Exploration of an alternative to body mass index to characterize the relationship between height and weight for prediction of metabolic phenotypes and cardiovascular outcomes
Abstract
Objective
Body mass index (BMI) is the most commonly used predictor of weight-related comorbidities and outcomes. However, the presumed relationship between height and weight intrinsic to BMI may introduce bias with respect to prediction of clinical outcomes. A series of analyses comparing the performance of models representing weight and height as separate interacting variables to models using BMI were performed using Vanderbilt University Medical Center's deidentified electronic health records and landmark methodology.
Methods
Use of BMI or height-weight interaction in prediction models for established weight-related cardiometabolic traits and metabolic syndrome was evaluated. Specifically, prediction models for hypertension, diabetes mellitus, low high-density lipoprotein, and elevated triglycerides, atrial fibrillation, coronary artery disease, heart failure, and peripheral artery disease were developed. Model performance was evaluated using likelihood ratio, R2, and Somers' Dxy rank correlation. Differences in model predictions were visualized using heat maps.
Results
Compared to BMI, the maximally flexible height-weight interaction model demonstrated improved prediction, higher likelihood ratio, R2, and Somers' Dxy rank correlation, for event-free probability for all outcomes. The degree of improvement to the prediction model differed based on the outcome and across the height and weight range.
Conclusions
Because alternative measures of body composition such as waist-to-hip ratio are not routinely collected in the clinic clinical risk models quantifying risk based on height and weight measurements alone are essential to improve practice. Compared to BMI, modeling height and weight as independent, interacting variables results in less bias and improved predictive accuracy for all tested traits. Considering an individual's height and weight opposed to BMI is a better method for quantifying individual disease risk.
1 INTRODUCTION
The most common proxy-measure of body fat in the clinical setting is body mass index (BMI),1, 2 a derived value where height and weight are assumed to act according to the fixed relation of weight divided by the square of height (kg/m2). While BMI has been consistently associated with multiple weight-related outcomes,3, 4 the intrinsic assumptions of BMI may result in limitations and biases as a predictive variable.5, 6 For example, recent work has demonstrated that BMI undervalues the predictive potential of height for blood pressure variation and the addition of height to measures such as waist circumference improves cardiometabolic risk prediction.7-10
Although BMI has limitations as a clinical measure of adiposity,5 the routine collection of height and weight and the general familiarity with BMI in clinical settings has perpetuated its use in clinical care, and it is likely that height and weight will continue to be the primary measures available to quantify adiposity. Therefore, understanding the performance of BMI versus a more flexible modeling of height and weight with respect to prediction of weight-related outcomes is critical to both research-based analyses and estimation of clinical risk.
Electronic health records (EHRs) are increasingly being used as a source of prospective clinical data for research. Importantly, researcher conducted using EHRs are limited to data collected as part of clinical care, and frequently will not have access to measures assesses in prospective epidemiologic cohorts. Consequently, researchers using EHRs may have to modify definitions of exposures and outcomes of interest based on available data. One example is metabolic syndrome. The criteria for clinical diagnosis is the presence of three or more of the following components: waist circumference >102 cm in males and 88 cm in females, blood pressure >130/85, fasting glucose >100 mg/dL, triglycerides >150 mg/dL, or reduced high density lipoprotein (HDL) <40 mg/dL in males and <50 mg/dL in females.11 The definition has expanded to include alternative criteria that are more commonly available to clinicians in the clinic, including elevated fasting glucose being replaced with anti-diabetic treatment and similar adjustments for blood pressure and lipid measurements being replaced with treatment. In this EHR-based study, as well as others, this surrogate diagnosis of metabolic syndrome is used in place of the classic definition due to data limitations as well as to reflect diagnostic practices.
As mentioned above, typical clinical assessments of adiposity do not routinely incorporate waist circumference or measures of central adiposity such as waist-to-hip ratio despite these measures being preferential predictors of disease risk. Because height and weight are so readily available and collected clinically, BMI continues the most frequently used surrogate measure of adiposity in both the clinic and in clinical research. Recognizing the limitations of BMI, we sought to investigate whether an alternative method to quantify the relationship between height and weight could provide a better surrogate of adiposity than BMI. This would provide an improved measurement to researchers leveraging the decades of clinical research in EHRs for which height and weight measurements are the primary data source.
Because excess weight is known to increase risk for the components of metabolic syndrome as well as cardiovascular disease,12-14 risk predictions models for these individual outcomes using BMI were compared to models using a maximally flexible, interacting model of the height/weight relationship. Specifically, model performance for metabolic syndrome, metabolic syndrome components, and a range of cardiovascular outcomes was evaluated.
