Circadian rhythm parameters and physical activity associated with cardiometabolic risk factors in the PREVIEW lifestyle study
Funding information: Netherlands Organization of Scientific Research (NWO), Grant/Award Numbers: Aspasia, VIDI grant 917.15.350; NUTRIM, School of Nutrition and Translational Research in Metabolism, Maastricht University, Maastricht, the Netherlands; Seventh Framework Programme, Grant/Award Number: 312057; University of Groningen and EU Co-Fund, Grant/Award Number: Rosalind Franklin Fellowship
Abstract
Objective
The aim of this study was an assessment of post hoc associations among circadian rhythm parameters, physical activity (PA), and cardiometabolic risk factors in adults with obesity and prediabetes after 3 years of weight loss maintenance.
Methods
Circadian rhythm parameters (continuous wrist-temperature measurements), PA, systolic and diastolic blood pressure (SBP, DBP), heart rate (HR), plasma high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol, remnant cholesterol, triacylglycerol, and C-reactive protein (CRP) concentrations were determined in 91 free-living participants (mean [SD], age = 56.6 [10] years; BMI = 28.2 [4.0]; homeostatic model assessment of insulin resistance [HOMA-IR] = 3.2 [3.1]) and in 38 participants in sedentary respiration chamber conditions (age = 56.6 [10] years; BMI = 28.5 [4.0]; HOMA-IR = 3.3 [1.4]). Associations of circadian rhythm parameters and PA with cardiometabolic risk factors were determined using factor analyses followed by Pearson correlations.
Results
Values of cardiometabolic risk factors were similar, whereas circadian rhythm parameters and PA differed significantly (p < 0.05) between conditions. In both conditions, parameters indicating a robust circadian rhythm associated inversely with CRP and positively with plasma HDL-C concentrations. In free-living conditions, PA associated inversely with SBP and HR and positively with HDL-C and robust circadian rhythm parameters. In sedentary conditions, PA associated positively with HR and inversely with robust circadian rhythm parameters. PA mediated the inverse association of parameters indicating a robust circadian rhythm with SBP in free-living conditions.
Conclusions
In adults with obesity and prediabetes, parameters indicating a robust circadian rhythm were, independently of PA, associated with lower cardiometabolic risk and CRP. Only in free-living conditions, PA mediated the association of higher circadian stability with lower SBP.
Study Importance
What is already known?
- Parameters indicating a robust circadian rhythm have been positively associated with control of body weight and insulin sensitivity.
- Circadian misalignment showed increases in cardiometabolic risk factors.
- Physical activity (PA) has been inversely associated with cardiovascular risk factors.
What does this study add?
- Parameters indicating a robust circadian rhythm (wrist-temperature measurements) were associated inversely with C-reactive protein and positively with plasma high-density lipoprotein cholesterol concentrations.
- Inverse associations of parameters indicating a robust circadian rhythm with systolic blood pressure in free-living conditions were mediated by PA.
- In free-living conditions, higher PA (but lower PA in sedentary conditions) was associated with parameters indicating a robust circadian rhythm.
How might these results change the direction of research or the focus of clinical practice?
- Assessment of relations between cardiometabolic risk factors and lifestyle requires an integrated approach including circadian rhythm parameters and physical activity.
INTRODUCTION
Obesity and insulin resistance are associated with increased cardiometabolic risk, represented by hypertension, a prothrombotic state, and dyslipidemia [(1, 2)]. Dyslipidemia reflects reduced levels of plasma high-density lipoprotein cholesterol (HDL-C), elevated plasma levels of small-dense low-density lipoprotein cholesterol (LDL-C), triacylglycerol (TAG), and remnant cholesterol (RC) [(1-4)]. Elevated plasma RC and TAG may lead to low-grade inflammation and atherogenesis [(4-6)]. Suboptimal cardiometabolic health accompanied by low-grade inflammation has been shown already in a prediabetic state [(7)].
Not only lifestyle in terms of diet and exercise [(8, 9)], but also circadian rhythm may play a role in control of cardiometabolic risk [(10-18)]. Circadian alignment, i.e., timing and regularity of meals, physical activity (PA), and sleep, synchronized with the individual's circadian rhythm, seems crucial for cardiometabolic health. The circadian system synchronizes physiological processes in the body with the environment. From the central biological clock, located in the suprachiasmatic nucleus (SCN) in the hypothalamus, neural and humoral signals are conducted to other brain areas and to peripheral tissues and organs, such as the heart, aorta, vascular cells, and cardiomyocytes [(10)]. The central biological clock is considered to be representative of both intrinsic (autonomic nervous activity, humoral factors) and exogenous factors, such as timing of meals, physical activity, and the sleep/wake state [(11, 12)]. Several cardiac functions, such as resting heart rate (HR) and blood pressure (BP), show circadian variation that is tightly related to the SCN [(12)]. Misalignment studies disturbing synchronization between central and peripheral clocks, such as in shift workers, showed increases in cardiometabolic risk by increased HR, BP, plasma LDL-C, TAG, and C-reactive protein (CRP) and decreased HDL-C concentrations [(13-18)]. In addition, increased PA has been associated with decreased cardiovascular risk [(19-23)], whereas at the same time, PA is suggested to be associated with circadian rhythm [(19-23)]. For example, a field study estimating circadian rhythm parameters using continuous wrist-temperature (WT) measurements for seven consecutive days, in two groups of men differing in metabolic health, body fat, and PA, observed a significant difference in circadian amplitude and stability between participants with at least a single metabolic syndrome risk factor and those with no metabolic syndrome risk. The variance in circadian amplitude and stability was explained by PA, showing PA as an important measure associated with differences in circadian rhythm parameters [(19)].
