Glucagon-like peptide-1 agonists combined with sodium-glucose cotransporter-2 inhibitors reduce weight in type 1 diabetes
Funding information: Kuwait Foundation for the Advisement of Sciences, Kuwait; Ministry of Health, Kuwait
Abstract
Objective
This study evaluated whether adding sodium-glucose cotransporter-2 inhibitors (SGLT2i) and/or glucagon-like peptide-1 receptor agonists (GLP1-RA) to insulin reduced weight and glycemia in people with type 1 diabetes.
Methods
This retrospective analysis of electronic health records evaluated 296 people with type 1 diabetes over 12 months after medications were first prescribed. Four groups were defined: control n = 80, SGLT2i n = 94, GLP1-RA n = 82, and combination of drugs (Combo) n = 40. We measured changes at 1 year in weight and glycated hemoglobin (HbA1c).
Results
The control group did not have changes in weight or glycemic control. The mean (SD) percentage weight loss after 12 months was 4.4% (6.0%), 8.2% (8.5%), and 9.0% (8.4%) in the SGLT2i, GLP1-RA, and Combo groups, respectively (p < 0.001). The Combo group lost the most weight (p < 0.001). The HbA1c reduction was 0.4% (0.7%), 0.3% (0.7%), and 0.6% (0.8%) in the SGLT2i, GLP1-RA, and Combo groups, respectively (p < 0.001). The Combo group had the biggest improvements in glycemic control and total and low-density lipoprotein cholesterol compared with baseline (all p < 0.01). Severe adverse events were similar between all the groups, with no increased risk of diabetic ketoacidosis.
Conclusions
The SGLT2i and GLP1-RA agents on their own improved body weight and glycemia, but combining the medications resulted in more weight loss. Treatment intensification appears to result in benefits with no difference in severe adverse events.
Study Importance
What is already known?
- Sodium-glucose cotransporter-2 inhibitors (SGLT2i) and glucagon-like peptide-1 receptor agonists (GLP1-RA) provide multiple benefits in people with type 2 diabetes, but the benefits and risks of these drugs for type 1 diabetes are not fully known.
What does this study add?
- In people with type 1 diabetes, SGLT2i and GLP1-RA alone reduced body weight and glycemic control, but coadministration of both medications was more effective for weight reduction.
- Weight loss was greater in patients with obesity compared with those who did not have obesity. In this sample, there was no significant increased risk of diabetic ketoacidosis or hypoglycemia with SGLT2i or GLP-1 RA therapy or the combination.
How might these results change the direction of research or the focus of clinical practice?
- Patients with type 1 diabetes and obesity may benefit from the use of SGLT2i and GLP1-RA either alone or in combination to improve body weight and glycemia.
INTRODUCTION
Type 1 diabetes mellitus is a chronic autoimmune disease characterized by an inability to produce insulin, resulting in hyperglycemia [(1)]. Recurrent exposure to severe hyperglycemia increases the risk of macro- and microvascular complications [(2, 3)]. Although exogenous insulin therapy remains the mainstay of treatment, intensified insulin therapy increases glucose variability, severe hypoglycemia, and body weight [(4-6)]. Many people fail to achieve optimal glycemic control despite intensive insulin therapy [(4, 7)] and instead develop central adiposity, insulin resistance, and dyslipidemia [(6, 8)]. These conditions amplify the risk of metabolic syndrome [(8)]. Therefore, therapy must improve glycemic control without increasing the risk of hypoglycemia and weight gain.
