Early meal timing attenuates high polygenic risk of obesity
Rocío De la Peña-Armada and María Rodríguez-Martín contributed equally and share first authorship.
Richa Saxena and Marta Garaulet contributed equally and share last authorship.
Abstract
Objective
We examined whether meal timing is associated with long-term weight-loss maintenance and whether meal timing interacts with a genome-wide polygenic score (PRS-BMI) on body weight-related outcomes. We then examined the interaction of meal timing with 97 BMI-related single-nucleotide polymorphisms on obesity outcome.
Methods
Participants (N = 1195, mean age 41.07 [SD 12.68] years, female 80.8%, baseline mean BMI 31.32 [SD 5.53] kg/m2) were adults with overweight or obesity from the Obesity, Nutrigenetics, Timing, and Mediterranean (ONTIME) study. We developed a PRS-BMI to assess the genetic risk for obesity and estimated the timing of the midpoint of meal intake. We also calculated the success in long-term weight-loss maintenance after a dietary obesity treatment (at least 3 years). Linear regression analyses were performed for association and interaction assessments.
Results
Each hour of delay in meal timing was associated with 2.2% higher long-term body weight (β [SE] = 2.177% [1.067%]; p = 0.042) (i.e., with lower weight-loss maintenance following dietary obesity treatment). There was a significant interaction between meal timing and PRS-BMI (p = 0.008); BMI increased by more than 2 kg/m2 for every hour of delay in meal timing in individuals with high PRS-BMI (β [SE] = 2.208 [0.502] kg/m2; p = 1.0E-5), whereas no associations were evident for those with lower genetic risk.
Conclusions
Meal timing is associated with weight-loss maintenance and may influence the association between obesity genetics and BMI. Findings underscore the importance of personalized obesity management.
Study Importance
What is already known?
- Meal timing and genetic factors individually influence obesity risk and weight management, but their interaction is not well understood.
- Polygenic risk scores for BMI (PRS-BMI) are used to estimate genetic predisposition to obesity, although their relationship with lifestyle factors such as meal timing has not been explored in detail, to our knowledge.
What does this study add?
- Late eating is associated with poorer long-term weight maintenance after a dietary intervention for obesity.
- Late eating as a risk factor for obesity is particularly pronounced in individuals with a higher genetic predisposition to obesity, in whom late eating is linked to higher BMI values, whereas, in those with a lower genetic predisposition, this relationship is not observed.
How might these results change the direction of research or the focus of clinical practice?
- These findings underscore the importance of personalized nutrition strategies.
- Future research may focus on integrating lifestyle adjustments, such as meal timing, with genetic profiling to improve obesity prevention and treatment strategies.
INTRODUCTION
The timing of food consumption is an emerging lifestyle factor that has drawn considerable attention because it can impact obesity and weight management [(1)]. Research has suggested that meal timing may impact metabolism, energy expenditure, and physiological processes related to body weight regulation [(2, 3)]. Food timing can also act as a zeitgeber, or synchronizing time cue for peripheral oscillators, especially in metabolic tissues such as the liver, pancreas, and adipose tissue [(4, 5)]. In this way, a change in food timing can lead to a change in the timing of the molecular circadian clocks and, consequently, circadian rhythms in metabolic function [(6),(7)]. In addition, mistimed food intake can cause internal circadian misalignment [(4)]. Indeed, peripheral oscillators in metabolic organs and tissues, which are highly sensitive to food timing as a zeitgeber, may become desynchronized from the central clock, which is highly sensitive to environmental light [(8)]. It is hypothesized that such internal circadian misalignment may contribute to adverse cardiometabolic traits and obesity [(9-11)].
It is essential to recognize that individual responses to meal timing can vary. Whereas some people may benefit from specific food timing strategies, others may not experience significant changes in body weight. Knowing whether food timing is relevant for every person or whether the metabolic outcome may differ depending on the individual's genetic background is relevant. More importantly, not everybody with a high genetic risk for obesity develops obesity, and the timing of food intake may be involved in this variation. Indeed, in our previous work, it has been demonstrated that the genetic risk for obesity is associated with the timing of objectively measured workplace food purchases [(12)].
Genetic factors play a role in obesity susceptibility, and researchers have identified several genes associated with an increased risk of obesity [(13)]. Polygenic risk scores (PRS) are used in genetic research to estimate an individual's genetic susceptibility to a particular trait or condition, such as obesity.
Despite these new tools to estimate the individual's genetic background, it is essential to note that genetics alone do not determine whether an individual will develop obesity [(14)]. A complex interplay between our genetic makeup and life experiences gives rise to what is known as “genetic-environment interactions” [(15)]. Whereas much research has focused on factors related to “what we eat” concerning dietary intake [(16)], relatively little attention has been given to understanding the impact of “when we eat” on this complex relationship [(17)].
