Aliphatic isocyanurates and polyisocyanurate networks†
Abstract
The production, processing, and application of aliphatic isocyanate (NCO)-based thermosets such as polyurethane coatings and adhesives are generally limited by the surprisingly high viscosity of tri-functionality and higher-functionality isocyanurates. These compounds are essential crosslinking additives for network formation. However, the mechanism by which these high viscosities are caused is not yet understood. In this work, model aliphatic isocyanurates were synthesized and isolated in high purity (>99%), and their viscosities were accurately determined. It was shown that the presence of the NCO group has a strong influence on the viscosity of the system. From density functional theory calculations, a novel and significant bimolecular binding potential of −8.7 kJ/mol was identified between NCO groups and isocyanurate rings, confirming the important role of the NCO group. This NCO-to-ring interaction was proposed to be the root cause for the high viscosities observed for NCO-functional isocyanurate systems. Molecular dynamics simulations carried out to further confirm this influence also suggest that the NCO-to-ring interaction causes a significant additional contribution to viscosity. Finally, model functional isocyanurates were further reacted into densely crosslinked polyisocyanurate networks which showed interesting material properties. Copyright © 2016 The Authors Polymers for Advanced Technologies published by John Wiley & Sons, Ltd.
Introduction
The current work is aimed at better understanding of the viscosity characteristics of isocyanate (NCO)-based crosslinkers, an essential parameter in their production and processing. Isocyanates, as primary ingredients of polyurethanes, have been of wide interest in both industry and academia ever since polyurethanes were invented.1-4 Aliphatic diisocyanates such as 1,6-hexamethylenediisocyanate (HDI), and its derivatives are produced on a large scale worldwide. They are well-known for their high performance regarding ultraviolet resistance, as well as their excellent mechanical, chemical, optical, and thermal properties in polyurethane coatings and adhesives. Of particular significance in the performance of these materials are the higher-functionality trimerized isocyanurate forms of the diisocyanates, key additives as crosslinking units for network formation. The chemical and physical stability of these crosslinking units, resulting from the deep thermodynamic minimum of the isocyanurate ring structure,5, 6 is key in the high performance of modern polyurethane coatings and adhesives. Furthermore, the possibility of trimerizing such trifunctional isocyanurate additives in a pure form has been investigated. This results in the formation of highly stable densely crosslinked polyisocyanurate networks, which show interesting material properties. In particular, the good optical properties and high chemical and thermal stability of these materials are attractive qualities.7-10
- to quantify accurately the viscosity increase upon trimerization of aliphatic diisocyanates into NCO-functional aliphatic isocyanurates, essential precursors for many isocyanate-based thermosets,
- to investigate the underlying chemistry to explain the unexpectedly high viscosities observed for NCO-functional aliphatic isocyanurates and
- to further react model isocyanurates into densely crosslinked polyisocyanurate networks and characterize the physical and thermal properties of the resulting materials.
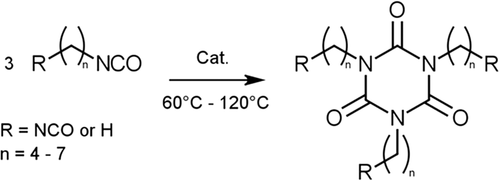
To investigate the viscosity characteristics of functional (network-forming) isocyanurates, a series of model diisocyanates with varying carbon chain lengths based on and including HDI was prepared. Also, a similar series of monofunctional isocyanates, yielding non-functional (non-network-forming) isocyanurates, was included as a reference. The relevant structures and general synthetic approach are presented in Scheme 1.
Experimental
Materials and methods
α-ω-linear diisocyanates were taken from internal industrial production (Covestro Deutschland AG) and vacuum distilled before use. n-Butylisocyanate and n-hexylisocyanate were purchased at Aldrich and distilled before use. n-Pentylisocyanate and n-heptylisocyanate were synthesized by reaction of corresponding amines (Sigma-Aldrich) with 1,6-hexamethylenediisocyanate. Dodecylbenzene sulfonic acid was supplied by Sigma-Aldrich and used as received, as were all other chemicals described. Viscosity measurements were performed at room temperature (RT) on an Anton-Paar MCR501 rheometer (Anton Paar Germany GmbH, Ostfildern-Scharnhausen, Germany) using either a 50 mm cone-plate setup (CP50-2, d = 0.214 mm) or a CC27 beaker-cup setup, in rotational mode at increasing shear rate from 0.1–100 s−1 (0.1–1000 s−1 for beaker-cup). Differential Scanning Calorimetry measurements were performed under nitrogen on 10 mg samples on a Perkin-Elmer (PerkinElmer, Rodgau, Germany) Calorimeter DSC-7 in two heating runs from −20°C to200°C by 20°C/min. Thermal gravimetric analysis measurements were performed under nitrogen on 4 mg samples on a Perkin-Elmer (PerkinElmer, Rodgau, Germany) Microthermowage TGA-7 from RT to 600°C by 20°C/min. Nuclear Magnetic Resonance-spectra were collected on a Bruker Avance III-700 (Bruker BioSpin GmbH, Rheinstetten, Germany). Fourier transform infrared spectroscopy-spectra were recorded on a Bruker FT-IR Spectrometer Tensor II (Bruker BioSpin GmbH, Rheinstetten, Germany) with Platinum-ATR-unit with diamond crystal.