2 METHODS
2.1 Study population
All data were extracted from a de-identified copy of the Vanderbilt University Medical Center EHR on 08/2019.15 Measures of height and weight after 18 years of age were cleaned and units harmonized to centimeters and kilograms, respectively, based on a previous method.16 Briefly the cleaning protocol included, removing nonphysiologic heights and weights including heights, <90 cm or >230 cm, removing heights that varied more than 3% from an individual's median value, and removing weights varying more than 33% of an individual's median value within 12 months.16 Individual BMIs were calculated from the remaining measurements.
Subjects were included in landmark analyses based on a prespecified 3-year qualification period that required four height and weight measures separated by approximately one year (1 year ± 4 months). For each outcome, subjects were excluded if the first occurrence of the particular outcome was before or during the qualification period. Validated data extraction methods were used to define outcomes: low high-density lipoprotein (HDL <40 mg/dL in males and <50 mg/dL in females), elevated triglycerides (triglycerides >150 mg/dL), hypertension, diabetes mellitus, atrial fibrillation (AF), coronary artery disease (CAD), heart failure (HF), and peripheral artery disease (PAD).17, 18 Because waist circumference and fasting glucose are infrequently ascertained in the clinical setting, we used a modified definition of metabolic syndrome, defined as two or more of the following events: diabetes mellitus, hypertension, low HDL, or elevated triglycerides.
2.2 Statistical analyses
Descriptive statistics were presented as count and frequencies for categorical variables and median and interquartile range for continuous variables. Comparisons were made using Pearson's chi-squared or Wilcoxon signed-rank, as appropriate. Cox regression analyses were conducted to examine how weight and height at a single time point, the end of the qualification period (i.e., t3), impact the hazard of developing each outcome. Analyses utilized two models: a maximally flexible model representing height and weight (each log transformed) as separate, non-linear (restricted cubic spline with three knots) terms and allowing for interactions; and a log transformed BMI model. All analyses were adjusted for sex, age (with restricted cubic spline with three knots), and race. Model performances were evaluated by likelihood ratio, R square, and Somers' Dxy rank correlation (index of discrimination between predicted score and observed responses). To quantify whether any of our findings could just be the result of overfitting a more complex model, we performed a bootstrap validation of each model to estimate the optimism in the calibration slope (slope of predicted vs. observed values).19
Event-free probability at 5 years was estimated across a wide range of height-weight combinations (weight range: 50–200 kg by 5 kg; height 160–200 cm by 5 cm [total 270 predictions]). In predicting event-free probability at five years, patient demographic characteristics were set to the population's median age of 51.2 years of age, white race, and female sex. Heat maps were presented to visualize predicted a 5-year event-free probability and the discrepancy between the two models across a full range of heights and weights. All statistical analyses were performed with R (version 3.3.1).
3 RESULTS
The demographics of included subjects are available in Tables S1 and S2. Metabolic syndrome event frequency was 16.7% and the frequencies for individual components were 5.5% for DM, 19.5%, for hypertension, 6.3% for low HDL, and 4.9% for elevated triglycerides. For cardiovascular outcomes, the event frequencies were 9.6% for AF, 21.8% for CAD, 8.8% for HF, and 2.8% for PAD.
Performances of the two body composition models for each outcome are summarized in Table 1. Briefly, the maximally flexible height*weight model had a better log likelihood ratio, R2, and discrimination ability (Somers' Dxy) than BMI with a maximum difference in model performance of 47.046, 0.0008, and 0.003, respectively. The worst calibration slope over all models was 0.99 (perfect is 1.0 when there is no overfitting) (Table S3). Loosely speaking, in the worst case 0.01 of what we learned is estimated to be from noise instead of signal. Figure S1 illustrates the calibration plot differences between the two models for metabolic dysregulation and its components and Figure S2 illustrates these differences for the cardiovascular outcomes.