Previously, we have shown that food timing, measured as midpoint of meal intake, is related to obesity, obesity-related disorders, obesogenic behaviors, and weight-loss trajectory during an obesity program [(18)]. However, it is unclear how it may affect long-term weight-loss maintenance (WLM). In addition, the potential interactions of food timing with the body mass index (BMI)–genetic background of the individual for obesity, in terms of the response to a dietary obesity program and for long-term WLM, have not yet been explored, to our knowledge.
The midpoint of meal intake serves as an indicator of overall meal timing [(19)]. It is analogous to the sleep midpoint, which reflects overall sleep timing and is defined as the midpoint between sleep onset and wake time [(20)]. In addition, the midpoint of meal intake correlates with morning-evening chronotype scores [(21)] and shares genetic overlap with sleep timing and chronotype, suggesting common biological mechanisms [(19)]. It is considered as a heritable trait (with 64% heritability) [(19)], and, unlike methods based on energy distribution, which suit irregular eating patterns, the midpoint of meal intake is particularly relevant in structured meal patterns, such as those in Mediterranean populations.
Our primary aims were to examine the following: 1) the associations of the timing of food intake with obesity (measured using BMI), weight-loss effectiveness, and long-term WLM; 2) the associations of PRS-BMI with obesity, weight-loss effectiveness, and WLM; and the interaction of both timing of food intake and PRS-BMI for obesity, for weight-loss effectiveness, and for WLM.
As an exploratory aim, we also tested whether any of the 97 specific genetic variants already known to be associated with BMI [(13)] interacted with food timing for obesity.
Understanding the role of food timing in obesity, weight-loss effectiveness, and long-term WLM and its interaction with genetics may advance the development of novel prevention programs or targeted behavioral obesity interventions, including precision nutrition.
METHODS
Participants
This is an observational cross-sectional and prospective study. Participants (N = 1195) were adults with overweight or obesity enrolled in the Obesity, Nutrigenetics, Timing, and Mediterranean (ONTIME) study (ClinicalTrials.gov identifier: NCT02829619). A flowchart of the included population is represented in Figure 1. Participants were recruited from six weight-loss clinics in Spain. Excluded patients are described in the Extended Methods section in online Supporting Information.
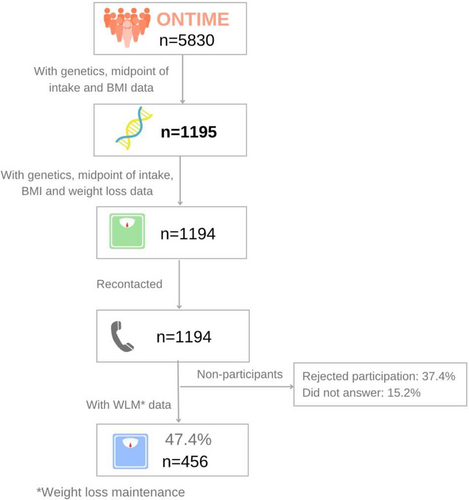
All participants followed a uniform multimodal weight-loss program based on nutrition education, physical activity, and behavioral techniques [(22)]. Meal timing was not controlled or specifically addressed in the program, which allowed for study participants' natural meal timing behaviors without being influenced by the treatment.
Those participants who attended the clinical center to lose weight more than 3 years ago were recontacted to assess their current weight (n = 456) to determine their success in long-term WLM.
Sample size was determined based on our previous study regarding significant associations between single-nucleotide polymorphisms (SNPs) and weight loss, which included a similar sample size (n = 1287) from the same ONTIME study population [(23)].
Participation in the study was voluntary, and all participants provided written informed consent before the study procedures. Study procedures were approved by the Committee of Research Ethics of the University of Murcia (identifier: 3092/2020) before recruitment, and the protocol followed Good Clinical Practice.
General characteristics
Age, sex, and BMI were recorded at baseline at enrollment, after the weight-loss treatment, and after at least 3 years of finishing the weight-loss treatment. A total of 98% of the studied population lost weight during treatment.
Weekly weight loss was evaluated during the dietary program for weight loss based on behavioral treatment. The mean number of weeks was 16 (SD 9). The characteristics of the weight-loss program have been described elsewhere in detail [(22)]. We calculated weight loss (percentage) as total weight loss in kilograms by comparing the “final weight” assessed at the end of the treatment with respect to the “initial weight” (weight in the first visit of the weight-loss treatment). Then, we divided participants into a group of those with success in weight loss (≥5%) and another group including those participants with non-success (<5%) [(24-26)]. The rate or speed of weight loss was determined as the average kilograms of body weight lost per week during the participant's intervention period.
Those participants who attended the clinical center to lose weight were recontacted to assess their current weight in the follow-up (after mean 12 [SD 3] years of finishing the weight-loss treatment). Current weight was measured mostly in the clinical center, in similar conditions to those at baseline. Weight regain percentage (WR%) was calculated as follows: (current weight − final weight) × 100/final weight. Final weight refers to body weight at the end of the obesity treatment.
Serum concentrations of glucose, cholesterol, triglycerides, and lipoproteins were determined at baseline during the first visit in fasting conditions [(27, 28)]. Metabolic syndrome (MetS) score was also calculated as described previously [(29)]. Serum leptin was also measured by radioimmunoassay (RIA) (LINCO Research, Inc.).