Several new therapies are available to manage type 2 diabetes [(9)]. The popularity of sodium-glucose cotransporter-2 inhibitors (SGLT2i) and glucagon-like peptide-1 receptor agonists (GLP1-RA) for type 2 diabetes management continues to grow. GLP1-RA stimulates insulin secretion in a glucose-dependent manner and suppresses glucagon secretion and appetite [(10, 11)]. SGLT2i prevents glucose reabsorption in kidney tubules, increases glucosuria independently of insulin action, and subsequently improves glycemia without increasing the risk of hypoglycemia in type 2 diabetes [(12)] while inducing benefits, such as reduction in weight, myocardial infarctions, and heart failure [(13)]. These beneficial effects observed in type 2 diabetes have encouraged some physicians to initiate GLP1-RA and/or SGLT2i in people with type 1 diabetes. Previous studies evaluated the efficacies and safety of GLP1-RA and SGLT2i therapy in individuals with type 1 diabetes, but the combination of the medications is not yet described [(14-17)]. In clinical trials, the addition of GLP1-RA liraglutide to insulin led to a reduction in glycated hemoglobin (HbA1c), body weight, and total insulin dose; however, episodes of hypoglycemia or hyperglycemia with ketosis increased [(14, 15)]. Similarly, adding SGLT2i dapagliflozin to insulin therapy was associated with improved HbA1c and reduced body weight and insulin dose [(16-18)]. The hypoglycemic episodes were comparable in the treatment and placebo groups, but the episodes of diabetic ketoacidosis (DKA) were higher in the treatment group. A similar result was also reported with the SGLT2i sotagliflozin when initiated by people with type 1 diabetes [(19, 20)]. Therefore, the risks and benefits of adjunctive agents continue to be evaluated in people with type 1 diabetes. The SGLT2i and GLP1-RA medications used in the treatment groups in our practice were predominantly dapagliflozin and liraglutide. We used the electronic health records at Dasman Diabetes Institute in Kuwait to retrospectively evaluate these drugs' impact on body weight and HbA1c in people of Arab descent with type 1 diabetes.
METHODS
Design
This observational study was conducted on prospectively collected clinical data during routine clinical care at the Dasman Diabetes Institute.
Study sample
After receiving approval from the Institutional Review Board (435/2016), we retrospectively collected the demographic and biomedical parameters of people with type 1 diabetes from their electronic health records. We include patients 18 years and older with at least 1 year of follow-up since the new therapy initiation. All patients had a documented diagnosis of type 1 diabetes (per American Diabetes Association 2022 definition/criteria), which was confirmed by an undetectable C-peptide level at diagnosis and the presence of autoantibodies consistent with the diagnosis of type 1 diabetes. All people with type 1 diabetes were enrolled in the Dose Adjustment for Normal Eating (DAFNE) program, a structured education program for type 1 diabetes. The DAFNE team had regular contact, collected information, and recommended therapy changes while monitoring adverse events, vitals, and laboratory reports. All participants were on insulin treatment using US Food and Drug Administration-approved insulin pumps (continuous subcutaneous insulin infusion) or multiple daily injections. Patients were on stable insulin doses for 3 months. All patients had a blood ketone meter and urine reagent strips and they were on a flash or continuous glucose monitoring device for at least 2 months. The clinical reasons for starting either SGLT2i (dapagliflozin), GLP1-RA (liraglutide), or the combination were often the high burden of obesity in the people with type 1 diabetes cared for in Kuwait, especially those with suboptimal glycemic control. The exclusion criteria included normal weight, pregnancy, dementia, psychosis, a hospitalization history within the past 6 months, DKA, or severe hypoglycemia within 6 months. Patient data were anonymized entirely before analysis.
Demographic and biomedical data
Demographic and biomedical data included age, gender, duration of type 1 diabetes, weight (kg), height (cm), and HbA1c. BMI was calculated as weight in kilograms divided by height in meters squared. Secondary data included complications, adverse events experienced by patients, and biochemical parameters. Discontinuation of the prescribed drug was defined as a gap of at least 30 days from the first prescription end date.
Statistical analyses
Data were analyzed using SPSS Statistics software version 24.0 (IBM Corp.). Paired t test was employed to compare values before and after the adjunct therapy initiation. The outcomes were expressed as mean difference (MD), mean ± standard deviation (SD), or frequency with percentage. One way ANOVA was employed to compare continuous variables between groups. The χ2 test was used to compare categorical variables across all groups. A two-sided p value ≤ 0.05 was considered statistically significant.