| Prediction model | LR | DF | R2 | DI |
|---|---|---|---|---|
| Metabolic syndrome | ||||
| log Height-adjusted weight interaction | 3074.44 | 15 | 0.0424 | 0.3060 |
| log BMI | 3053.02 | 10 | 0.0418 | 0.3050 |
| Diabetes mellitus | ||||
| log Height-adjusted weight interaction | 2735.044 | 15 | 0.0431 | 0.4319 |
| log BMI | 2687.998 | 10 | 0.0423 | 0.4289 |
| High density lipoprotein | ||||
| log Height-adjusted weight interaction | 1102.552 | 15 | 0.0161 | 0.2645 |
| log BMI | 1084.594 | 10 | 0.0159 | 0.2636 |
| Hypertension | ||||
| log Height-adjusted weight interaction | 2900.190 | 15 | 0.0538 | 0.3221 |
| log BMI | 2877.066 | 10 | 0.0534 | 0.3214 |
| Triglycerides | ||||
| log Height-adjusted weight interaction | 1134.645 | 15 | 0.0184 | 0.2981 |
| log BMI | 1112.862 | 10 | 0.0181 | 0.2959 |
| Atrial fibrillation | ||||
| log Height-adjusted weight interaction | 5024.154 | 15 | 0.0591 | 0.4244 |
| log BMI | 4983.270 | 10 | 0.0586 | 0.4237 |
| Coronary artery disease | ||||
| log Height-adjusted weight interaction | 3375.364 | 15 | 0.0481 | 0.2752 |
| log BMI | 3347.507 | 10 | 0.0477 | 0.2743 |
| Heart failure | ||||
| log Height-adjusted weight interaction | 5486.422 | 15 | 0.0665 | 0.4390 |
| Log BMI | 5470.308 | 10 | 0.0664 | 0.4380 |
| Peripheral artery disease | ||||
| log Height-adjusted weight interaction | 2486.560 | 15 | 0.0503 | 0.5049 |
| log BMI | 2468.629 | 10 | 0.0500 | 0.5030 |
- Abbreviations: BMI, body mass index; DF, degrees of freedom; DI, discrimination index (Somers’ Dxy); LR, likelihood ratio.
Figures 1 and 2 (columns 1 and 2) present heat maps displaying the pattern of predicted a 5-year event-free probability for each outcome across the range of height and weight measures at t3. Similarly, gray-scaled heat maps were used to display the absolute discrepancy between model predictions ( ) (Figures 1 and 2, column 3). In addition, for each outcome we present a histogram of prediction differences (Figures 1 and 2, column 4). The distribution of a 5-year outcome risk prediction across the height-weight spectrum is presented in Figure S3.
) (Figures 1 and 2, column 3). In addition, for each outcome we present a histogram of prediction differences (Figures 1 and 2, column 4). The distribution of a 5-year outcome risk prediction across the height-weight spectrum is presented in Figure S3.
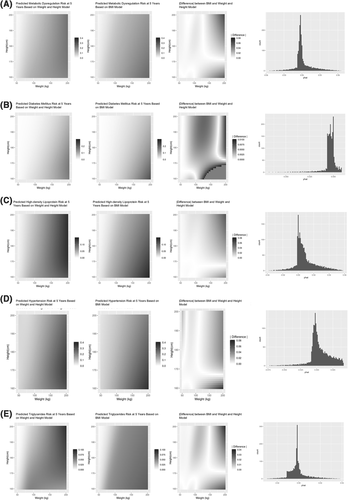
Comparison of predictive models for metabolic dysregulation and its components. (A) Metabolic dysregulation; (B) diabetes mellitus; (C) high density lipoprotein; (D) hypertension; (E) triglycerides. Graphics from left to right for each panel are height-adjusted weight model prediction of a 5-year free event probability across heights and weights, body mass index (BMI) model prediction of a 5-year free event probability across heights and weights, difference in model prediction ( ), and distribution of the difference in the prediction difference
), and distribution of the difference in the prediction difference
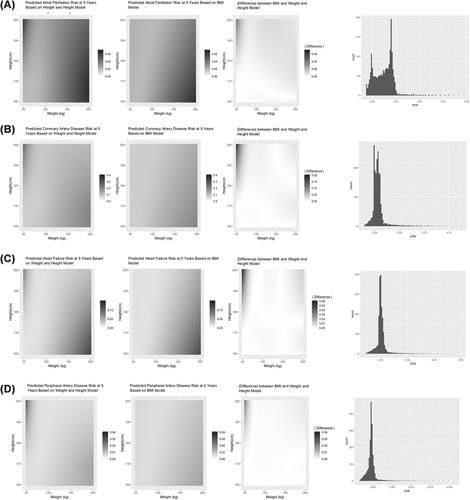
Comparison of predictive models for cardiovascular diseases. (A) Atrial fibrillation; (B) coronary artery disease; (C) heart failure; (D) peripheral artery disease. Graphics from left to right for each panel are height-adjusted weight model prediction of a 5-year free event probability across heights and weights, body mass index (BMI) model prediction of a 5-year free event probability across heights and weights, difference in model prediction ( ), and distribution of the difference in the prediction difference
), and distribution of the difference in the prediction difference
4 DISCUSSION
Using EHR-derived data to conduct a comparative analysis of prediction models using either BMI or an unbiased height*weight interaction model. Principal findings were that models with maximum flexibility outperform those using BMI across a wide range of cardiometabolic outcomes, and that discrepancies between models vary by outcome and location within the height-weight variable space.