Synthesis of n-pentylisocyanate and n-heptylisocyanate
In a typical experiment, in a 1000 ml two-neck round-bottom flask with distillation bridge, 15.45 g of n-alkylamine was dripped into 412.7 g of pure HDI at 80°C under formation of white deposit. The mixture was heated to 170°C, and pressure was gradually reduced to 50 mbar. After 4 hr, distillation was complete. The distillate fraction was redistilled at 175°C under atmospheric pressure, collecting the pure products as transparent liquid, analyzed by 1H-NMR and FTIR.
Synthesis of functional isocyanurates
In a typical experiment, in a cylindrical flask equipped with a continuous near infraRed (NIR) probe-head (Bruker NIR Vector 22/N), 150 g of α-ω-linear diisocyanate was heated to 60°C and 0.15 wt% of catalyst solution (1 wt% of N,N-methyl-butyl-pyrrolidiniumhydroxide in isopropanol) was added, until trimerization started, followed by continuous NIR and temperature (for 1,4-butyldiisocyanate, N,N-dimethyltrimethylsilylamine was used as catalyst at 120°C). After 5–20% reduction of NCO absorption-intensity in NIR (30–90 min), an equimolar amount, compared with active catalyst, of dodecylbenzene sulfonic acid was added to quench the reaction (1,4-butanediol for the reaction of 1,4-butyldiisocyanate). The mixture was transferred to a dripping funnel and evaporated using a thin-film short-path evaporator at 150°C and 1°10−1 mbar, collecting the resin as highly viscous brown oil. Reusing the distillate as starting material and supplementing with freshly distilled diisocyanate, this procedure was repeated six times, combining all resins. The combined resin-fraction was then distilled in a thin-film short-path evaporator at 220°C and <10−7 mbar, collecting the distillate as a very slightly greenish transparent viscous oil. The distillate was evaporated using a thin-film short-path evaporator at 160°C and <10−7 mbar, collecting the pure product as resin as a very slightly greenish transparent viscous oil, characterized by (numbers for HDI-trimer) 1H-NMR (700 MHz, C6D6): δ = 3.78 (2H, t), 2.54 (2H, t), 1.53 (2H, quin), and 1.03–0.92 (6H, m) ppm; 13C-NMR (700 MHz, C6D6): δ = 149.01, 122.92, 42.78, 42.60, 31.07, 27.99, 26.17, and 26.16 ppm and gel permeation chromatography (GPC).
Synthesis of non-functional isocyanurates
In a typical experiment, in a 100 ml round-bottom flask, 34.63 g of n-alkyl isocyanate was heated to 60°C. Dropwise, 2.46 g of catalyst solution (1 wt% of N,N-methyl-butyl-pyrrolidiniumhydroxide in isopropanol) was added, until trimerization started, followed by FTIR and temperature. In 2–4 hr, the reaction was run to completion, followed by disappearance of the 2275 cm−1 NCO-band in FTIR. The mixture was trap-to-trap distilled under reduced pressure (<10−7 mbar) at 200°C, and products were isolated as intermediate distillate fraction as transparent oils, analyzed by (numbers for n-hexylisocyanate trimer) 1H-NMR (700 MHz, C6D6): δ = 3.82 (2H, t), 1.63 (2H, quin), 1.22–1.14 (6H, m), and 0.82 (3H, t) ppm; 13C-NMR (700 MHz, C6D6): δ = 149.06, 42.98, 31.73, 28.26, 26.72, 22.91, and 14.19 ppm and GPC.