RESULTS
Baseline characteristics
This study included 80 people with type 1 diabetes who received usual care as a control group and 216 people with type 1 diabetes in whom SGLT2i and/or GLP1-RA therapy was initiated. The baseline characteristics of all four groups are presented in Table 1. Of the total people, 150 (51%) were female, with a mean (SD) age of 36.9 (9.3) years (range, 18–72 years). The mean duration of diabetes was 18.9 (8.6) years (range, 2–46 years). The mean BMI was 30.8 (4.0) kg/m2, with 54% of patients having BMI > 30 kg/m2. The mean HbA1c was 66.3 (13.2) mmol/mol (8.2% [1.2%]), with 84% of patients having baseline HbA1c >53.0 mmol/mol (7.0%).
| Control (n = 80) | SGLT2i (n = 94) | GLP1-RA (n = 82) | Comboa (n = 40) | p value | |
|---|---|---|---|---|---|
| Gender | |||||
| Male, n (%) | 42 (52.5) | 52 (55.3) | 31 (37.8) | 21 (52.5) | 0.12 |
| Female, n (%) | 38 (47.5) | 42 (44.7) | 51 (62.2) | 19 (47.5) | |
| Age (y), mean (SD) | 33.8 (9.0) | 38.6 (8.9) | 36.5 (9.2) | 38.0 (10.1) | 0.01 |
| Diabetes duration (y), mean (SD) | 18.7 (10.2) | 19.1 (8.2) | 18.7 (8.6) | 19.5 (7.1) | 0.96 |
| HbA1c (%), mean (SD) | 8.2 (1.3) | 8.2 (1.1) | 8.1 (1.2) | 8.4 (1.2) | 0.63 |
| HbA1c (mmol/mol), mean (SD) | 66.3 (14.7) | 66.1 (11.8) | 65.3 (13.0) | 68.6 (13.4) | 0.63 |
| HbA1c > 7% (> 53.0 mmol/mol), n (%) | 65 (81.3) | 83 (88.3) | 65 (79.3) | 36 (90.0) | 0.24 |
| HbA1c ≤ 7% (≤ 53.0 mmol/mol), n (%) | 15 (18.8) | 11 (11.7) | 17 (20.7) | 4 (10.0) | |
| Body weight (kg), mean (SD) | 82.3 (12.8) | 83.6 (13.8) | 84.8 (14.8) | 93.3 (16.3) | 0.001 |
| BMI (kg/m2), mean (SD) | 30.1 (2.9) | 30.0 (4.4) | 31.3 (3.7) | 33.3 (4.7) | 0.001 |
| BMI < 30 kg/m2, n (%) | 44 (55.0) | 48 (51.1) | 33 (40.2) | 11 (27.5) | 0.02 |
| BMI >30 kg/m2, n (%) | 36 (45.0) | 46 (48.9) | 49 (59.8) | 29 (72.5) | |
| Systolic BP (mmHg), mean (SD) | 118.7 (14.0) | 121.5 (13.8) | 119.9 (12.4) | 118.2 (19.8) | 0.51 |
| Diastolic BP (mmHg), mean (SD) | 74.3 (9.2) | 74.2 (9.2) | 74.0 (9.5) | 73.4 (13.9) | 0.97 |
| Creatinine (mmol/L), mean (SD) | 71.9 (27.3) | 77.3 (26.1) | 81.7 (28.6) | 79.3 (28.4) | 0.16 |
| Albumin-creatinine ratio, mean (SD) | 44.4 (167.3) | 74.9 (252.3) | 89.9 (330.9) | 116.3 (413.8) | 0.73 |
| Total cholesterol (mmol/L), mean (SD) | 4.8 (1.0) | 4.6 (1.1) | 4.5 (1.0) | 4.8 (1.2) | 0.22 |
| HDL cholesterol (mmol/L), mean (SD) | 1.6 (0.5) | 1.6 (0.4) | 1.6 (0.4) | 1.5 (0.5) | 0.69 |
| LDL cholesterol (mmol/L), mean (SD) | 2.8 (0.9) | 2.6 (1.0) | 2.4 (1.0) | 2.8 (1.1) | 0.05 |
| Triglyceride (mmol/L), mean (SD) | 0.8 (0.4) | 1.0 (0.6) | 0.8 (0.5) | 1.1 (0.9) | 0.001 |
| Insulin basal (units/day), mean (SD) | 26.4 (10.2) | 30.6 (13.3) | 29.2 (14.0) | 34.1 (17.4) | 0.03 |
| Insulin bolus (units/day), mean (SD) | 32.6 (16.4) | 33.2 (16.9) | 31.2 (14.3) | 31.6 (14.2) | 0.87 |
| Total insulin (units/day), mean (SD) | 59.2 (22.0) | 63.8 (26.3) | 58.7 (23.1) | 65.9 (26.3) | 0.33 |
| Dyslipidemia, n (%) | 35 (43.8) | 82 (91.1) | 44 (60.3) | 39 (97.5) | < 0.001 |
| Hypertension, n (%) | 8 (10.0) | 31 (41.9) | 17 (28.3) | 15 (53.6) | < 0.001 |
| Retinopathy, n (%) | 20 (69.0) | 59 (66.3) | 28 (43.8) | 29 (78.4) | 0.002 |
| Nephropathy, n (%) | 1 (1.30) | 10 (14.1) | 4 (7.7) | 2 (8.7) | 0.03 |
| Neuropathy, n (%) | 8 (10.0) | 14 (19.4) | 7 (13.2) | 5 (21.7) | 0.31 |
- Abbreviations: BP, blood pressure; GLP1-RA, glucagon-like peptide-1 receptor agonist; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SGLT2i, sodium-glucose cotransporter-2 inhibitor.
