Where does the time go when children don't sleep? A randomized crossover study
Funding information: Karitane Fellowship, Grant/Award Number: N/A; Marsden Fund Royal Society of New Zealand, Grant/Award Number: 19-UOO-225
Abstract
Objective
This study aimed to describe how mild sleep deprivation in children changes time spent physically active and sedentary.
Methods
In 2018 through 2020, children (n = 105) with normal sleep were randomized to go to bed 1 hour earlier (extension) or 1 hour later (restriction) than their usual bedtime for 1 week, each separated by a 1-week washout. Twenty-four-hour movement behaviors were measured with waist-worn actigraphy and expressed in minutes and proportions (percentages). Mixed-effects regression models determined mean differences in time use (95% CI) between conditions. Time gained from sleep lost that was reallocated to other movement behaviors in the 24-hour day was modeled using regression.
Results
Children (n = 96) gained ~49 minutes of awake time when sleep was restricted compared with extended. This time was mostly reallocated to sedentary behavior (28 minutes; 95% CI: 19-37), followed by physical activity (22 minutes; 95% CI: 14-30). When time was expressed as a percentage, the overall composition of movement behavior remained similar across both sleep conditions.
Conclusions
Children were not less physically active when mildly sleep deprived. Time gained from sleeping less was proportionally, rather than preferentially, reallocated to sedentary time and physical activity. These findings suggest that decreased physical activity seems unlikely to explain the association between short sleep and obesity in children.
Study Importance
What is already known?
- Inadequate sleep increases the risk of obesity in children, but the reasons why remain uncertain.
- Recent reviews have suggested that the effect of sleep deprivation on weight is most likely a result of changes in diet and sedentary behaviors rather than physical activity.
What does this study add?
- With 24-hour accelerometry data, we show that children were not less physically active with mild sleep deprivation.
- Time gained from sleeping less was reallocated to sedentary time and physical activity proportionally rather than preferentially.
How might these results change the direction of research?
- Analysis of compositional 24-hour time-use data with obesity outcomes should focus on proportional (not preferential) reallocation.
- Exploration of which types of sedentary behavior may increase with mild sleep deprivation is needed.
INTRODUCTION
Although studies have consistently demonstrated that insufficient sleep elevates the risk of obesity in children [(1, 2)], why this occurs remains uncertain. Reviews have suggested that changes in dietary intake and/or sedentary behaviors are more likely explanations than reductions in physical activity [(3, 4)]. However, because the limited evidence to date has mostly been observational and cannot determine causality, experimental studies are needed that reduce sleep duration and determine how children spend this extra time awake to answer this question appropriately.
It is also important to acknowledge that physical activity, sedentary time, and sleep are codependent and constrained within the 24-hour day [(5)], meaning that appropriate statistical techniques are required to account for the “compositional” nature of such data [(6, 7)]. Although studies are starting to examine how time can be reallocated from one behavior to another, which could help answer why insufficient sleep leads to obesity [(7-11)], these cross-sectional studies cannot truly assess how time in one component of the 24-hour day is “reallocated” to other components of the day because they are simply theoretical estimates from a single time point. Similarly, although analyses have demonstrated that habitually short sleepers use their waking time both similarly [(12)] and differently [(13)] from adequate sleepers, these cross-sectional studies do not reveal whether the amount of sleep is a causal mechanism for waking behaviors. Indeed, habitually short sleepers are likely to be different from habitually adequate sleepers in many ways, not just regarding sleep duration, and it is unknown which factors actually influence waking behaviors.
To date, no studies, to our knowledge, have reduced the amount of time children spend asleep and described how the sleep time that was lost was reallocated to physical activity and sedentary time within the 24-hour day. The aim of the present study was to experimentally produce a state of mild sleep deprivation to describe how the wake time gained was reallocated to other time-use behaviors in the 24-hour day. We hypothesized that children would be more sedentary when their sleep was restricted.
METHODS
Study design
Data were obtained as part of the Daily, Rest, Eating, and Activity Monitoring (DREAM) study, for which published protocol [(14)] and primary outcome [(15)] papers are available. In brief, DREAM was a randomized crossover trial that both extended and restricted sleep for 1 week each in 8- to 12-year-old children in their home environment to create a level of mild sleep deprivation that might be experienced by children today [(16, 17)]. All participants provided written informed consent (parent) or assent (child) prior to commencing the study. Ethical approval (#18/146) was provided by the University of Otago Human Ethics Committee (Dunedin, New Zealand).
Participants
Recruitment via social media commenced on October 15, 2018, and ran until March 23, 2020. Recruitment and data collection were affected by the New Zealand COVID-19 pandemic restrictions (effective from March 23, 2020), but only to a minor degree: one fewer participant was recruited than initially anticipated (n = 106), and two children were unable to complete the last week of actigraphy measures, meaning that their data could not be used in its entirety, as this was a crossover trial. Children were eligible if they were 8 to 12 years old, had no underlying medical conditions, and had a score of 39 or less on the Sleep Disturbance Scale for Children (indicated that they had minimal sleep issues) [(18)]. Children were excluded if they were outside of this age, if they took medications, or if they had medical conditions that could affect sleep. Only children with a reported time in bed of 8 to 11 hours per night were included to ensure that the sleep intervention (i.e., extension and restriction) did not place the child in the “not recommended” category of sleep duration (<7 or >12 hours) per National Sleep Foundation recommendations [(19)].