As part of the ONTIME study, a medical record was obtained from each participant, in which the obesity grade history was collected (see online Supporting Information).
Food timing and dietary composition
Upon enrollment (i.e., baseline), participants were asked the following question: “On weekdays (or weekends), at what time do you usually have breakfast (lunch or dinner)?” and responses were given in 15-min increments. Weighted weekly average breakfast and dinner times were computed with 5/7 weighting for weekdays and 2/7 for weekends. Based on our previous work, the midpoint of meal intake was determined by calculating the midpoint between the weekly averages for the first meal of the day (usually breakfast) and the last meal of the day (usually dinner), including post-dinner snacks [(19, 21)]. Then, we divided the participants into groups of early and late eaters using the midpoint of intake tertiles (first and third tertile). We also performed latent class analyses as complementary analyses to capture meal timing across all three meals. Dietary intake was assessed at baseline using a single 24-h dietary recall (online Supporting Information). In addition, 24-h and per-meal energy intake and macronutrient composition were determined using the nutritional evaluation software program GRUNUMUR based on Spanish food composition tables [(30)].
Physical activity
Habitual physical activity at baseline was evaluated using the International Physical Activity Questionnaire, which assesses physical activity over the past 7 days [(31)].
Genetics determination
Genotyping was conducted using the Infinium Global Screening Array version 3.0, whereas imputation was performed via the Michigan Imputation Server with the Haplotype Reference Consortium panel, and quality control was performed. Ancestry was determined using TRACE and the Human Genome Diversity Project (HGDP) as a reference panel (see online Supporting Information for more details).
We calculated a genome-wide PRS with 900,492 SNPs per individual. Each participant's genome-wide PRS-BMI was calculated using polygenic scores of continuous shrinkage (PRS-CS) [(32)]. PRS-CS provides a substantial improvement over existing methods for polygenic prediction [(32)] because it no longer considers only those significant gene variants in the genome-wide association studies (GWAS), but it is based on a Bayesian regression framework and places a continuous shrinkage for all SNP effect sizes. In the current work, effect estimates for PRS were derived from a meta-analysis of GWAS for BMI in ~700,000 individuals of European ancestry led by the Genetic Investigation of ANthropometric Traits (GIANT) consortium [(33)]. The PRS was standardized with a mean (SD) of 0 (1).
Statistical analyses
Linear regression analyses were conducted to determine the following primary outcomes: 1) the associations of the timing of the midpoint of meal intake with participants' BMI at baseline, weight-loss effectiveness (weight loss percentage and weight loss rate), and long-term WLM; and 2) the associations of PRS-BMI with participants' BMI at baseline and long-term WLM (measured as WR%). The association between PRS-BMI and weight loss has already been published by our team [(34)]. To determine for the first time the potential interactions of the timing of the midpoint of meal intake with PRS-BMI for BMI at baseline, weight-loss effectiveness, and long-term WLM, interaction models were performed. For this purpose, we added to the previous model the interaction variable of PRS-BMI × midpoint of meal intake and the independent variables (PRS-BMI and midpoint of meal intake).
In brief, for the primary outcomes, the variables used were as follows: 1) midpoint of meal intake (main exposure); 2) PRS-BMI (main exposure); 3) BMI at baseline (main outcome); 4) weight loss percentage and weight loss rate (main outcome); and 5) long-term WLM (WR%, main outcome). All of these were continuous variables. The description of main outcomes, main exposures, and covariables is presented in Table 1. A summary of the statistical analyses used is presented in Table S1A, and a logical framework of the study design is also presented in Table S1B.