- a Combo means both SGLT2i and GLP1-RA simultaneously started.
Reduction of weight and HbA1c
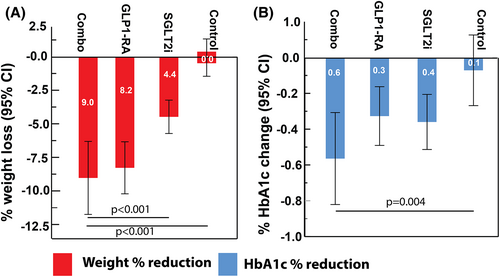
Table 2 presents the effects of SGLT2i and GLP1-RA drugs alone and in combination (Combo) on weight and glycemia. The 80 patients in the control group who received usual care had mean (SD) BMI of 30.1 (2.9) kg/m2 and HbA1c of 66.3 (14.7) mmol/mol (8.2% [1.3%]). They did not significantly change in weight or HbA1c after 1 year (all p > 0.06). The 94 people in the SGLT2i group had BMI of 30.0 (4.4) kg/m2 and HbA1c of 66.1 (11.8) mmol/mol (8.2% [1.1%]). They had a reduction in weight and HbA1c of 3.7 (5.2) kg (4.4% [6.0%]) and 3.9 (8.0) mmol/mol (0.4% [0.7%]), respectively, after 1 year of medication initiation (all p < 0.001). The 82 people in the GLP1-RA group had BMI of 31.3 (3.7) kg/m2 and HbA1c of 65.3 (13.0) mmol/mol (8.1% [1.2%]). They had a reduction in weight of 7.1 (7.8) kg (8.2% [8.5%]) and HbA1c of 3.6 (7.7) mmol/mol (0.3% [0.7%]) (p ≤ 0.001). The 40 people treated with a combination of SGLT2i and GLP1-RA (Combo) had BMI of 33.3 (4.7) kg/m2 and HbA1c of 68.6 (13.4) mmol/mol (8.4% [1.2%]). They had a reduction in weight of 8.5 (8.6) kg (9.0% [8.4%]) and HbA1c reduction of 6.2 (8.5) mmol/mol (0.6% [0.8%]) (all p ≤ 0.007). Weight loss was superior in the Combo group compared with the control and the SGLT2i groups after 6 months and after 1 year (p < 0.001), with superior HbA1c improvements at 1 year in the combo group compared with controls (p = 0.004) (Table 2; Figure 1). The decreased body weight in the treatment groups translated into BMI reductions of 1.4 (2.0), 2.7 (2.9), and 3.1 (3.0) kg/m2 in the SGLT2i, GLP1-RA, and Combo groups after 1 year, respectively (all p < 0.001 from baseline; Supporting Information Table S1). Interestingly, the weight reduction in those with a starting BMI > 30 kg/m2 was greater than in those with BMI ≤ 30 kg/m2 for the SGLT2i group (5.1 [6.7] kg vs. 2.7 [3.4] kg), GLP1-RA group (8.9 [9.3] kg vs. 4.7 [4.1] kg), and Combo group (9.8 [9.6] kg vs. 5.3 [4.1] kg). Similarly, HbA1c reduction was greater in people who had baseline HbA1c > 7% for the SGLT2i (0.4%), GLP1-RA (0.4%), and Combo groups (0.6%) compared with those who had HbA1c ≤ 7% (all p < 0.01; Supporting Information Table S1).
