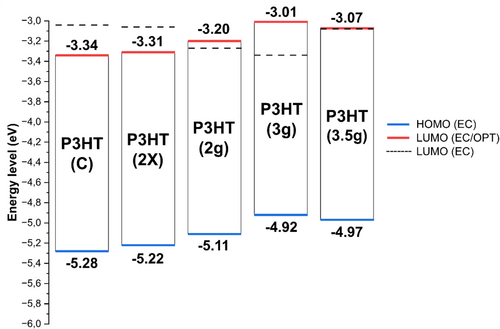
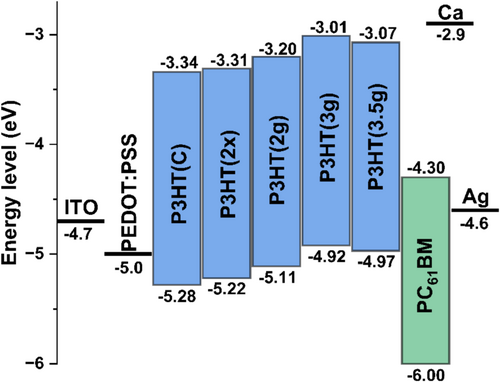
3.9 Device Evaluation of Synthesized P3HT
Figure 9 shows the energy diagram of the devices manufactured using conventional architecture on a glass/ITO substrate, with PEDOT:PSS (40 nm) as the HTL, P3HT:PC61BM (1:1 mass ratio) as the active layer, Ca (20 nm) as a cathode buffer layer, and silver as the metallic contact (100 nm). The optimization process involved varying the concentration of P3HT(C) (10, 20, 30, 40 mg/mL), spin-coating speed (500–1500 rpm), spin-coating duration, heat treatment, and/or solvent vapor annealing. Additionally, different solvent ratios (chloroform (CHCl3), chlorobenzene (CB), and dichlorobenzene) were explored, along with thickness measurements to ensure optimal film formation. The thickness of the active layer was standardized to 200 nm according to the optimized results compared to commercial P3HT. The solution concentration was 20 mg/mL in CHCl3:CB (4:1 volume ratio), deposited in dynamic mode at 500 rpm for 20 s. Solvent vapor annealing treatment was performed for 5 min in CHCl3:CB (4:1), followed by thermal annealing at 140°C for 10 min.
The data presented pertain to two pixel sizes: smaller (0.16 cm2) and larger (1.00 cm2). Measurements under illumination were conducted using a shadow mask to define the active area of the pixel. The substrate size was fixed at 5.0 × 5.0 cm to allow the evaluation of pixel size influence without altering the design parameters or variables related to film deposition and the sheet resistance of the electronic components. This approach facilitates a realistic scale-up analysis, as the only variable related to infrastructure is the pixel size.
On the other hand, the efficiency of smaller pixels tends to decrease due to the presence of a region where the transparent conductive electrode does not generate an active area, referred to as a dead zone. This region is highly conductive, leading to the dissipation or escape of charges generated within the pixel to this low-resistance area. Additionally, increasing the distance that a charge must travel amplifies the resistive effects, reducing the system's power generation capacity. In logic circuit systems, the complete device has a specific resistance associated with charge transport between the cathode and anode, known as series resistance. In this context, all components that drain charges or hinder charge transport toward the electrodes can be viewed as a system in parallel with a resistance known as shunt resistance. These factors influence the photovoltaic parameters extracted from the device's operational analysis. Furthermore, using a substrate much larger than the pixel size presents practical challenges, as it is necessary to cover a larger area uniformly. This increases the likelihood of defects or issues in film morphology, which can further reduce efficiency.
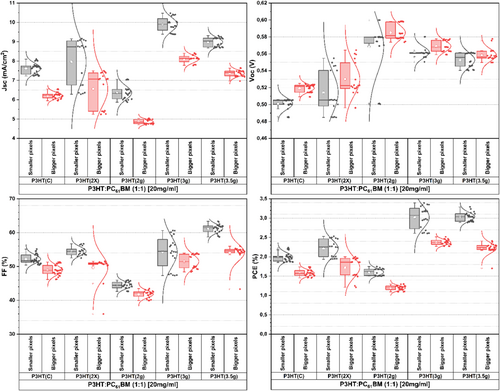
Based on the current density analysis as a function of the voltage of the devices under 1 Sun illumination, the photovoltaic parameters Voc, Jsc, FF, and PCE were obtained. These results are presented in Figure 10 according to the boxplot statistical analysis. Firstly, it can be observed that larger pixels exhibit lower Jsc, FF, and PCE. This trend is expected due to the probabilistic effects of defects occurring over a larger analyzed area, resulting in a higher likelihood of charge recombination and a reduced photocurrent generation capacity of the material. Furthermore, these three photovoltaic parameters demonstrate similar behavior depending on the synthesis methods used for P3HT. Voc tends to show excellent values for P3HT(2 g), reaching record levels close to 0.6 V.