Synthesis of polyisocyanurate networks
In a typical experiment for the trifunctional isocyanurate to 100 g of isocyanurate precursor, 3.140 g of catalyst solution (0.148 g KOAc, 0.396 g 18-crown-6-ether, 2.596 g diethyleneglycol) was added and mixed in a Hauschild & Co KG, Hamm, Germany DAC 150.1 FVZ speedmixer at 2000 rpm for 60 sec. The mixture was poured out into 6.3 cm aluminum lids in 4, 8, or 16 g samples and kept at 180°C for 15 min. Materials were collected as rigid transparent thermosets, showing slight yellowing and in some cases formation of a few small bubbles. For difunctional isocyanates, mixtures were allowed to react in a glass vial under nitrogen for 1–2 hr before similar pouring and annealing.
Caution: Because of the strongly exothermic character of the trimerization reaction, care should be taken with dissipation of heat especially during the pretrimerization of difunctional diisocyanates.
Quantum calculations details
All density functional theory (DFT) calculations were performed with gaussian '09 software15 using a B3-LYP hybrid functional and the 6–31 + g(d,p) basis set, and have been carried out using the ultrafine integration grid. The accuracy of the calculations was improved considering empirical dispersion corrections, as prescribed by the Grimme's D3 scheme16 with the Becke-Johnson damping.17 For all molecules, stable geometries were obtained and characterized in terms of the relevant geometrical parameters (bonds, angles, dihedrals, and impropers).
Free energy calculations
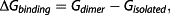
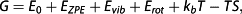
Molecular dynamics simulations
- Force field parametrization. A fully flexible all-atom representation of the molecules was adopted, and the general amber 18 force field was used and parametrized using the data from the quantum calculations. In particular, bond angles and lengths at equilibrium were slightly tuned to match the results from DFT. Partial charges on each atom were obtained using the restrained electrostatic potential method.19 To account for charge screening effects, all the Coulomb interactions were scaled by a factor of two. To check the validity of the force field, the interaction energies calculated by molecular mechanics were compared with those calculated by DFT for various configurations of the bimolecular complexes along a specified reaction coordinate, reported in Figure S1 (Supporting Information). There is a slight underestimation of the well depth by molecular mechanics, but the long-range part of the interaction energy is very well described. As further validation, the equilibrium density obtained for the HDI trimer liquid at 300 K and 1 atm, using molecular dynamics simulations, has been compared with the experimental density at 300 K and 1 atm. An agreement of up to 1% was found.
- Molecular dynamics (MD) calculations setup. MD simulations were performed using the lammps20 software package. The velocity verlet integration method has been used. A timestep of 1 fs has been used, along with the reversible reference system propagation algorithm,21 using an inner timestep of 0.125 fs to keep the energy drift under control. A general cut off of 13 Å for pair forces calculations was considered. Above that cut off, long range electrostatic interactions have been evaluated using the Ewald Particle Mesh method.22 For each molecular liquid, six independent runs were performed. Preparation of the system consisted of a high temperature (480 K) run of 1 ns to randomize the initial conditions, followed by an equilibration step toward the target temperature. This was performed under constant temperature and pressure conditions, implemented by the Nosé-Hoover23 thermostat and Parrinello-Rahman24 barostat. Input files for data production runs were chosen to match both the equilibrium density and the target temperature. Production runs consisted of 5 ns runs (five million steps) under NVE (constant Number of particles, Volume and Energy) conditions.
Calculation of viscosity

Results and Discussion
Synthesis of isocyanurates
All syntheses were performed based on previously described methods,27, 28 using an N,N-methyl-butyl-pyrrolidiniumhydroxide catalyst, as summarized in Scheme 1. Because even small amounts of impurities can cause deviations in viscosity measurements, extreme care was taken throughout with the purification and isolation of isocyanurate products. On top of the already high sensitivity of isocyanates to undergo various side reactions, difunctional isocyanates (R = NCO) will inevitably form higher molecular weight oligomers, because of the similar reactivities of the starting material and product. The optimal conditions for isolating the functionalized trimers in pure form were hence obtained by early quenching of the reaction. Specifically, at about 10% conversion of the NCO groups (determined by continuous near-infraRed spectroscopy), the catalyst was quenched by addition of an equimolar amount of a suitable catalyst poison. Because isocyanates tend to exhibit reactivity toward most column-chromatography media, the generally preferred purification technique is distillation. Therefore, despite the high molecular weights of the products, workup of the reaction products was performed by means of a multiplestep thin-film distillation process under very low pressures (<10−7 mbar) using a short-path evaporator, as described in the next sections. In a typical experiment, first all unreacted monomer was removed by thin-film evaporation at 150°C and 10−1 mbar. Then, the targeted trimers were separated from higher molecular weight oligomers by thin-film distillation at 220°C and <10−7 mbar, thereby giving the products as the distillate. Finally, from this distillate, any reformed monomer and other low-boiling impurities formed during the purification process were removed by similar thin-film evaporation at 160°C and <10−7 mbar, yielding the product in pure form as the residue.