| Main outcomes | Assessment |
|---|---|
| Body weight-related outcomes | |
| BMI. kg/m2 | At baseline, weight determined while the participant was wearing light clothes and barefoot with a digital scale to the nearest 0.1 kg; height measured using a Harpenden digital stadiometer at the same time of day |
| Body fat percentage | Measured by bioelectrical impedance, using TANITA TBF-300 equipment, at baseline |
| Weight loss percentage | Percentage calculated as total weight loss in kilograms with respect to the initial weight |
| Rate of weight loss, kg/wk | Average kilograms of body weight lost per week during the participant's intervention period |
| Weight-loss success | Success defined as weight loss percentage ≥5% and non-success as weight loss percentage <5% |
| Weight regain (WR), % | (Current weight − final weight after treatment) × 100/final weight after treatment; thus values >100% represent weight regain, whereas values <100% represent further weight loss after completion of the intervention |
| Main exposures | |
| Meal timing (midpoint of intake) (hh:mm) | Determined by calculating the midpoint between the weekly averages for the first meal of the day (usually breakfast) and the last meal of the day (usually dinner), including post-dinner snacks |
| PRS-BMI | Each participant's genome-wide polygenic BMI score was calculated using PRS-CSa with 900,492 SNPs |
| Covariables | |
| Main analyses | |
| Age, y | Recorded at baseline, when participants started the weight-loss treatment |
| Sex, % women/men | Participants categorized as woman or man according to their self-stated biological sex |
| Clinic site | Categorization of the site where participants underwent weight-loss treatment |
| Principal components of ancestry | Principal component analysis on genomic data used to adjust for ancestry differences |
| Baseline weight, kg | Weight determined at baseline wearing light clothes and barefoot with a digital scale to the nearest 0.1 kg |
| Treatment weeks | Weeks from treatment start to treatment finish, when participants achieved their weight goal |
| Years from end of treatment | Computed as years from the end of weight-loss treatment to date of recontact |
| Sensitivity analyses | |
| Energy intake, kcal | Using a single 24-h dietary recall, energy intake determined using a nutritional evaluation software program based on Spanish food composition tables |
| Physical activity, MET-min/wk | Using the International Physical Activity Questionnaire |
| Nighttime sleep duration, h | Computed as the interval between self-reported nighttime sleep onset and offset |
| Educational level | Educational level was self-reported as primary school, secondary school, and university |
| Macronutrient intake (carbohydrates, fat, protein, g) | Using a single 24-h dietary recall, macronutrient composition determined using a nutritional evaluation software program based on Spanish food composition tables |
- Abbreviations: MET, metabolic equivalent; PRS-BMI, polygenic score of BMI; PRS-CS, polygenic score of continuous shrinkage; SNP, single-nucleotide polymorphism.
- a Ge et al. [(32)].
All linear regression analyses were performed applying the correction for age, sex, clinic site, and principal component analysis for BMI, plus baseline weight and treatment weeks for weight-loss success and years since the weight-loss treatment for WR%. Sensitivity analyses included in the model total energy intake, physical activity levels, nighttime sleep duration, educational level, and macronutrient composition (i.e., carbohydrates, fat, and protein). We ensured the goodness of fit of all models by testing whether the residuals were approximately symmetrical.
We further divided PRS-BMI into groups according to the individual's genetic predisposition for obesity tertiles, namely, high, medium, or low, and tested for differences among the three groups in the characteristics of the populations using ANCOVA (adjusted by sex, age, clinical site, and principal components) for the second primary aim.
Exploratory analyses were performed for exploratory outcomes. We tested 97 SNPs that have been previously associated with BMI in GWAS [(13)] to examine whether the findings were driven entirely by a single variant. We also looked for potential interactions with the midpoint of meal intake for BMI at baseline. Analyses were performed separately for each of the 97 SNPs.
Other exploratory analyses were linear regression analyses between PRS-BMI and obesity traits, including body fat percentage, fasting serum leptin levels, and MetS score. Furthermore, we explored for heritability (mother and father obesity degree) and periods of life of obesity onset. Given the exploratory nature of these analyses, multiple comparison correction was not applied.
All statistical analyses were performed using SPSS Statistics software (IBM Corp.). A p value of <0.05 was considered statistically significant. Given the nature of our study and the pattern of the missing data, we chose not to impute the missing values, aiming to avoid potential biases introduced through imputation techniques. We proceeded with the analysis using the available data, and no missing values were included in the final analysis.
RESULTS
Table 2 presents the general characteristics of the total population. Participants were adults with an average age of 41 years; 80.8% were female, 36.1% had overweight, and 53.7% had obesity, with an average population BMI at baseline of 31.3 kg/m2. Of the 1195 individuals studied, 94% had an education level higher than secondary school, with 68% holding university degrees (Table 2). The meal timings were as follows: mean (SD) hh:mm, breakfast at 8:28 (0:55), lunch at 14:33 (0:32), and dinner at 21:20 (0:35). The midpoint of meal intake was 14:54 (0:35) hh:mm. Total weight loss during the treatment was 9.3% (SD 5.3%) of initial body weight, and those individuals with success in weight loss (≥5% of weight loss, as previously described [(26)]) were 78.9%. The percentage of participants who maintained their body weight at the long-term follow-up was 23.9%.