Voc is directly related to the energy levels of the HOMO and LUMO orbitals of the donor and acceptor components. Generally, it is desirable to maximize the difference between the donor's HOMO level and the acceptor's LUMO to achieve a higher Voc. One common strategy mentioned in the literature is to reduce the donor's HOMO level. However, if this difference becomes too large, the likelihood of energy loss processes also increases [62, 63]. Another limitation regarding the difference between the donor's HOMO and the acceptor's LUMO is the bandgap of the donor.
Furthermore, these considerations assume that effective exciton dissociation has already occurred, meaning the electron is in the LUMO of the acceptor, and the hole is in the HOMO of the acceptor. Before this can happen, the electron must be excited with sufficient energy to overcome the bandgap. Subsequently, the exciton must dissociate and overcome the energy potential barrier to be transported from the donor LUMO to the acceptor LUMO. Therefore, the difference between the LUMOs must be greater than or equal to the exciton binding energy, which in donor polymers typically ranges from 0.3 to 0.5 eV [62].
In polymeric solar cells, effective exciton dissociation occurs only at the donor/acceptor interface. When the exciton reaches this interface, there must be a sufficient potential difference (ΔELUMO) for dissociation to take place and generate free charge carriers. If ΔELUMO is not large enough, exciton dissociation will not occur. Conversely, if ΔELUMO is too large, it can lead to losses in Voc, as it reduces the intensity of the electromagnetic field at the interface that is responsible for efficient exciton dissociation. The greater the distance between two points with a potential difference, the lower the intensity of the electric or electromagnetic field.
In this context, it is believed that the P3HT(2 g) sample achieved a more suitable balance between ΔELUMO (1.1 eV) and the difference between the donor's HOMO and the acceptor's LUMO, thereby reducing Voc losses. The samples P3HT(C) and P3HT(2×) exhibit lower HOMO levels, with ΔELUMO values of 1.04 and 1.01 eV, respectively. This suggests that these samples may face greater difficulty in dissociating excitons. Both samples displayed the largest difference between the optical and electrochemical bandgaps. Bredas (2014) and Kumar et al. (2019) indicate that a greater difference in bandgaps from these two analyses corresponds to a higher exciton binding energy [60, 61].
As these two samples exhibit very similar LUMO levels, the main hypothesis is that P3HT(C) has a higher exciton binding energy, as indicated by the greater difference in bandgap among the analyzed samples. P3HT(3 g) and P3HT(3.5 g) show a slight reduction in Voc, despite having higher LUMO levels. This reduction in short-circuit voltage is attributed to these samples also having higher HOMO levels, which decreases the difference between the donor's HOMO and the acceptor's LUMO. Therefore, the behavior of Voc can be directly understood through the energy diagram. The Voc of larger pixels may be slightly higher, which is expected since the applied voltage is better distributed over a larger area, leading to a more effective measurement of Voc. It is important to note that this effect occurs because the area of the transparent conductive electrode is the same for both, while the pixel size differs.
Table 9 presents the average values with standard deviation and the 95% confidence interval for the data obtained from the devices manufactured with the synthesized P3HT. P3HT(C) showed an efficiency of 1.99% ± 0.11% for the small pixel and 1.58% ± 0.08% for the large pixel. These values are consistent with the performance of typical P3HT devices, as reported in the literature [64, 65]. Except for P3HT(2 g), all synthesized materials exhibited superior efficiency. P3HT(3 g) and P3HT(3.5 g) demonstrated average efficiencies that were 50% higher (< 3% PCE). P3HT(3 g) presented a higher Jsc, while P3HT(3.5 g) showed a higher FF. Both samples exhibited a higher degree of crystallinity, which is an important morphological factor. The FF is directly related to morphological quality and effective charge collection. P3HT(3.5 g) displayed larger crystallite size, greater molar mass, a higher degree of crystallinity, and a more pronounced shoulder in the UV–Vis spectrum corresponding to π–π stacking, all of which contribute to better charge transport and a higher FF.