During trimerization of the monofunctional isocyanates (R = H) of the reference series, oligomerization does not occur, so the reactions could be carried out to completion (as determined followed by the disappearance of the 2275 cm−1 NCO band in the FTIR spectrum). Subsequently, the reaction products were purified in a fashion to that described previously, in a multistep trap-to-trap distillation process under very low pressures (<10−7 mbar). The products were collected as the intermediate distillate fractions.
All products were isolated in >99% purity with the exception of the sample with n = 4 and R = NCO (>98%), as confirmed by 700-MHz 1H NMR and 13C NMR spectroscopies and GPC, in most cases limited only by the detection level of the equipment (data not shown). Because of the strong emphasis on purity, no yields were determined.
Quantification of the viscosities of isocyanurates
For the isolated trimers, viscosities were measured on an Anton-Paar MCR501 rheometer using a 50 mm cone-plate (CP50-2) setup. For validation, several samples were also measured using a more consuming but more precise CC27 beaker-cup setup, yielding similar results. The measured viscosity values are depicted in Fig. 1 as a function of the carbon chain length of the isocyanate reactants.
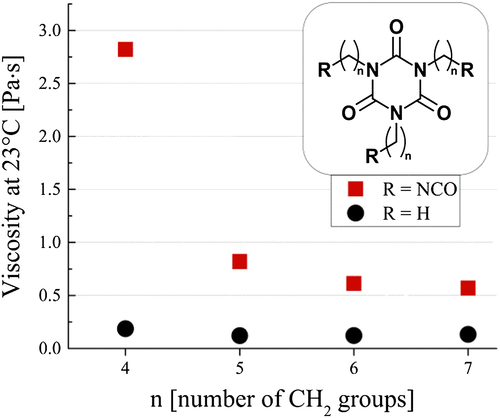
As can be clearly seen from the figure, the viscosities of all functionalized samples (R = NCO) were significantly higher than those of the non-functionalized samples (R = H), while also increasing for higher NCO and isocyanurate contents (i.e. lower value of n). Within the present series, the side chains are assumed to be too short to show any macromolecular behavior and no significant contribution to viscosity is expected from entanglements. Because the molecular shapes of the two series are practically identical, differing only in the fact that the linear NCO groups are slightly more rigid than alkyl chain ends, no significant effects are expected based on molecular shape either. Hence, any difference between the two series can be attributed entirely to the presence/absence of the NCO group or, more specifically, to electronic/chemical interactions involving the NCO group. However, it should be noted that in monomeric (di)isocyanates such as HDI, NCO groups are abundantly present as well, although here this does not lead to increased viscosities. The specific chemical mechanism by which the presence of the NCO group influences viscosity in these mixtures seems to be somewhat complicated. To investigate this influence in more detail, DFT calculations were performed on the same series of model compounds.
Density functional theory calculations of bimolecular interaction potentials
To investigate the underlying mechanism causing the unexpectedly high viscosities of functional aliphatic isocyanurates, quantum mechanical calculations and viscosity simulations were carried out. To study the molecular binding between NCO groups and the carbonyls of adjacent isocyanurate rings, as well as between adjacent NCO groups, several model bimolecular assembly systems were simulated using DFT. For the NCO-to-ring interaction (Fig. 2a), surprisingly, a stable configuration was found. A significant free energy of binding of −8.7 kJ/mol was calculated for the NCO-to-ring interaction under standard conditions (298 K, 1 atm). This is clearly above the thermal energy threshold. However, the free energy of binding of the NCO-NCO bimolecular complex (Fig. 2b) was found to be positive (+23.1 kJ/mol), clearly suggesting the absence of any relevant interaction between NCO groups themselves.
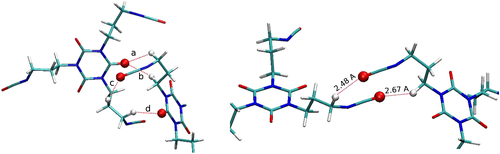
To verify the influence of these bimolecular interactions on the viscosity of the system, MD simulations were carried out for both model series using the Green-Kubo25 formalism. All calculated viscosity values were in agreement with experimental results, showing the same trends. From the MD simulations, it was found that, indeed, the additional electronic interaction potential between NCO groups and isocyanurate rings gives rise to an increase in the final viscosity.