| 6-Month follow-up | p valuec | 12-Month follow-up | p valuec | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | 95% CIa | N | 95% CIa | ||||||
| Group | MD (SD)b | Lower | Upper | MD (SD)b | Lower | Upper | ||||
| Weight (kg) | ||||||||||
| Control | 78 | 0.4 (4.6) | −0.7 | 1.4 | 0.48 | 80 | −0.1 (5.1) | −1.2 | 1.1 | 0.91 |
| SGLT2i | 94 | −2.4 (3.7) | −3.2 | −1.6 | <0.01 | 93 | −3.7 (5.2) | −4.8 | −2.6 | <0.01 |
| GLP1-RA | 82 | −6.3 (5.8) | −7.5 | −5.0 | <0.01 | 77 | −7.1 (7.8) | −8.9 | −5.3 | <0.01 |
| Combod | 40 | −7.8 (6.5) | −9.9 | −5.8 | <0.01 | 39 | −8.5 (8.6) | −11.3 | −5.7 | <0.01 |
| HbA1c (%) | ||||||||||
| Control | 78 | −0.2 (0.8) | −0.4 | 0.0 | 0.02 | 79 | 0.0 (0.9) | −0.2 | 0.2 | 0.74 |
| SGLT2i | 92 | −0.4 (0.7) | −0.6 | −0.3 | <0.01 | 94 | −0.4 (0.7) | −0.5 | −0.2 | <0.01 |
| GLP1-RA | 82 | −0.4 (0.7) | −0.5 | −0.2 | <0.01 | 74 | −0.3 (0.7) | −0.5 | −0.2 | <0.01 |
| Combod | 40 | −0.5 (0.8) | −0.8 | −0.3 | <0.01 | 38 | −0.6 (0.8) | −0.8 | −0.3 | <0.01 |
| HbA1c (mmol/mol) | ||||||||||
| Control | 78 | −2.5 (9.2) | −4.6 | −0.4 | 0.02 | 79 | −0.4 (9.9) | −2.6 | 1.8 | 0.74 |
| SGLT2i | 92 | −4.5 (7.9) | −6.1 | −2.9 | <0.01 | 94 | −3.9 (8.0) | −5.5 | −2.2 | <0.01 |
| GLP1-RA | 82 | −3.9 (8.1) | −5.7 | −2.1 | <0.01 | 74 | −3.6 (7.7) | −5.3 | −1.8 | <0.01 |
| Combod | 40 | −5.9 (8.5) | −8.6 | −3.1 | <0.01 | 38 | −6.2 (8.5) | −9.0 | −3.3 | <0.01 |
- Abbreviations: GLP1-RA, glucagon-like peptide-1 receptor agonist; SGLT2i, sodium-glucose cotransporter-2 inhibitor.
- a 95% confidence interval.
- b Mean difference (± standard deviation).
- c Paired t test.
- d Combo—both SGLT2i and GLP1-RA simultaneously started.
Supporting Information Table S1 presents the changes in blood pressure, lipid profile, serum creatinine, and insulin dose. Systolic and diastolic blood pressure at baseline were within the reference range and it did not differ between the groups. The GLP1-RA group demonstrated significant systolic blood pressure changes, but the changes were within the reference range. The Combo group had significantly reduced total cholesterol and low-density lipoprotein cholesterol (LDL-C) levels (p < 0.001 for both). This change was not observed in the single-drug groups. None of the groups demonstrated changes in serum creatinine and urine albumin-creatine ratios. The basal insulin dose reduction in the SGLT2i group was 3.7 (10.7) units/day and 3.7 (8.5) units/day in the GLP1-RA group (both p < 0.01). However, the Combo and the control group did not exhibit reduced basal or bolus insulin doses (all p > 0.40). The total insulin reduction was 5.1 (17.0) units/day (p = 0.02), 5.2 (18.5) units/day (p = 0.04), 0.7 (17.1) units/day (p = 0.83), and 1.7 (23.8) units/day (p = 0.54) in the SGLT2i, GLP1-RA, Combo, and control groups, respectively; however, no differences were observed between the groups (p = 0.56) (Supporting Information Table S2).