| Sample | Pixel size | Voc (V) | Jsc (mA/cm2) | FF (%) | PCE (%) |
|---|---|---|---|---|---|
| P3HT(C) | Smaller | 0.501 ± 0.007 [0.498–0.504] | 7.62 ± 0.27 [7.52–7.72] | 52.2 ± 1.3 [51.7–52.7] | 1.99 ± 0.11 [1.95–2.04] |
| Bigger | 0.519 ± 0.005 [0.518–0.521] | 6.22 ± 0.16 [6.16–6.28] | 48.9 ± 1.5 [48.3–49.5] | 1.58 ± 0.08 [1.5–1.6] | |
| P3HT(2×) | Smaller | 0.514 ± 0.026 [0.501–0.527] | 7.96 ± 1.21 [7.34–8.58] | 54.5 ± 1.3 [53.8–55.2] | 2.21 ± 0.23 [2.1–2.33] |
| Bigger | 0.53 ± 0.02 [0.52–0.54] | 6.56 ± 0.94 [6.08–7.04] | 49.7 ± 3.8 [47.7–51.6] | 1.72 ± 0.26 [1.6–1.9] | |
| P3HT(2 g) | Smaller | 0.568 ± 0.03 [0.554–0.582] | 6.36 ± 0.36 [6.19–6.53] | 44.4 ± 1.1 [43.9–44.9] | 1.6 ± 0.09 [1.56–1.65] |
| Bigger | 0.585 ± 0.009 [0.581–0.589] | 4.87 ± 0.12 [4.81–4.92] | 41.8 ± 1.1 [41.3–42.3] | 1.19 ± 0.06 [1.2–1.2] | |
| P3HT(3 g) | Smaller | 0.562 ± 0.007 [0.559–0.565] | 9.90 ± 0.37 [9.72–10.07] | 54.3 ± 4.7 [52.1–56.5] | 3.02 ± 0.28 [2.89–3.15] |
| Bigger | 0.569 ± 0.007 [0.566–0.572] | 8.10 ± 0.2 [8.01–8.2] | 51.5 ± 2.1 [50.5–52.5] | 2.37 ± 0.07 [2.3–2.4] | |
| P3HT(3.5) | Smaller | 0.553 ± 0.009 [0.549–0.557] | 8.98 ± 0.24 [8.87–9.08] | 61.2 ± 1.3 [60.6–61.8] | 3.04 ± 0.11 [2.99–3.09] |
| Bigger | 0.558 ± 0.007 [0.555–0.562] | 7.37 ± 0.17 [7.29–7.44] | 54.1 ± 2.5 [53–55.2] | 2.23 ± 0.14 [2.2–2.3] |
On the other hand, high crystallinity can significantly increase the size of the donor domain relative to the acceptor, which tends to reduce the available interface and photocurrent. In this context, P3HT(3 g), with its slightly lower crystallinity, does not excessively increase the donor domain beyond the optimal value, resulting in a Jsc approximately 1 mA/cm2 higher. This leads to better donor/acceptor dispersion, which increases the interfaces that generate photocurrent. Additionally, due to its lower crystalline fraction and molar mass, P3HT(3 g) experiences reduced charge transport throughout the crystals and polymer chain. In other words, there is a balance between these properties, which perfectly describes the situation for these two P3HTs, with each sample optimizing different parameters while both achieving excellent efficiencies.
P3HT, with a lower molar mass, exhibits a lower degree of crystallinity and reduced charge mobility. This leads to increased recombination processes, diminishing photocurrent generation capacity and resulting in a lower FF and Jsc [66] This trend was observed in P3HT(2 g), which had the lowest FF and Jsc. Conversely, a molar mass greater than the optimal value compromises crystallinity, which negatively impacts both the FF and Jsc [66] The P3HT(2×) sample exhibited the greatest molar mass dispersion, leading to increased difficulty in organizing the domains. However, it is important to note that all samples showed parameters consistent with P3HT, which is of good quality compared to the literature.
As mentioned, increasing the active area, pixel size, and substrate tends to significantly reduce the photocurrent and FF (Gupta, Bag, and Narayan 2008). This trend was observed across all devices, while Voc remained unchanged or saw a slight increase. Park et al. (2011) achieved a 0.38 cm2 P3HT device with a very high efficiency of 3.13% by employing sub-electrodes and grid electrodes [67]. These additional electrodes resulted in an FF of 64.2% and a Jsc of 8.42 mA/cm2, aiding in charge collection around the pixel and above the ITO. Li et al. (2019) reached maximum efficiencies of 2.51% after complete optimization of the P3HT layer in roll-to-roll devices measuring 0.09 cm2 with an inverted architecture [68]. Chalal et al. (2016) manufactured a P3HT device with a 2 cm2 pixel on a 2.5 × 2.5 cm substrate, achieving a high efficiency of 3.2% by using silver and gold double sub-electrodes in stripes to reduce resistive losses associated with the ITO transparent conductive electrode [69]. Additionally, this work demonstrated that minimizing the dead area is essential for developing high-efficiency devices. The present study successfully synthesized two P3HT variants that could produce large-area devices with excellent power conversion efficiencies of approximately 2.3% without the need for sub-electrodes, grids, or substrate modifications.