Until now, lifestyle studies that investigated possible effects of changes in diet and increases in PA on reduction of obesity and related cardiometabolic risk have not included circadian rhythm parameters as part of a lifestyle. Also, including PA while investigating effects of circadian rhythm parameters [(19)] has hardly been shown. The relevance of including PA assessment in studies on modulation of cardiovascular risk markers by the circadian system was shown by Scheer et al., who showed modulation of cardiovascular risk markers by the circadian system at rest as well as their reactivity to exercise, with resultant profiles that could contribute to the day/night pattern of adverse cardiovascular events [(20)]. Moreover, experimental circadian misalignment in humans modified the skeletal muscle clocks and led to disturbed energy metabolism and insulin resistance [(21, 22)]. Observed upregulated skeletal muscle clock gene expression in an exercised versus nonexercised leg suggests that exercise may alter gene expression of skeletal muscle clocks [(21)]. Exercise, and especially timing of exercise, may contribute to circadian alignment [(20-22)].
Although associations of circadian rhythm with cardiometabolic risk [(13-18)], as well as associations of PA with cardiometabolic risk and with circadian rhythm [(19-23)], have been reported, possible associations between these three factors within one study have not been shown. The aim of the present study was to investigate possible associations of circadian rhythm parameters measured by WT [(23-28)] with cardiometabolic risk factors and plasma CRP concentrations in adults with increased cardiometabolic risk due to obesity and prediabetes, during seven consecutive days in the free-living condition. At the same time, possible associations of PA with cardiometabolic risk factors and with circadian rhythm parameters were investigated. To further assess the role of PA, the same associations were investigated during 48 hours in a controlled, sedentary respiration chamber condition.
METHODS
Main study
The present study is a substudy of the PREVIEW intervention study [(29, 30)]. Briefly, the PREVIEW study (trial registration: ClinicalTrials.gov: NCT01777893) was a multicenter randomized controlled trial that compared the impact of two long-term weight maintenance diets (high-protein, low-glycemic index [GI] and moderate-protein, moderate GI) combined with high-intensity or moderate-intensity PA on incidence of type 2 diabetes during 34 months of weight maintenance after 8 weeks of rapid weight loss in adults with overweight/obesity and prediabetes [(29)]. Prediabetes was defined as either (i) increased fasting glucose, with venous plasma glucose concentration of 5.6 to 6.9 mmol L−1 and/or (ii) impaired glucose tolerance, with venous plasma glucose concentration of 7.8 to 11.0 mmol L−1 at 2 hours and fasting plasma glucose < 7.0 mmol L−1. [(29, 30)]. A total of 2326 adults (age 25–70 years, BMI ≥ 25 kg/m2) with prediabetes participated in the PREVIEW study, from 2013 to 2018 (Figure 1). Secondary outcomes included body weight, glucose and insulin concentrations, homeostatic model assessment of insulin resistance (HOMA-IR), HR, systolic BP (SBP), diastolic BP (DBP), total plasma cholesterol, HDL-C, LDL-C, TAG, and CRP concentrations [(29, 30)], and they did not differ significantly between groups [(30)].
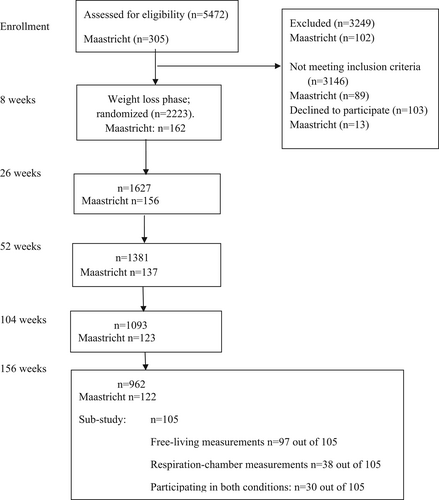
Design of the present substudy
The present study was conducted in 105 adult PREVIEW participants at Maastricht University in the Netherlands, during 2017 and 2018 (Figure 1). Apart from following the original PREVIEW inclusion and exclusion criteria [(29)], those with shift work or sleep disorders, as indicated by a score > 6 on the Pittsburgh Sleep Quality Index [(31)], were excluded from the present study. The Medical Ethical Committee of Maastricht University approved the study, and all participants gave written informed consent. The substudy was registered on ClinicalTrials.gov with identifier NCT01777893 [(29, 30)]. Because no differences among the four original intervention groups were observed [(30)], the data were pooled and used for the present post hoc associations.
During 2017 and 2018, at 3 years of weight loss maintenance, cardiometabolic risk factors, BMI, HOMA-IR, and PA were determined. During the week preceding the final clinical investigation day of the main PREVIEW study, circadian rhythm parameters were determined in 97 out of 105 participants on seven consecutive days in the free-living condition. Randomly, either 3 to 4 weeks before or after the free-living measurements, the measurements were conducted in the 48-hour sedentary respiration chamber condition in 38 out of 105 participants [(11)] (Figure 1). A total of 30 participants underwent both conditions; these data were used for comparisons of the values between both conditions. Power calculations to test, with a paired design, a study power of 0.8, and an effect size of 10%, differences in the circadian rhythm parameters amplitude, interdaily stability (IS), and intradaily variability (IV) between the two conditions indicated that 24 participants were necessary [(11, 23, 24)].