| N/n of total population | %, categorical variables | Mean ± SD, continuous variables | |
|---|---|---|---|
| Age, y | 1195 | 41.07 ± 12.68 | |
| Female, %a | 965 | 80.8 | |
| University education, % yes | 324 | 68.4 | |
| BMI (at baseline), kg/m2 | 1195 | 31.32 ± 5.53 | |
| Overweight, % yes | 431 | 36.1 | |
| Obesity, % yes | 642 | 53.7 | |
| Total weight loss, % of initial body weight | 1194 | 9.34 ± 5.28 | |
| Weight loss, % of success | 942 | 78.9 | |
| Rate of weight loss, kg/wk | 1194 | 0.60 ± 0.43 | |
| Weight regain, % of success | 109 | 23.9 | |
| Weight regain, % of body weight | 456 | 8.90 ± 13.28 | |
| Obesity onset, % | |||
| Childhood | 272 | 24.6 | |
| Adolescence | 247 | 22.3 | |
| Adulthood | 374 | 33.8 | |
| Never | 213 | 19.3 | |
| Mother with overweight/obesity, % | 661 | 56 | |
| Father with overweight/obesity, % | 529 | 44.9 | |
| Number of children with obesity | 934 | 0.25 ± 0.58 | |
| MetS score | 1042 | 2.13 ± 1.16 | |
| Glucose, mg/dL | 1178 | 86.63 ± 13.29 | |
| Cholesterol, mg/dL | 1192 | 193.23 ± 37.52 | |
| HDL cholesterol, mg/dL | 1192 | 57.76 ± 15.52 | |
| LDL cholesterol, mg/dL | 1191 | 114.91 ± 32.19 | |
| VLDL, mg/dL | 1182 | 20.36 ± 10.62 | |
| Triglycerides, mg/dL | 1191 | 102.15 ± 52.57 | |
| Blood pressure systolic, mm Hg | 1046 | 117.19 ± 14.89 | |
| Blood pressure diastolic, mm Hg | 1046 | 72.85 ± 10.16 | |
| Leptin, ng/mL | 188 | 21.38 ± 14.42 | |
| PRS-BMI | 1195 | −0.02 ± 0.95 | |
| Physical activity MET-min/wkb | 1080 | 3653 ± 6299 | |
| Midpoint of meal intake, h | 1195 | 14:54 ± 0:35 | |
| Timing of breakfast, h | 1195 | 8:28 ± 0:55 | |
| Timing of lunch, h | 1185 | 14:33 ± 0:32 | |
| Timing of dinner, h | 1195 | 21:20 ± 0:35 | |
| Midpoint of meal intake, h, early eaters | 399 | 14:17 ± 0:19 | |
| Midpoint of meal intake, h, intermediate eaters | 392 | 14:52 ± 0:08 | |
| Midpoint of meal intake, h, late eaters | 404 | 15:32 ± 0:20 | |
| Energy intake, kcal | 984 | 1969 ± 718 | |
| Protein, % of total kcala | 984 | 17.52 ± 5.21 | |
| Fat, % of total kcala | 980 | 41.10 ± 11.78 | |
| Carbohydrates, % of total kcala | 984 | 40.80 ± 10.92 | |
| Protein, g | 984 | 83.86 ± 33.25 | |
| Fat, g | 980 | 91.36 ± 45.95 | |
| Carbohydrates, g | 984 | 199.85 ± 88.18 | |
| Fiber, g | 986 | 16.75 ± 10.35 |
- Note: Characteristics and lifestyle traits of participants were measured at baseline, except weight loss and long-term weight regain.
- Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; MET, metabolic equivalent; MetS, metabolic syndrome; PRS, polygenic score; VLDL, very low-density lipoprotein..
- a Values in the percentage of the total population.
- b Trait log-transformed in the analytical model.
Association of midpoint of meal intake with obesity, weight-loss effectiveness, and long-term WLM
A summary of these results is represented in Figure 2. As a primary outcome, our midpoint of meal intake exhibited a significant association with BMI in the current population. Each hour of increment in the midpoint of meal intake was associated with a BMI at baseline increment of 0.952 kg/m2 (R2: 0.149; β [SE] = 0.952 [0.258] kg/m2; p = 2.0E-4). Further sensitivity analyses showed that the significance of the association was maintained even after adjusting for total energy intake, physical activity, nighttime sleep, educational level, and intake of the three macronutrients (i.e., carbohydrates, fat, and protein; all p < 0.05; see Extended Results in online Supporting Information).

We also found a significant association between the midpoint of meal intake and the speed or rate of weight loss during the treatment. Each hour of increment in the midpoint of meal intake was associated with a decrease in the rate of weight loss of −0.046 kg/week (R2: 0.265; β [SE] = −0.046 [0.019] kg/week; p = 0.013).
Regarding long-term WLM, we found a significant association between the midpoint of meal intake and WR%. Importantly, each hour of increment in the midpoint of meal intake was associated with an increment of 2.2% in body weight in the long-term follow-up (after mean 12 [SD 3] years of finishing the weight-loss treatment) (R2: 0.084; β [SE] = 2.177% [1.067%]; p = 0.042). When further adjusting for total daily energy intake, each hour of increment in the midpoint of meal intake was associated with an increment of 2.7% in body weight in the long term (R2: 0.094; β [SE] = 2.734% [1.210%]; p = 0.024).
Association of PRS-BMI with obesity and related heritability traits
Another primary outcome was that our global PRS for BMI exhibited a significant association with BMI of the participants (Figure 2). Each SD increment in PRS was significantly associated with a 1.763-kg/m2 higher BMI value (R2: 0.235; β [SE] = 1.763 [0.150] kg/m2; p = 2.728E-30). Further sensitivity analyses showed that the significance of the association was maintained even after adjusting for total energy intake, physical activity, nighttime sleep duration, educational level, and intake of the three macronutrients (i.e., carbohydrates, fat, and protein; overall p = 8.0E-5; online Supporting Information).
The PRS-BMI, however, was not significantly associated with long-term WR% (R2 = 0.098; β [SE] = 0.963% [0.663%]; p = 0.147).