A total of five people experienced DKA, of which one episode was observed in the GLP1-RA group, one in the SGLT2i group, and three in the control group. All patients had a ketone meter and urine reagent strips, but no other patients reported increased urinary ketones between clinic visits. None of the episodes was severe and none required hospital admission. These were treated with the support of the DAFNE hotline, emphasizing sick day rules.
One individual in the GLP1-RA group and four in the control group reported one severe hypoglycemic event requiring third-party help during the 1-year follow-up period. The control group had 1.2 ± 1.0 hypoglycemic events per week at baseline and 1.3 ± 1.0 events per week after 1 year (p = 0.74). The SGLT2i group had 1.7 ± 2.1 hypoglycemic events per week before and 1.8 ± 1.6 events per week after the intervention (p = 0.71). The GLP1-RA group had 1.5 ± 1.8 events per week before and 2.2 ± 2.0 events per week after the intervention (p = 0.07), and the Combo group had 1.2 ± 1.4 events per week before and 1.9 ± 2.3 events per week after the intervention (p = 0.18). The proportion of participants who reported increases in hypoglycemic events per week was 28% (1.3 ± 0.9 events) in control, 29% (1.7 ± 1.0 events) in SGLT2i, 23% (2.6 ± 1.5 events) in GLP1-RA, and 15% (3.0 ± 2.5 events) in the Combo groups (Table 3). The overall change in all of these patients' reported hypoglycemic events per week was significant (p = 0.004).
| Group | Participants with increased hypoglycemia events, N (%) | Events/week, MD (SD)a | p valueb |
|---|---|---|---|
| Control | 25 (28) | 1.3 (0.9) | 0.004 |
| SGLT2i | 27 (29) | 1.7 (1.0) | |
| GLP1-RA | 19 (23) | 2.6 (1.5) | |
| Comboc | 6 (15) | 3.0 (2.5) |
- Abbreviations: GLP1-RA, glucagon-like peptide-1 receptor agonist; SGLT2i, sodium-glucose cotransporter-2 inhibitor.
- a Mean difference (± standard deviation).
- b ANOVA test between all groups.
- c Combo means both SGLT2i and GLP1-RA simultaneously started.
DISCUSSION
This study presents the clinical benefits of SGLT2i and GLP1-RA drugs alone or in combination in Arab people with type 1 diabetes. GLP1-RA reduced weight, HbA1c, and daily insulin doses. This finding is consistent with other clinical study results, in which HbA1c was reduced by 0.43% and weight by 4.6 kg after 6 months of liraglutide without severe episodes of hypoglycemia [(21)]. GLP1-RA appears to reduce weight, total daily insulin dose, and glycemia without increasing hypoglycemic risk in people with type 1 diabetes [(22, 23)]. In addition, SGLT2i reduces weight, HbA1c, and daily insulin doses without changing systolic or diastolic blood pressure, a finding consistent with those of several other studies [(24-26)].
The strength of our study was that the combined effect of SGLT2i/GLP1-RA appeared additive in reducing weight and HbA1c, as well as total cholesterol and LDL-C. To our knowledge, this is the first study to report the combination of SGLT2i/GLP1-RA in people with type 1 diabetes.
Body weight reduction was highest in the Combo group, but weight loss was most profound in people with obesity in all three groups. The reduction of glycemia in the GLP1-RA and the SGLT2i groups during the first 6 and 12 months was almost identical. Still, the reduction of glycemia in the combo group trended to be even better. However, the reduction of weight and glycemia in the Combo group occurred without a significant reduction in exogenous insulin dose. Insulin dose reduction was based on the treating physician's judgment and individual patient requirements. The physicians were aware that avoiding excessive insulin dose reductions after initiating adjunct dapagliflozin therapy may mitigate the risk of DKA [(16, 17, 27)]. All patients were graduates of the DAFNE program where they underwent intensive, structured education of more than 25 hours.
The mechanisms of action of the drugs in type 1 diabetes may differ or depend on the remaining number of β-cells. Our patients had no residual β-cell function left, evidenced by undetectable C-peptide levels; hence, the underlying mechanism of action of GLP1-RA in our patients is likely different from those with type 2 diabetes. In the latter group, there is a direct augmentation of β-cell function and an indirect improvement of insulin sensitivity by promoting weight loss. SGLT2i indirectly augments β-cell function in type 2 diabetes by reversing glucotoxicity, thus improving insulin sensitivity and weight loss [(28)]. Therefore, the additive effects of SGLT2i/GLP1-RA can be expected to improve β-cell function and enhance peripheral tissue sensitivity to insulin in type 2 diabetes.