Measurements
Body weight, HOMA-IR, cardiovascular risk factors, and CRP
Body weight was measured using a calibrated scale. Height was measured using a wall-mounted stadiometer during screening. BMI was calculated as weight in kilograms divided by height in meters squared [(29, 30)]. At 8:30 a.m. at the clinical investigation day at year 3, and at 8:30 a.m. at day 2 of the respiration chamber measurements, resting HR, SBP, and DBP were measured in the fasting condition, and fasting blood samples were taken from an antecubital vein, according to standard operating procedures. Blood samples were initially stored locally at −80 °C and then transported and analyzed centrally at the National Institution for Health and Welfare (THL) in Helsinki, Finland, where they were analyzed for glucose, insulin, plasma TAG, total cholesterol, LDL-C, HDL-C, and high-sensitivity CRP concentrations [(29)]. HOMA-IR was calculated as fasting insulin in mU L−1 × fasting glucose in mmol L−1/22.5 [(29, 30)]. RC concentration was calculated as plasma total cholesterol—(HDL-C + LDL-C) concentration [(5)].
PA and sleep duration estimation
To quantify PA and sleep duration, the ActiSleep+ (ActiGraph LLC) accelerometer was worn. It was attached to an elastic waist belt and worn over the right midaxillary line 24 h/d for seven consecutive days in the free-living condition and continuously in the respiration chamber condition, removing it only for water-based activities [(29, 30)]. Data were collected using 60-second epochs, and nonwear was classified as 60 minutes of consecutive zeros with the allowance of interruptions for up to 2 minutes [(32)]. After the removal of nocturnal sleep episodes [(32)], participants providing at least 4 days, including at least one weekend day, of valid data (≥ 10 h/d of waking wear time) were included in the analysis [(32)]. Sleep time was determined using a fully automated algorithm developed for use with a 24-hour waist-worn accelerometer protocol validated in adults [(32)]. Counts per minute were derived and used to estimate PA [(33)]. Counts per minute has been shown to correlate well with total activity energy expenditure measured by the doubly labeled water technique [(33)].
Respiration chamber
Participants stayed in the respiration chamber for 48 hours for assessment of circadian rhythm variables and cardiometabolic risk factors in a controlled sedentary condition implying fixed bed and meal times, PA level (total energy expenditure/basal metabolic rate) of 1.35, and fixed room temperature at 21 °C [(11)]. The respiration chamber stay started at 9:30 a.m. and finished 2 days later at 9:30 a.m. Bedtimes were fixed from 11:30 p.m. to 7:30 a.m., based upon deviating maximally 30 minutes from the participants' regular bed- and wake-up times. Breakfast was offered at 9:00 a.m., lunch at 1:00 p.m., and dinner at 5:45 p.m.; participants were instructed to finish their meals within 30 minutes. Participants were fed based on their individual daily energy requirements, which were calculated as basal metabolic rate multiplied by a PA level of 1.35 [(11)]. Water consumption was allowed ad libitum between the meals; no other foods or beverages were available. During daytime, participants were not allowed to sleep or to perform exercise. Ambient light was gradually dimmed from 8:00 to 11:30 p.m., and using phones or tablets was not allowed after 9:00 p.m., in order to avoid circadian disruption [(11)].
Circadian rhythm measurements
In the free-living condition and in the respiration chamber, WT was measured continuously [(23-28)]. WT presents as a mirrored pattern compared with core body temperature with lowest values during the day and highest values during the night. In previous BP research, a concordance of 78.6% was shown between actual and WT indicated BP pattern [(27)]. Circadian rhythm parameters as measured by WT are partially under the regulation of the circadian system and they do not assess circadian rhythm directly. However, WT measured circadian rhythm parameters are associated with the timing of light exposure and amplitude of melatonin secretion, thus reflecting circadian rhythm [(26)].
A temperature sensor (Thermochron, iButton DS1921H, Dallas Semiconductor/Maxim) was fixed to the inside of the wrist using medical tape, and the sensor surface was placed over the radial artery of the nondominant hand [(11)]. The sensitivity of the sensor was 0.125 °C; the temperature was measured every 10 minutes. Temperature, date, and clock time were stored in the iButton and transferred to a computer using iButton Viewer (version 3.22, Dallas Semiconductor/Maxim) [(23-25)]. During the free-living as well as the respiration chamber condition, the fixed iButtons were covered by a light elastic sports band, which had to be worn continuously for 7 days or 48 hours. Collected data had to be complete; incomplete data were not used for analyses. Temperature data were processed using an automatic rejection procedure that excluded extreme changes in temperature and replaced these with intrapolated values while correcting for PA induced temperature changes [(23-25)]. The oscillations in temperature were analyzed by cosinor-based analysis of circadian rhythm, using the software program “Circadianware,” which corrects for temperature excursions due to high PA [(23-25)].
Characterization of circadian rhythm parameters
Circadian rhythm parameters obtained from this cosinor-based analysis were acrophase time (the time of peak activity); amplitude (difference between the model fitted peak value and the mesor); relative amplitude (AR; difference between maximum or minimum fitted cosinor function and mesor, the mean activity of the fitted 24-hour pattern); Circadian Function Index (CFI) determining the circadian robustness, based on IS, IV, and AR; L5 (average temperature value during the 5-hour period with lowest temperature recordings); M10 (average temperature value during the 10-hour period with highest temperature recordings); IS (stability of the 24-hour rhythm over days); IV (fragmentation of the rhythm); percentage rhythmicity (PR; consistency of the 24-hour rhythmic pattern over days); and Rayleigh (phase stability during successive days) [(11, 23-26)]. Higher values of amplitude, AR, IS, CFI, PR, and Rayleigh and lower IV indicate a robust WT measured circadian rhythm [(26)].