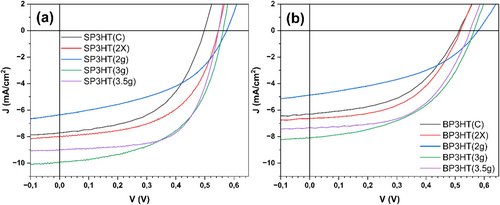
Geethu et al. (2019) reported that their optimized P3HT devices achieved a thickness of 200 nm and a power conversion efficiency (PCE) of approximately 1.4%, with Voc around 0.56 V, Jsc about 7.5 mA/cm2, and a FF of 35% [65]. Lee, Choi, and Moon (2016) attained a Jsc of 6.9 mA/cm2, Voc of 0.596 V, FF of 49.9%, and a PCE of 2.02%. In general, the literature indicates that results for P3HT can vary up to approximately 4% under optimized conditions for small-area devices (< 0.10 cm2). Xian et al. (2023) reported that achieving high-efficiency P3HT-based devices requires the use of one or two non-fullerene acceptors. Notably, they demonstrated that the ternary blend P3HT:ZY-4Cl:BTP-2Br significantly improved all photovoltaic parameters, achieving a power conversion efficiency of up to 11.4% using a small active area of 0.04 cm2 [70]. P3HT(3 g) and P3HT(3.5 g) demonstrated superior PCE in large-area devices (1 cm2) compared to those from small-area devices such as P3HT(C), P3HT(2×), and P3HT(2 g), as well as the studies mentioned in this paragraph. It is important to note that the optimization was conducted concerning P3HT(C) in the present work, suggesting that the synthesized samples may be further improved through different solvent methodologies, thickness adjustments, layer combinations, and thermal treatments. Finally, Figure 11 shows the JV curves of the P3HT-based devices in the smallest and largest pixels. At the x-axis intercept, Voc is identified. At the y-axis intercept, Jsc is observed. The FF is assessed as the curve approaches a rectangle defined by Voc and Jsc.
4 Conclusion
In conclusion, this study provides valuable insights into the relationship between the synthesis scale and the optoelectronic properties of P3HT. By optimizing the synthesis conditions, it is possible to enhance the efficiency and stability of polymer-based solar cells, paving the way for more sustainable and cost-effective solar energy solutions.
It was demonstrated that scaling down the synthesis to 50% of the reaction system leads to a decline in optoelectronic properties and a reduction in the performance of the photovoltaic device. Conversely, synthesis using proportions close to 3.5 g of monomer leads to improved optoelectronic properties, higher molar mass, and enhanced device efficiencies. Specifically, devices employing the polymer synthesized with 3.5 g of monomer achieved efficiencies of up to 3.04% ± 0.11% for a 0.16 cm2 pixel and 2.23% ± 0.14% for a larger area pixel of 1 cm2. These results underscore the importance of carefully controlled synthesis conditions in achieving optimal photovoltaic performance. Beyond these technical findings, this study also highlights the need for deeper investigations into the effects of synthesis downscaling. Future research should focus on systematically comparing different synthesis scales with the resulting polymer characteristics—including molar mass distribution, regioregularity, and optoelectronic behavior—in order to understand the critical parameters that govern performance losses during miniaturization.
Moreover, it becomes essential to invest in alternative synthesis methodologies—such as continuous-flow polymerization and microwave-assisted synthesis—that enable reaction downscaling while maintaining the consistency and quality of the resulting materials. Such approaches may offer both improved reproducibility and better alignment between synthetic efficiency and device performance, supporting the broader transition from lab-scale development to industrially relevant production of high-performance organic semiconductors.
Acknowledgments
The authors thank the Brazilian Council for Scientific and Technological Development (CNPq), the Coordination for the Improvement of Higher Education Personnel (CAPES), the Carlos Chagas Filho Foundation for Supporting Research in the State of Rio de Janeiro (FAPERJ), the São Paulo State Research Support Foundation (FAPESP) and Fraunhofer IAP for the financial support. The Article Processing Charge for the publication of this research was funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) (ROR identifier: 00x0ma614).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