Based on the combined experimental and computational results, it is proposed that the newly identified bimolecular NCO-to-ring interaction potential is the main cause of the high viscosities observed in functional aliphatic isocyanurate systems. As a result, increased viscosities should be expected only when NCO groups and isocyanurate rings are simultaneously present. This is perfectly supported by the experimental data. Furthermore, viscosity is expected to increase with increasing relative contents of NCO groups and isocyanurate rings. This was also effectively demonstrated experimentally. Because of the lack of interaction between adjacent NCO groups, as indicated by the binding free energy calculations, no additional contribution to viscosity is expected in systems containing only NCO groups. This was also indeed observed, noting the very low viscosities of monomeric (di)isocyanates such as HDI. Given that these interactions involve only the functional groups, it is expected that the proposed mechanism will be applicable to a much wider range of materials than just these model systems based on linear aliphatic diisocyanates. In fact, in any system where both NCO groups and isocyanurate rings are simultaneously present, this interaction should be considered.
Synthesis and characterization of densely crosslinked isocyanurate networks
For the most common functional model precursor (HDI, n = 6), initial attempts to prepare densely crosslinked networks by complete trimerization were carried out. The resulting thermosets were investigated in terms of mechanical and thermal properties. A comparison was made between the use of two different catalysts (potassium acetate (KOAc) and tin(II) 2-ethylhexanoate (SnOct)), and the difference between using the difunctional diisocyanate or the trifunctional isocyanurate as the starting material was investigated. The physical properties of the obtained materials are reported in Table 1. All samples were collected as rigid transparent thermosets, exhibiting slight yellowing, and in some cases, the formation of a few small bubbles, depending on the conditions and catalyst (see Picture 1).
| Onset of mass loss (°C) | Decomposition (°C) | Mass loss (%) | Tga (°C) | E modulus (GPa) | Ultimate strength (MPa) | Elongation at break (%) | ||
|---|---|---|---|---|---|---|---|---|
| 150°C | 250°C | |||||||
| HDI trimerb | 275 | 440 | 0.1 | 0.5 | 118 | 2.1c | 60c | 5.5c |
| HDIb | 95 | 285 | 0.6 | 1.7 | 108 | — | — | — |
| HDId | 95 | 280 | 1.4 | 2.6 | 122 | — | — | — |
- a Glass transition temperature.
- b Catalyst: potassium acetate (KOAc) in diethylene glycol (DEG).
- c Covestro internal report.
- d Catalyst: tin(II) 2-ethylhexanoate (SnOct).

For these preliminary results, it is noted that the glass transition temperatures Tg are somewhat lower than those reported in the literature (140°C7, 8) and that they exhibit some slight variation between samples. Based on both the literature and our own findings, the differences in physical properties can most likely be attributed to the degree and quality of network formation. Because the network density increases rapidly during the reaction, the probability of trimerizing three NCO groups quickly decreases, and complete network formation is difficult to achieve. Furthermore, diffusion of the catalyst quickly becomes restricted, limiting the rate of reaction even further. Based on these preliminary results, thermosets prepared from NCO-functional isocyanurates as the starting material seem to show better physical properties than those prepared from the linear diisocyanates. Future work will be needed to find optimized processing conditions.
Conclusions
Model aliphatic isocyanurates of varying carbon chain length were synthesized and isolated in high purities (>99%). The viscosities of these model systems were accurately quantified, and viscosities were found to be significantly higher for NCO-functional isocyanurates than for non-functional isocyanurates. Viscosities also increased with increasing relative contents of NCO groups and isocyanurate rings. Through DFT calculations, a newly identified strong intermolecular binding potential was found to be present between the NCO groups and the isocyanurate-ring carbonyl groups. Also a less significant interaction between the NCO groups themselves was identified. At standard conditions (298 K, 1 atm), a binding free energy of −8.7 kJ/mol was calculated for the NCO-to-ring interaction. This value is clearly above the thermal energy threshold. MD simulations further confirmed the influence of this NCO-to-ring interaction as a contributor to the viscosity of the system.
Based on the combined experimental and computational results, it is proposed that this NCO-to-ring interaction is the main cause of the high viscosities generally observed in functional aliphatic isocyanurate systems. Consequently, the presence and relative content of the combination of NCO groups and isocyanurate rings has a significant effect on increasing the viscosity of such systems.
Finally, densely crosslinked HDI-based isocyanurate networks were synthesized. These were found to exhibit interesting but varying mechanical and thermal properties depending on catalyst use and the degree of crosslinking achieved.
Acknowledgements
This project has received funding from the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement no. 642890 (http://thelink-project.eu/), and it was partially supported by the Portuguese Foundation for Science and Technology (FCT) in the framework of the strategic funding UID/FIS/04650/2013, and under the project Search-ON2: NORTE-07-0162-FEDER-000086.