Statistical analysis
Because no differences between the four original intervention groups were observed [(30)], the data were pooled and used for present post hoc associations. Statistical tests were performed using SPSS Statistics for Macintosh (version 25; IBM Corp.). Data were expressed as means and standard deviations (SD) and were normally distributed. Significance was defined as p < 0.05. Differences in participant characteristics and circadian rhythm parameters between the free-living condition and the respiration chamber condition at 3 years were determined using paired samples t tests; associations between the data in the two different conditions were assessed using Pearson correlation analyses. For assessment of associations of circadian rhythm parameters, PA, and sleep duration with cardiometabolic risk factors, BMI, HOMA-IR, age, and sex, dimension reduction was achieved by factor analyses according to the analyses by Corbalan-Tutau in a similar 3-day study that included 20 participants with normal weight and 50 participants with overweight [(24)]. Factor analyses were applied to find patterns of relationships, being more relevant than individual p values. Factors were determined based upon a high eigen value (> 1), accounting for a substantial amount of variance, and varimax rotation was applied, as well as suppression of small loadings [(11, 23)]. Pearson correlations for associations of circadian rhythm parameters, PA, and cardiometabolic risk factors were presented as follow-up, including only those circadian rhythm parameters that showed the maximal variability in the factor analyses (0.7), in order to avoid multiple testing [(24)]. Here it also needs to be considered that some circadian rhythm parameters are partly redundant, such as amplitude with AR and CFI with amplitude, IV, and IS [(24)]. When circadian rhythm parameters and physical activity were associated with each other and with cardiometabolic outcomes, mediation of PA in associations between circadian rhythm parameters and cardiometabolic factors was tested with PROCESS mediation analysis [(34)]. Previously, we have reported inverse associations of BMI (r = −0.22; p < 0.05) and HOMA-IR (r = −0.21; p < 0.05) with circadian rhythm parameters from the same study, so these will not be included here [(11)].
RESULTS
Comparison of circadian rhythm parameters, PA, sleep duration, and cardiometabolic risk factors between conditions
PA and sleep duration
In 30 participants who underwent both conditions, a statistically significant difference in PA was confirmed, being higher in the free-living than in the respiration chamber condition. PA data from the two conditions were not associated with each other. Sleep durations were similar and significantly associated with each other between the two conditions (Table 1).
| Free-living | Respiration chamber | Difference | Association, r | |
|---|---|---|---|---|
| Age (y) | 56.6±10.0 | 56.6±10.0 | ||
| BMI (kg/m2) | 28.2±4.0 | 28.5±4.0 | −0.3±2.4 | 0.81*** |
| HOMA-IR | 3.2±3.1 | 3.3±1.4 | −0.1±2.0 | 0.40* |
| Physical activity (CPM) | 278.5±85.3 | 145.3±36.8 | 133.2±91.6*** | 0.04 |
| Sleep duration (min) | 490±75 | 471±7 | 19±79 | 0.61** |
| HR (beats/min) | 65.9±9.2 | 59.4±6.5 | 6.3±6.8*** | 0.63*** |
| SBP (mm Hg) | 129.0±13.6 | 126.7±11.0 | 2.3±0.7 | 0.71*** |
| DBP (mm Hg) | 75.9±6.8 | 76.3±6.2 | −0.4±0.1 | 0.34* |
| HDL-C (mmol/L) | 1.41±0.26 | 1.37±0.24 | 0.04±0.13 | 0.86*** |
| LDL-C (mmol/L) | 3.65±0.94 | 3.37±0.80 | 0.28±0.54** | 0.82*** |
| RC (mmol/L) | 0.62±0.27 | 0.79±0.35 | −0.17±0.19*** | 0.83*** |
| TAG (mmol/L) | 1.33±0.60 | 1.67±0.78 | −0.35±0.42*** | 0.84*** |
| CRP (mg/L) | 2.30±3.68 | 2.07±1.86 | 0.23±3.88 | 0.14 |
| Circadian rhythm variables | ||||
| Mesor (°C) | 33.4±0.8 | 32.4±1.1 | 1.0±1.0*** | 0.49** |
| Acrophase time (hh:mm) | 1:34±1:39 | 2:34±0.85 | 1:00±0.9* | 0.10 |
| M10 (°C) | 35.2±0.55 | 35.3±0.68 | 0.1±0.00 | 0.52** |
| L5 (°C) | 32.3±1.03 | 30.7±1.39 | 1.6±0.01 | 0.48* |
| Amplitude | 1.8±0.7 | 2.6±0.8 | −0.7±0.9*** | 0.22 |
| AR | 0.04±0.02 | 0.09±0.04 | 0.05±0.03** | 0.21 |
| Rayleigh | 0.75±0.24 | 0.95±0.20 | −0.20±0.3** | 0.25 |
| PR (%) | 38.8±13.6 | 61.4±16.6 | −22.6±18.6*** | 0.25 |
| IS | 0.53±0.13 | 0.96±0.17 | −0.43±0.18*** | 0.28 |
| IV | 0.17±0.08 | 0.12±0.09 | 0.05±0.11* | 0.16 |
| CFI | 0.51±0.06 | 0.67±0.06 | −0.16±0.08*** | 0.17 |
- Note: Data given as mean ± SD. Acrophase time: time of peak activity or difference between the mesor and the peak values; M10: average temperature value during the 10-hour period with highest temperature recordings; L5: average temperature value during the 5-hour period with lowest temperature recordings; Rayleigh test: measure of the rhythm's phase stability during successive days.
- Abbreviations: AR, relative amplitude; CFI, Circadian Function Index; CPM, counts per minute; CRP, C-reactive protein; DBP, diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment of insulin resistance; HR, heart rate; IS, interdaily stability; IV, intradaily variability; LDL-C, low-density lipoprotein cholesterol; mesor, mean activity of the fitted 24-hour pattern; PR, percentage rhythmicity; RC, remnant cholesterol (total cholesterol-[HDL-C + LDL-C]) concentration; SBP, systolic blood pressure; TAG, triacylglycerol.