Differences among the three tertiles of PRS-BMI in the characteristics of the populations are presented in Table S2. Furthermore, the model predicted BMI values for each individual case based on the linear regression equations. Thus, predicted BMI according to PRS in tertiles is represented in Figure S1.
Interaction between PRS-BMI and the timing of food intake for obesity, weight-loss treatment effectiveness, and long-term WLM
We did not find a significant association between PRS-BMI and midpoint of meal intake (R2 = 0.028; β [SE] = −0.010 [0.018]h; p = 0.572). Nevertheless, our primary analyses results revealed a significant interaction between both variables for BMI, i.e., the timing of the midpoint of meal intake modified the effect of PRS-BMI on BMI (R2 = 0.251; β [SE] = 0.687 [0.257] kg/m2; p = 0.008; Figure 2). Further sensitivity analyses showed that the significance of the interaction was maintained even after adjusting for total energy intake, physical activity, nighttime sleep duration, educational level, and intake of the three macronutrients (i.e., carbohydrates, fat, and protein; overall p = 0.007; online Supporting Information).
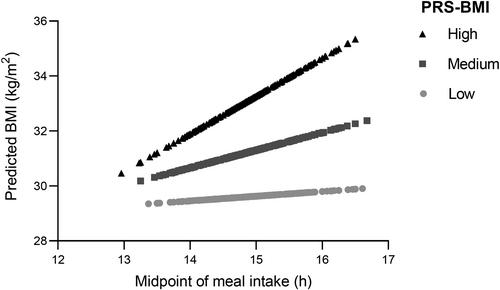
To further explore this relationship, we conducted an analysis stratified by tertiles of PRS-BMI. Linear regression analysis within each tertile of PRS-BMI indicated that the association between the timing of the midpoint of meal intake and BMI was significant for the highest PRS-BMI tertile (R2 = 0.196; β [SE] = 2.208 [0.502] kg/m2; p = 1.0E-5). For example, in this top tertile PRS-BMI group, BMI increased by more than 2 kg/m2 for every hour delay in meal timing. In other words, individuals in the third tertile of PRS-BMI, with the highest genetic predisposition for obesity and later meal timing, exhibited a higher BMI (p = 2.056E-7). Specifically, in this high genetic risk group, there was a difference of 3.29 kg/m2 in BMI between early and late eaters (mean [SD] BMI for the earliest midpoint of meal intake tertile = 31.97 [5.60] kg/m2 and BMI for latest tertile = 35.26 [7.27] kg/m2). Similar results were obtained after using the latent class analyses (online Supporting Information).
However, the midpoint of meal intake was not significantly associated with BMI of the individuals in the second tertile of PRS-BMI (R2 = 0.195; β [SE] = 0.191 [0.388] kg/m2 per hour in meal timing; p = 0.623) or the first tertile (R2 = 0.198; β [SE] = 0.720 [0.382] kg/m2 per hour in meal timing; p = 0.063).
Figure 3 illustrates the interaction between the midpoint of meal intake and PRS for predicted BMI within each PRS-BMI tertile. For this representation, we used regression equations to predict the value of BMI (dependent variable) for observations of the midpoint of meal intake (independent variable) in each of the calculated PRS-BMI tertiles (high, medium, and low genetic predisposition for obesity). This allows better visualization of data tendency. Figure S2 presents the adjusted BMI using the raw data.
When we studied the potential interaction between PRS-BMI and meal timing for weight loss (percentage) during the dietary treatment and for rate of weight loss, no significant interactions were found for weight loss (R2 = 0.024; β [SE] = −0.336 [0.280]% of weight loss per hour in meal timing; p = 0.230) or for rate of weight loss (R2 = 0.073; β [SE] = 0.005 [0.023]kg/week per hour in meal timing; p = 0.822). With respect to long-term WLM, no significant interaction was found for WR% (R2 = 0.055; β [SE] = −1.116 [1.177]% per hour in meal timing; p = 0.344).
Secondary outcomes (exploratory analyses)
We also explored the associations between the global PRS for BMI and other obesity traits, heritability, and periods of life of obesity onset. Our results showed that the global PRS for BMI was significantly associated with body fat (percentage), fasting serum leptin levels, and MetS score (online Supporting Information).
Further analyses showed that the global PRS for BMI was significantly associated with the period of life when the individuals started to develop obesity, with higher PRS-BMI when the obesity onset was earlier in life. The PRS-BMI was also significantly associated with the degree of obesity of the mother (online Supporting Information).
As secondary outcomes, additional exploratory analyses were conducted to identify those genetic variants or SNPs that individually interacted with meal timing for BMI (Tables S3 and S4 and Figure S3). Our results revealed that, from the 97 genetic variants that have previously been shown to be associated with BMI [(13)], 2 genetic variants (i.e., rs9925964 and rs1018218) nominally interacted with meal timing for BMI in different directions (positive or negative sign of effect size) depending on whether they may be protective or promoters of obesity (Table S4 and Figure S3).