DKA is a potential side effect of SGLT2i, but its incidence can vary [(26, 29)]. Rare cases of DKA were reported in patients with type 1 diabetes using GLP1-RA [(14)]. In this study, one episode of DKA was observed in a person who used SGLT2i and in another who used GLP1-RA. Several studies report episodes of DKA in people with type 1 diabetes treated with SGLT2 inhibitors [(17, 18)]. The risk of DKA markedly increased in those who substantially decreased insulin doses to mitigate the risk of hypoglycemia [(30)]. DKA episodes were rare in our study, but the risk remains important. The relatively low number of DKA episodes in our study may be attributable to a growing understanding of the pathogenesis of DKA, identification of responsible factors, and its management, especially given the comprehensive DAFNE course and the absence of alcohol use in our population [(31-33)].
Self-reported hypoglycemic events were not similar between all our groups. The number of hypoglycemic episodes in the GLP1-RA and the Combo groups was almost equal, but this was double what was observed in the SGLT2i and the control groups. Usually, symptomatic hypoglycemia experienced by people with type 1 diabetes is, on average, two events per week [(34)]. Comparatively fewer hypoglycemia events in the SGLT2i group could be associated with the slight reduction of insulin dose and an improvement in glycemic control [(17)]. The hypoglycemic events in the GLP1-RA group were consistent with the evidence in the ADJUNCT ONE and ADJUNCT TWO trials, in which people with type 1 diabetes treated with liraglutide increased symptomatic hypoglycemia [(14, 35)]. They reported that hypoglycemic events were uniformly distributed throughout the trial [(35)]. More hypoglycemic events in the Combo group could be associated with unchanged daily insulin doses.
Our study's limitations include the retrospective nature of our analysis and the failure to randomize the patients to different treatments. However, our data provide the basis for conducting a power calculation for future studies. Adverse events were self-reported and captured as part of clinical care and the DAFNE database. The lack of evidence guiding practice and the nonrandomized nature of this study resulted in no patterns emerging as to which medications were used in our study's various retrospectively defined groups. Our nonrandomized study is also not enough to clearly define the place for combinations of SGLT2i and GLP-1 in patients with type 1 diabetes. Still, a rationale can be made to treat patients with type 1 diabetes and obesity. This may, in the future, lead to the exploration of whether these drugs may also have renoprotective and cardioprotective effects in people with type 1 diabetes [(36)].
This is the first real-world evidence of the impact of SGLT2i, GLP-1RA, and their combination in Arab patients with type 1 diabetes. These drugs individually had a positive effect on weight loss and glycemic control; however, the combination of these drugs appears even more beneficial for weight loss while remaining safe. To change clinical practice, evidence from randomized controlled trials in patients with type 1 diabetes and obesity is necessary.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to this study. EA contributed to conception and design, supervised the data collection and analysis, wrote the manuscript, and agreed to be accountable for all aspects of the work to ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; MI contributed to data analysis and interpretation and wrote the manuscript; LS and ET contributed to data collection and ensured data accuracy; JA and DA reviewed the literature and wrote the manuscript. ClR critically reviewed the manuscript's important intellectual content, reviewed the data analysis, and provided final approval of the version to be published.
ACKNOWLEDGMENTS
The authors thank the staff members of the DAFNE unit who have helped in the retrospective data collection and Dasman Diabetes Institute for administrative support, in addition to the clinical laboratory service at Dasman Diabetes Institute.
CONFLICT OF INTEREST
ClR reports grants from the Irish Research Council, Science Foundation Ireland, Anabio, and the Health Research Board. He serves on advisory boards of Novo Nordisk, Herbalife, GI Dynamics, Eli Lilly, Johnson & Johnson, Glia, and Boehringer Ingelheim. ClR is a member of the Irish Society for Nutrition and Metabolism outside the area of work commented on here. He was the chief medical officer and director of the Medical Device Division of Keyron in 2011. Both of these were unremunerated positions. He continues to provide scientific advice to Keyron for no remuneration. The other authors declared no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data will be available upon reasonable request from the corresponding author.