- * p < 0.05,
- ** p < 0.01,
- *** p < 0.001.
Circadian rhythm parameters
Between conditions, circadian rhythm parameters differed significantly (Table 1). During the 48-hour respiration chamber study, mesor and IV were lower, acrophase time was later, and amplitude, rayleigh, PR, IS, and CFI were higher than during the 7-day free-living condition, indicating a more robust circadian rhythm (Table 1), which was controlled for by setting the bedtime and wake time. The values of mesor, M10, and L5 were significantly associated between conditions (Table 1).
Cardiometabolic risk factors
Values of SBP, DBP, plasma HDL-C, and BMI and HOMA-IR did not differ significantly between the two conditions, and they were significantly associated with each other (Table 1). HR and plasma LDL-C concentrations were lower, whereas RC and TAG concentrations were higher in the respiration chamber than in the free-living condition. Values of all cardiometabolic risk factors were significantly associated with each other between conditions (Table 1). CRP concentrations were similar but not associated between conditions (Table 1).
In subsequent factor analyses, sleep duration did not appear in any of the factors. Therefore, it was omitted from these analyses.
Associations of circadian rhythm variables with HR, SBP, DBP and PA
Free-living condition
A total of 97 participants underwent the measurements during the 7-day free-living condition. Data from 6 out of 97 participants were excluded from further analyses because of incomplete wearing times of the devices.
Factor analyses including circadian rhythm parameters, PA, HR, SBP, and DBP showed that four factors explained 58.1% of the total variability of the construct. Circadian rhythm parameters, SBP, and DBP appeared together within factor 1, explaining 28.7% of the variability (Table 2). Follow-up with Pearson correlations showed that amplitude, AR, and IS were inversely associated with both SBP and DBP; CFI was inversely associated with DBP (Table 3). PA was inversely associated with SBP and HR. PA was positively associated with IS, CFI, and PR (Table 3). Mediation analysis showed that PA mediated an indirect effect of IS on SBP (Table 4).
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | |
|---|---|---|---|---|
| BMI | 0.376 | |||
| HOMA-IR | 0.528 | |||
| HR | 0.507 | 0.581 | ||
| SBP | −0.306 | 0.687 | ||
| DBP | −0.344 | 0.707 | ||
| PA CPM | −0.450 | |||
| Mesor | −0.569 | −0.456 | ||
| Amplitude | 0.908 | |||
| Rayleigh | 0.318 | 0.747 | ||
| PR | 0.910 | |||
| IS | 0.865 | |||
| IV | −0.551 | 0.428 | ||
| CFI | 0.924 | |||
| AR | 0.911 | |||
| Acrophase time | −0.394 | 0.538 | ||
| M10 | −0.318 | 0.368 | ||
| L5 | −0.698 | |||
| Age | −0.337 | 0.320 | ||
| Sex | 0.514 | 0.329 | ||
| % of Total variability (58.1%) | 28.7 | 11.7 | 10.0 | 7.7 |
- Note: Four factors explained 58.1% of the total variance. Factors with eigenvalues < 1 are not shown. Varimax rotation was applied, as well as suppression of small loadings. Mesor: mean activity of the fitted 24-hour pattern; Rayleigh test: measure of the rhythm's phase stability during successive days; acrophase time: time of peak activity or difference between the mesor and the peak values; M10: average temperature value during the 10-hour period with highest temperature recordings; L5: average temperature value during the 5-hour period with lowest temperature recordings; sex = 1 women, sex = 2 men.
- Abbreviations: AR, relative amplitude; CFI, Circadian Function Index; CPM, counts per minute; DBP, diastolic blood pressure; HOMA-IR, homeostatic model assessment of insulin resistance; HR, heart rate; IS, interdaily stability; IV, intradaily variability; PA, physical activity; PR, percentage rhythmicity; SBP, systolic blood pressure.
| Amplitude, r | AR, r | IS, r | CFI, r | PR, r | PA, r | |
|---|---|---|---|---|---|---|
| HR | −0.24* | |||||
| SBP | −0.22* | −0.22* | −0.18* | −0.29** | ||
| DBP | −0.23* | −0.24* | −0.22* | −0.22* | ||
| PA | 0.22* | 0.22** | 0.20* |
- Abbreviations: AR, relative amplitude; CFI, Circadian Function Index; DBP, diastolic blood pressure; HR, heart rate; IS, interdaily stability; PA, physical activity; PR, percentage rhythmicity; SBP, systolic blood pressure.
- * p < 0.05,
- ** p < 0.01.
| Direct effect on PA | Direct effect on SBP | Indirect effect on SBP | ||||
|---|---|---|---|---|---|---|
| B (95% CI) | p | B (95% CI) | p | B (95% CI) | p | |
| IS | 184.17 (19.72, 348.61) | 0.03 | −12.99 (−35.70, 9.72) | 0.26 | −6.56 (−14.71, −0.61) | 0.01 |
| PA | −0.04 (−0.06, −0.01) | 0.01 | ||||
- Abbreviations: B, mean effect size; IS, interdaily stability; PA, physical activity; SBP, systolic blood pressure.