DISCUSSION
This study highlights the potential role of meal timing in the relationship between genetic predisposition and BMI. Our primary findings revealed that a later midpoint of meal intake is associated with less success in long-term WLM following weight loss. Furthermore, the timing of the midpoint of meal intake interacts with PRS for BMI, suggesting that early meal timing may attenuate high polygenic risk of obesity.
Meal timing is relevant for long-term WLM
The timing of food intake can impact various physiological processes related to body weight regulation and metabolism [(1, 3, 8)]. Meal timing has been previously shown to be relevant for obesity and for weight-loss effectiveness [(18)]. In the current study, we demonstrate that meal timing is relevant for long-term WLM, which, in most individuals, has proven painfully tricky, and subsequent weight gain is typical [(35)]. To our knowledge, this is the first study to test the potential involvement of food timing in long-term WLM.
Modifiable behavioral factors may be critical for the overall success of long-term WLM. Previously, it has been reported that individuals who succeed in the long term are those who report engaging in high levels of physical activity (i.e., 1 h/day), eating a low-calorie diet or a low-fat diet, eating breakfast regularly, self-monitoring body weight, and maintaining a consistent eating pattern across weekdays and weekends [(35)]. Now, we demonstrate that food timing is also a relevant modifiable factor in long-term maintenance of body weight, and advancing meal timing could be an adequate strategy for success in long-term WLM.
Early meal timing attenuates high polygenic risk of obesity
Our findings show that the association between meal timing and BMI varied depending on the individual's genetic background, specifically within the highest PRS tertile. Those individuals with a greater genetic predisposition for obesity and later meal timing had the highest BMI values, whereas those with earlier meal timing had lower BMI values.
Interestingly, the timing of food intake was not significantly associated with BMI in those individuals with a medium or low genetic predisposition for obesity who were in the second or first tertile of PRS-BMI. These findings suggest that early eating may be especially relevant for individuals with a genetic predisposition for obesity and not for others. Further studies should test whether the individual's responsiveness to meal timing interventions for weight management may differ depending on the genetic background.
Remarkably, there was no difference in predicted BMI among the three PRS-BMI tertiles for the earliest midpoint of meal intake (toward −2 h compared to the median), suggesting that early eating may mitigate the effects of genetic risk for obesity on BMI. Understanding the interplay between genetics and meal timing may help inform personalized approaches to obesity prevention and targeted behavioral interventions, such as precision nutrition.
Relevant mechanisms
It is important to note that the mechanisms involved in the interaction between meal timing and genetic background are still being actively researched.
Changes in circadian rhythms and clock genes may be implicated in these results [(36)]. The timing of food intake can influence the alignment of circadian rhythms (i.e., the 24-h biological cycles) [(4, 7, 37, 38)] that regulate various physiological processes, including metabolism [(39)]. Genetic variations in clock genes, i.e., those responsible for maintaining circadian rhythms, may affect an individual's sensitivity to meal timing and subsequent metabolic responses.
Meal timing may also influence the energy balance by regulating the timing and distribution of nutrient intake throughout the day [(40)]. Studies have shown that consuming larger meals later in the day may lower energy expenditure patterns [(3)] and alter the usage of macronutrients such as carbohydrates or protein [(41)]. In the current study, total energy intake and macronutrient composition are not involved in the significant interaction between PRS-BMI and the timing of food intake for BMI. Indeed, data show that the significance was maintained even after adjusting by both variables, which suggests that the increase in BMI that characterized those individuals with high genetic risk for BMI, who used to eat late, is independent of energy intake and dietary composition.
Genetic factors may influence the body's response to nutrient timing and use, contributing to variations in BMI based on meal timing. Meal timing can also affect the release and regulation of hormones involved in appetite control, satiety, and energy expenditure across the day and influence adipose tissue metabolic pathways [(3)]. Genetic factors may affect the sensitivity and responsiveness of these hormonal systems to meal timing cues, affecting energy intake, nutrient metabolism, and subsequent body weight [(23)].
Notably, the current study identified specific genetic variants (i.e., rs9925964 and rs1018218) that showed nominal interaction with meal timing for BMI. These genetic variants have been previously identified as BMI-associated loci through a GWAS and Metabochip meta-analysis of BMI in up to 339,224 individuals [(13)]. The mechanism involved in this nominal interaction is unknown, and the exploratory nature of these analyses may incur type I error. Further replication studies are needed to validate these genetic variants and their interaction with meal timing, and similar analyses considering correction for multiple comparisons are necessary.
The current population comes from a Mediterranean area in outheast Spain, in which meal timing is very well structured and rather stable [(42)]. Previous literature addressing meal timing patterns across different countries has revealed that Mediterranean countries had later timing of meals and snacks compared to Northern/Central European countries [(43)]. An interesting aspect of this is that, in these Mediterranean countries with delayed meal timing, the ratio in energy intake between later meals during the day (dinner) and earlier meals (such as breakfast) is lower than in those countries with earlier meal timing. This circumstance may account for the lower impact of late eating in the Mediterranean countries [(43)]. In addition, in Spain, lunch accounts for ~40% of the daily energy intake, whereas, in the US population, lunch represents 24% of the daily energy intake [(44)] and dinner represents about 35% of the daily energy intake [(45)]. It is remarkable that a high energy intake earlier in the day may positively influence health compared with later energy consumption [(43)]. More specifically, lower dietary consumption close to bedtime and higher dietary consumption after waking up have been associated with lower BMI values [(46)].