Abnormal body composition is an important determinant of clinical outcomes, and accurately modeling the effect of height and weight on outcomes is critical for risk prediction. In this context, the model findings have several important implications. First, although BMI is the most commonly used measure of body composition, it demonstrates inferior performance compared to a maximally flexible model across all outcomes, suggesting the assumed fixed relationship between weight and height (i.e., kg/m2) inadequately represents the clinical impact of body composition. Second, BMI introduces complex non-uniform biases across outcome and height-weight space. For example, predicted risk for hypertension and diabetes mellitus is similar between both approaches with the exception of high body weight individuals with short stature where BMI significantly underestimates risk. In contrast, BMI introduces considerable error into prediction of the features of atherogenic dyslipidemia (low HDL and high triglycerides), especially at higher weights, where the contribution of height is poorly modeled. Different patterns emerge for cardiovascular outcomes. BMI systematically overestimates the contribution of height for lower weight individuals and underestimates height for heavy individuals with respect to CAD, HF, and PAD risk. For instance, a decrease in the predicted CAD event free risk is observed in tall slender individuals, height >180 cm and weight <50 kgs, an observation that is missed by the BMI model. By comparison, BMI consistently overestimates the contribution of height for AF, though the bias is most pronounced for those at extremes of weight.
While the absolute magnitudes of discrepancies between flexible and BMI-based models were frequently modest, they are not clinically insignificant, as they are frequently not small compared to the absolute risk of the outcome in question. For example, for abnormal HDL, the BMI model overestimates the event free probability of individuals in tall patients with extreme obesity patients from 0.02 to 0.06 (2.0% to 6.0%). Considering the frequency of abnormal HDL in the total population is 6.3% this overestimate may be as frequent as the outcome alone. For abnormal HDL, a similar concern of underestimation arises in extremely heavy short patients.
The current analysis adds to the literature by systemically examining the limitations and biases of BMI across the height-weight space and a diverse set of cardiometabolic outcomes. Because BMI is calculated from height and weight, the use of BMI is a choice rather than an issue of data availability. The rationale for using BMI has often focused on the ease of calculation and interpretation, familiarity with its use among clinicians and scientists, and its established value as a predictor. Allowing height and weight to “speak for themselves”, rather than be forced to exert effects through the BMI relationship, results in more accurate risk prediction across a range of conditions and outcomes. Thus, to the extent that more accurate risk prediction translates to improved patient care, future efforts should consider more flexible approaches to modeling height and weight.
There are limitations to this study. For example, known clinical predictors for the various outcomes were not included in models. However, this choice was made to allow characterization of how choice of body weight (i.e., BMI vs. height*weight) impacts model performance across outcomes. As is common with use of EHR data there are always concerns related to data sparsity. While the study design did its best to minimize sparsity issues, it remains possible that there is potential for confounding due to this, for example, it is possible that a patient may have a particular outcome, however, it was missed either due to misclassification or its development outside of the follow-up period due to various reasons. Finally, we did not compare this model's predictive performance with models including waist-circumference, waist-to-height ratio, other measures of adiposity because these measures are less frequently collected in large EHRs.
5 CONCLUSION
A data-driven, maximally flexible, log height-adjusted weight interaction model has better log likelihood for the prediction of weight-related outcomes than BMI. The prediction performance of these two models varies across the full spectrum of heights-weights and the absolute difference in model prediction may exceed the frequency of a given outcome. The scientific community should consider avoiding BMI when studying weight-related outcomes in favor of more flexible modeling strategies. Similarly, a more flexible modeling strategy could refine estimation of risk for weight-related outcomes in the clinical setting and improve identification of high-risk individuals for targeted interventions.
ACKNOWLEDGMENTS
The dataset used for the analyses described was obtained from the Vanderbilt University Medical Center Synthetic Derivative, which is supported by institutional funding, the 1S10RR025141-01 instrumentation award, and by the CTSA grant UL1TR000445 from National Center for Advancing Translational Sciences/National Institutes of Health. The work was supported by an American Heart Association strategically focused research network award, 17SFRN33520017. In addition, FE Harrell's work on this paper was supported by CTSA award No. UL1TR002243 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
CONFLICT OF INTEREST
J. Beckman declares potential conflicts with Amgen, Bayer, Glaxo Smith Kline, Janssen, Novartis, and Sanofi in the past 12months. All other authors declare no conflicts of interest.