Respiration chamber condition
Factor analyses including circadian rhythm parameters, PA, HR, SBP, and DBP in the controlled respiration chamber condition (n = 38), showed that five factors explained 70.3% of total variability of the construct (Table 5). Circadian rhythm parameters, PA, HR, SBP, and DBP appeared together within factor 1, explaining 26.9% of the variability. Follow-up with Pearson correlations showed that M10 was positively associated with DBP (Table 6). AR, PR, and amplitude were inversely associated with HR and IV; M10 and PA were positively associated with HR (Table 6). PA was inversely associated with amplitude, AR, and PR and positively with IV and L5 (Table 6). Possible mediation by PA of an indirect effect of AR, amplitude, PR, or IV on HR did not reach significance.
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | |
|---|---|---|---|---|---|
| BMI | 0.595 | 0.536 | |||
| HOMA-IR | 0.395 | 0.460 | 0.417 | ||
| HR | 0.520 | −0.365 | 0.423 | ||
| SBP | 0.648 | 0.799 | |||
| DBP | −0.349 | 0.701 | 0.460 | ||
| PA CPM | 0.626 | ||||
| Acrophase time | 0.436 | 0.554 | |||
| Mesor | 0.686 | 0.561 | 0.313 | ||
| Amplitude | −0.887 | −0.581 | |||
| Rayleigh | 0.551 | 0.453 | |||
| PR | −0.827 | 0.347 | |||
| IS | 0.710 | 0.466 | −0.373 | ||
| IV | 0.834 | −0.399 | |||
| CFI | −0.358 | 0.605 | 0.44 | −0.382 | |
| AR | −0.705 | ||||
| M10 | 0.876 | ||||
| L5 | 0.821 | 0.381 | |||
| Age | 0.358 | 0.303 | 0.587 | ||
| sex | −0.540 | 0.526 | −0.389 | ||
| % of Total variability (70.3%) | 26.9 | 16.5 | 13.0 | 8.7 | 5.2 |
- Note: Five factors explained 70.3% of the total variance. Factors with eigenvalues < 1 are not shown. Varimax rotation was applied, as well as suppression of small loadings. Mesor: mean activity of the fitted 24-hour pattern; Rayleigh test: measure of the rhythm's phase stability during successive days; acrophase time: time of peak activity or difference between the mesor and the peak values; M10: average temperature value during the 10-hour period with highest temperature recordings; L5: average temperature value during the 5-hour period with lowest temperature recordings; sex = 1 women, sex = 2 men.
- Abbreviations: AR, relative amplitude; CFI, Circadian Function Index; CPM, counts per minute; DBP, diastolic blood pressure; HOMA-IR, homeostatic model assessment of insulin resistance; HR, heart rate; IS, interdaily stability; IV, intradaily variability; PA, physical activity; PR, percentage rhythmicity; SBP, systolic blood pressure.
| AR, r | Amplitude, r | PR, r | IV, r | M10, r | L5, r | PA, r | |
|---|---|---|---|---|---|---|---|
| HR | −0.47** | −0.41* | −0.47* | 0.38* | 0.31* | 0.29* | |
| DBP | 0.30* | ||||||
| PA | −0.35* | −0.42** | −0.51** | 0.57** | 0.37* |
- Note: M10: average temperature value during the 10-hour period with highest temperature recordings; L5: average temperature value during the 5-hour period with lowest temperature recordings.
- Abbreviations: AR: relative amplitude; DBP, diastolic blood pressure; HR, heart rate; IV, intradaily variability; PA, physical activity, PR, percentage rhythmicity.
- * p < 0.05,
- ** p < 0.01
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | |
|---|---|---|---|---|
| BMI | 0.384 | |||
| HOMA-IR | −0.428 | |||
| HDL-C | 0.440 | 0.521 | ||
| LDL-C | 0.591 | |||
| RC | 0.915 | |||
| TAG | 0.916 | |||
| CRP | −0.324 | 0.447 | ||
| PA CPM | 0.480 | |||
| Acrophase time | −0.347 | −0.408 | 0.360 | |
| Mesor | −0.707 | 0.616 | ||
| Amplitude | 0.910 | |||
| Rayleigh | 0.434 | 0.391 | ||
| PR | 0.838 | |||
| IS | 0.810 | |||
| IV | −0.512 | |||
| CFI | 0.881 | 0.304 | ||
| M10 | 0.845 | |||
| L5 | −0.896 | 0.421 | ||
| AR | 0.882 | |||
| Sex | −0.529 | |||
| % of Total variability (55.1%) | 20.6 | 16.9 | 9.7 | 7.9 |
- Note: Four factors explained 55.1% of the total variability. Factors with eigenvalues < 1 are not shown. Varimax rotation was applied, as well as suppression of small loadings. Mesor: mean activity of the fitted 24-hour pattern; Rayleigh test: measure of the rhythm's phase stability during successive days; acrophase time: time of peak activity or difference between the mesor and the peak values; M10: average temperature value during the 10-hour period with highest temperature recordings; L5: average temperature value during the 5-hour period with lowest temperature recordings; sex = 1 women, sex = 2 men.
- Abbreviations: AR, relative amplitude; CFI, Circadian Function Index; CPM, counts per minute; HDL-C, high density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment of insulin resistance; hs-CRP, high-sensitivity C-reactive protein; IS, interdaily stability; IV, intradaily variability; LDL-C, low-density lipoprotein cholesterol; PA, physical activity; PR, percentage rhythmicity; RC, remnant cholesterol; TAG, triacylglycerol.
| Amplitude, r | PR, r | IS, r | CFI, r | Mesor, r | L5, r | PA, r | |
|---|---|---|---|---|---|---|---|
| HDL-C | 0.22* | 0.20* | 0.29** | ||||
| CRP | −0.24* | −0.19* | −0.25** | ||||
| PA | 0.20* | 0.22* | 0.22** | 0.20* |
- Note: Mesor: mean activity of the fitted 24-hour pattern; L5: average temperature value during the 5-hour period with lowest temperature recordings.