It is essential to acknowledge the limitations of the study. The results come from a cross-sectional study based on a specific population of adults with overweight and obesity enrolled in the ONTIME study. Therefore, we should be cautious in generalizing the findings to other populations. Additionally, the study relied on self-reported meal timing and dietary intake, which may be subject to recall bias [(47)]. Moreover, to assess long-term WLM, the weight regain was assessed in 456 participants who responded to the recruitment. Recontacting individuals who attended an obesity program several years ago is challenging; therefore, the lack of interaction between PRS-BMI and meal timing for this variable may be related to the relatively lower sample size. Further research is needed to validate and expand upon these findings, including replication studies in diverse populations and the use of objective measures for assessing meal timing and dietary intake. In the current study, sensitivity analyses further adjusted by nighttime fasting showed similar significant association and interaction results (data not shown). Nevertheless, further studies controlling the fasting period are mandatory to ensure that the association with obesity is due to the time itself. Owing to the observational nature of the study, we cannot infer causality from our results. Some factors, such as stress, emotional eating, and social pressure, may affect meal timing. For example, the prevalence of less healthy food options during nighttime may also influence the dietary choices of late eaters [(48)].
In our study, the predominance of female participants (81%) over male participants reflects a well-documented trend in weight-management interventions, in which women are more likely to seek nutritional guidance and participate in related studies [(49, 50)]. Although no interaction between the main outcomes and sex was found, we accounted for sex as a covariable to mitigate potential biases, and it is important to acknowledge that differences in metabolic, behavioral, and psychosocial factors between men and women could influence the outcomes. Future research should aim to recruit a more balanced sample or conduct stratified analyses to better understand potential sex-specific responses to nutritional interventions.
In conclusion, this study sheds light on the potential interactions among genetics, meal timing, and obesity. From an intervention point of view, these results suggest that early meal timing may help to maintain the weight loss in the long term, and it may also mitigate the increased risk of obesity by genotype. Genetics may also have an impact in the association between meal timing and BMI, emphasizing the importance of considering genetic factors and lifestyle behaviors in obesity management. Nevertheless, BMI genetics is not associated with weight-loss effectiveness or long-term WLM. Future research exploring the underlying mechanisms and conducting intervention studies can provide further insights into how optimizing meal timing in individuals with specific genetic profiles can be leveraged to prevent and manage obesity effectively.
AUTHOR CONTRIBUTIONS
The authors' responsibilities were as follows: conceptualization, Marta Garaulet, Richa Saxena, and Frank A. J. L. Scheer; methodology, Rocío De la Peña-Armada, María Rodríguez-Martín, Hassan S. Dashti, Frank A. J. L. Scheer, Richa Saxena, and Marta Garaulet; Ana Isabel Cascales, María Rodríguez-Martín, and Marta Garaulet were instrumental in re-contacting patients during the follow-up; formal analysis, Rocío De la Peña-Armada, María Rodríguez-Martín, Hassan S. Dashti, and Marta Garaulet; data curation, Marta Garaulet; writing, original draft preparation, Rocío De la Peña-Armada, María Rodríguez-Martín, and Marta Garaulet; writing, review and editing, Rocío De la Peña-Armada, María Rodríguez-Martín, Hassan S. Dashti, Frank A. J. L. Scheer, Richa Saxena, and Marta Garaulet; visualization, Rocío De la Peña-Armada, María Rodríguez-Martín, and Marta Garaulet; and supervision, Richa Saxena and Marta Garaulet. All authors have read and approved the final version of the manuscript.
FUNDING INFORMATION
The ONTIME study received the following funding: PID2020-112768RB-I00 and PID2023-146183OB-I00, funded by MCIN/AEI/10.13039/501100011033, The Autonomous Community of the Region of Murcia through the Seneca Foundation (20795/PI/18), and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01DK105072 granted to Marta Garaulet. María Rodríguez-Martín was supported by the MICINN grant PID2020-112768RB-I00.
CONFLICT OF INTEREST STATEMENT
Frank A. J. L. Scheer served on the Board of Directors for the Sleep Research Society and has received consulting fees from the University of Alabama at Birmingham. Frank A. J. L. Scheer's interests were reviewed and managed by Brigham and Women's Hospital and Partners HealthCare following their conflict-of-interest policies. Frank A. J. L. Scheer's consultancies are not related to the current work. Richa Saxena is a cofounder of Magnet Biomedicine, which is unrelated to the current work. The other authors declared no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data described in the manuscript will be made available upon request pending to corresponding authors Marta Garaulet ([email protected]) and Richa Saxena ([email protected]).