- Abbreviations: CFI, Circadian Function Index; CRP, C-reactive protein; HDL-C, high-density lipoprotein cholesterol; IS, interdaily stability; PA, physical activity; PR, percentage rhythmicity.
- * p < 0.05,
- ** p < 0.01.
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | |
|---|---|---|---|---|
| BMI | 0.589 | 0.477 | ||
| HOMA-IR | 0.350 | 0.510 | ||
| HDL-C | −0.695 | |||
| LDL-C | −0.429 | |||
| RC | 0.380 | 0.744 | ||
| TAG | 0.380 | 0.744 | ||
| CRP | 0.372 | 0.324 | ||
| PA CPM | 0.615 | −0.423 | ||
| Acrophase time | 0.455 | 0.319 | ||
| Mesor | 0.718 | 0.411 | ||
| Amplitude | −0.881 | |||
| Rayleigh | 0.457 | 0.370 | ||
| PR | −0.809 | |||
| IS | 0.789 | |||
| IV | 0.823 | |||
| CFI | −0.306 | 0.716 | ||
| M10 | 0.768 | −0.312 | ||
| L5 | 0.843 | |||
| AR | −0.693 | |||
| Age | 0.422 | |||
| Sex | −0.480 | 0.564 | ||
| % of Total Variability (62.2%) | 24.1 | 15.4 | 14.1 | 8.6 |
- Note: Four factors explained 62.2% of the total variability. Factors with eigenvalues < 1 are not shown. Varimax rotation was applied, as well as suppression of small loadings. Mesor: mean activity of the fitted 24-hour pattern; Rayleigh test: measure of the rhythm's phase stability during successive days; AR: relative amplitude; M10: average temperature value during the 10-hour period with highest temperature recordings; L5: average temperature value during the 5-hour period with lowest temperature recordings; sex = 1: women; sex = 2: men.
- Abbreviations: CFI, Circadian Function Index; CPM, counts per minute; CRP, C-reactive protein; HOMA-IR, homeostatic model assessment of insulin resistance; HDL-C, high-density lipoprotein cholesterol; IS, interdaily stability; IV, intradaily variability; LDL-C, low-density lipoprotein cholesterol; PA, physical activity in counts per minute; PR, percentage rhythmicity; RC, remnant cholesterol; TAG, triacylglycerol.
| Amplitude, r | PR, r | IV, r | IS, r | Mesor, r | L5, r | |
|---|---|---|---|---|---|---|
| HDL-C | 0.28* | |||||
| RC | −0.26* | −0.24* | 0.28* | |||
| TAG | −0.26* | −0.24* | 0.28* | |||
| CRP | −0.22* | −0.21* | ||||
| PA | −0.42** | −0.51** | 0.57** | 0.30* | 0.37* |
- Note: Mesor: mean activity of the fitted 24-hour pattern; L5: average temperature value during the 5-hour period with lowest temperature recordings.
- Abbreviations: CRP, C-reactive protein; HDL-C, high-density lipoprotein cholesterol; IS, interdaily stability; IV, intradaily variability; RC, remnant cholesterol; PA, physical activity in counts per minute; PR, percentage rhythmicity; TAG, triacylglycerol.
- * p < 0.05,
- ** p < 0.01.
Associations of circadian rhythm variables and PA with lipids/lipoproteins and CRP
Free-living condition
Factor analyses including circadian rhythm parameters, PA, plasma HDL-C, LDL-C, RC, TAG, and CRP in the free-living condition showed that four factors explained 55.1% of the total variability. Circadian rhythm parameters appeared together with plasma HDL-C and CRP within factor 1 and 2, explaining 20.6% and 16.9% of the variability (Table 7). Follow-up with Pearson correlations showed that IS, L5, and PA were positively associated with plasma HDL-C concentrations. PR, amplitude, and CFI were inversely associated with CRP (Table 8). PA was associated positively with PR, IS, CFI, and Mesor (Table 8). Possible mediation by PA of an indirect effect of IS on HDL-C did not reach significance.
Respiration chamber condition
Factor analyses including circadian rhythm parameters, PA, plasma HDL-C, LDL-C, RC, TAG, and CRP concentrations of 38 participants in the respiration chamber condition showed that four factors explained 62.2% of the total variability. Circadian rhythm parameters and PA appeared together with HDL-C, RC, TAG, and CRP concentrations within factors 1, 2, and 3, explaining, respectively, 24.1%, 15.4%, and 14.1% of the total variability (Table 9). Follow-up with Pearson correlations showed that IS was positively associated with plasma HDL-C concentrations. PR and amplitude were inversely associated, and IV positively associated, with RC and TAG concentrations. Amplitude and IS were inversely associated with CRP (Table 10). PA was associated inversely with amplitude and PR and positively with IV, mesor, and L5 (Table 10).
DISCUSSION
The present study compared associations of circadian rhythm parameters and PA with cardiometabolic risk factors under two conditions differing in circadian rhythm parameters and PA. Circadian rhythm was likely stronger during the 48-hour respiration chamber condition, where control of meal and bedtimes may have played a role, than in the 7-day free-living condition. Also, a possible effect of the difference in duration between the two experiments cannot be ruled out. The associations of mesor, M10, and L5 between both conditions suggest a similarity in activity pattern, despite PA being higher in the free-living condition. Values of most cardiometabolic risk factors did not differ significantly and they were highly associated with each other between conditions, likely because of the short durations between the measurements. Differences in lipoprotein fractions suggest a shift between plasma RC and TAG concentrations and LDL-C concentrations.
In the free-living condition, parameters indicating a robust circadian rhythm, namely amplitude, AR, and IS, were associated with lower SBP and DBP. Associations of IS with SBP and DBP align with previous observations by Sohail et al., who observed positive associations of irregular 24-hour rhythms and metabolic syndrome parameters in older adults. [(35)]. Similarly, inverse associations of IV with cardiorespiratory fitness, positive associations with metabolic risk in adolescents [(36)], and inverse associations of AR with cardiovascular events in older men [(37)] have been observed. The inverse association of IS and amplitude with SBP and DBP and the mediation of the inverse association of IS with SBP by PA in the free-living condition align with observations by Tranel et al. [(19)]. The mediation by PA of the association of IS with SBP may be explained by habitual PA, lower SBP, and circadian stability over days being consistent long-term effects [(8, 9, 12, 14, 19, 20)]. In the sedentary respiration chamber condition, however, such associations were absent, likely because of the considerable higher values of IS, amplitude, and AR with a smaller range than in the free-living condition. The associations of parameters indicating a robust circadian rhythm with HR in the respiration chamber condition underscore the observations on resting heart rate being an emerging risk factor in cardiovascular disease [(38)].
Interestingly, in the free-living condition, higher PA was associated with parameters indicating a robust circadian rhythm and with lower HR, whereas in the sedentary condition, lower PA was associated with parameters indicating a robust circadian rhythm and lower HR. Possible mediation by PA of associations of circadian rhythm parameters with HR was absent. The duration of the unusually low PA may have been too short to show a stable consistent mediation effect [(28, 38, 40)].
In both conditions, parameters indicating a robust circadian rhythm were associated with higher plasma HDL-C and lower plasma CRP concentrations. The latter aligns with previous observations, emphasizing the potential relevance of circadian alignment for control of inflammation [(26)]. Only in the respiration chamber condition, parameters indicating a robust circadian rhythm were associated with reduced plasma RC and TAG concentrations, likely because of the larger range of these concentrations in the respiration chamber condition. Positive associations of IS with plasma HDL-C concentrations in both conditions add to previous observations by Hoopes et al. [(39)] who demonstrated inverse associations of circadian rhythm with cholesterol and non-HDL-C concentrations independently of PA [(39)]. Positive associations of PA as well as IS with plasma HDL-C concentrations did not result in significant mediation of a possible indirect effect of IS on HDL-C by PA, likely because of HDL-C also being affected by food intake [(8)].
Strengths of the present study are the objective measurements of all variables in two conditions that differed in circadian rhythm parameters and PA, which add to evidence from misalignment studies.
Because the study was conducted in adults with obesity and prediabetes, who have increased cardiometabolic risk, the outcomes of the study are limited to those adults. Limitations of the study are the difference in durations between the two conditions and the lack of information on regular meal times of the participants in the free-living condition. The 48-hour duration of the respiration chamber study was too short, regarding circadian rhythm parameter assessment. This should be at least 7 days, according to Calogiuri et al. [(40)]. However, the difference between conditions was not circadian misalignment but a more controlled condition close to regular sleep times. The waist-worn ActiSleep is not an ideal measurement of sleep duration, because sleep efficiency cannot be derived. In the respiration chamber condition, where sleep architecture was measured with polysomnography, sleep efficiency was 91.3%. Furthermore, endogenous measurements of HR, SBP, and DBP would have allowed observations on circadian patterns of these variables and on possible links with circadian rhythm parameters.
CONCLUSION
In adults with obesity and prediabetes, parameters indicating a robust circadian rhythm were associated with lower cardiometabolic risk, independently of associations of PA with circadian rhythm or cardiometabolic risk. Only in the case of SBP, PA mediated the association of higher circadian stability with lower SBP.
AUTHOR CONTRIBUTIONS
M.S.W.-P. attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. M.S.W.-P., T.C.A., and M.D. designed the part of the PREVIEW intervention study presented. M.S.W.-P., M.D. and T.C.A. were responsible for the statistical analyses. M.S.W.-P., T.C.A. and M.W. drafted the manuscript. A.R. was responsible for the main PREVIEW project. G.S. and N.S. were responsible for the PA measurements by the Actigraph. M.S.W.-P., M.D., L.T., and T.C.A. contributed to the implementation of the experimental trial and the analysis and interpretation of the data. All authors contributed to the critical revision of the manuscript and approved the final version of the manuscript. M.S.W.-P. and T.C.A. are the guarantors of this work and, as such, had full access to all the data in the study, taking responsibility for the integrity and the accuracy of the data.
ACKNOWLEDGMENTS
We thank all study participants for their time and commitment to the study. Specifically, we thank Bjorn Winkens (Maastricht University) and Lloyd Brants (Clinical Epidemiology and Medical Technology Assessment [KEMTA], MUMC) for statistical advice, and Jennie-Brand-Miller for editing the manuscript. The data are available upon request, addressed to M.S.W.-P. and T.C.A.
FUNDING INFORMATION
The PREVIEW study was sponsored by European Union Framework Program 7 (FP7/2007-2013) grant agreement 312057. The present part of this study was also sponsored by NUTRIM, School of Nutrition and Translational Research in Metabolism, Maastricht University, Maastricht, the Netherlands. M.W. is supported by VIDI grant 917.15.350 and an Aspasia grant from the Netherlands Organization of Scientific Research (NWO) and a Rosalind Franklin Fellowship with an EU Co-Fund attached from the University of Groningen. The funders had no role in discussing the outcome and interpretation of the study results.
CONFLICT OF INTEREST
A.R. has received honoraria from the International Sweeteners Association and Unilever. The other authors declared no conflict of interest.