Randomization and masking
After baseline assessment, eligible participants were randomized to intervention order using random block lengths (Stata version 15.1; StataCorp LLC, College Station, Texas) with a 1:1 allocation, stratified by gender and age (8-10 vs. 11-12 years). Researchers and participants were unable to be blinded to the order of conditions during the intervention phase of data collection.
Procedures
To achieve mild sleep deprivation, all children were asked to turn the lights out and be ready to sleep 1 hour earlier than their usual time (based on baseline information collated from a sleep diary and parental feedback regarding typical bedtimes to find the “best” measure of usual time) for seven nights (sleep extension) and 1 hour later per night for another seven nights (sleep restriction). Lights out time was calculated separately for weeknights (Sunday through Thursday) and weekend nights (Friday and Saturday). Daily personalized bedtime text reminders to the family supported adherence to the protocol. After the 1-week baseline assessment, children started with either restriction or extension. Each week of sleep intervention was separated by a 1-week washout to allow children sufficient time to return to their usual, baseline sleep habits. All research was conducted during the school term only and covered all seasons of the year. Sleep changes were made by changing evening bedtimes only, keeping wake times constant, and requiring families to wake the child, if necessary, which was particularly important during the sleep restriction week.
Outcomes
During restriction and extension weeks, overnight sleep was measured using an ActiGraph (wGT3X-BT; ActiGraph, Pensacola, Florida) attached to the skin (waterproof) or worn on an elastic belt (not waterproof) on the participant's right hip for 24 h/d over 8 days. Participants who wore the belt removed the ActiGraph for water-based activities. The ActiGraph was initialized using ActiLife software (version 9.0.0) to commence at 12:00 a.m. on the day of the first appointment. Data were cleaned and scored with an automated script developed in MatLab (MathWorks, Natick, Massachusetts), which searches 3 hours forward and 2 hours backward from preset time flags to detect actual sleep onset and offset [(20)]. All files were visually inspected to ensure that accurate onset and offsets were selected and that the device was not removed for or during sleep. Reporting of pediatric actigraphy scoring rules and sleep-wake variables followed the recommendations of Meltzer et al. [(21)]. Sleep onset was recorded as the start of the first 15 continuous minutes of sleep preceded by 5 minutes of awake time, and sleep offset was recorded as the final 15 continuous minutes of sleep followed by 5 minutes of awake time. Sleep period time (SPT) was defined as the time between sleep onset and sleep offset. Total sleep time (TST) was defined as SPT minus the duration of any nighttime awakenings. Waking after sleep onset (WASO) was identified as at least five consecutive minutes of awake time preceded by 10 minutes of sleep during the SPT. A valid day was defined as at least 8 hours of wear time during awake hours, at least 5 hours of sleep, and less than 2 hours of non-wear time while awake. Data were averaged across the week, weighted for weekend days (2/7) and weekdays (5/7), and normalized to 24 hours. An a priori “per-protocol” definition was set as a mean difference of at least 30 minutes in TST between treatment conditions [(22)].
Time spent sedentary and in light physical activity (LPA) and moderate-to-vigorous physical activity (MVPA; “movement behaviors”) was determined between sleep offset and sleep onset (i.e., awake time only) using the Evenson et al. [(23)] cut points as recommended [(24)]. Counts per minute (cpm) were also calculated as an indicator of overall activity during awake hours only. Non-wear time while awake was defined as at least 20 minutes of consecutive zeros.
At baseline, trained researchers measured height (WSA-HRP Portable Height Rod; Wedderburn, Auckland, New Zealand) to the nearest 0.5 cm and weight (electronic scale HD351; Tanita, Tokyo, Japan) to the nearest 0.1 kg, in duplicate, with children wearing light clothing and no shoes. Body mass index (BMI) z scores were determined according to World Health Organization (WHO) growth standards for 5- to 19-year-old individuals [(25)], with underweight defined as ≤−2 standard deviation (SD), normal weight as >−2 SD to +1 SD, overweight as >+1 SD to 2 SD, and obesity as >+2 SD.
Statistical analysis
We aimed to recruit 106 participants to detect relevant differences in our primary outcome (eating in the absence of hunger), with up to 40% allowance for dropout and incomplete data [(14)]. All statistical analyses were undertaken in Stata version 17.0. Mean differences in time (minutes) spent in each component (sedentary, LPA, MVPA, sleep, WASO) during sleep restriction compared with extension were estimated, including 95% confidence intervals (CIs) and p values, using mixed-effects regression models with participant as a random effect. The same process was undertaken when expressing time as a proportion of the 24-hour day in each component (rather than minutes) and the proportion of “wake time” (time between sleep offset and onset) spent in each movement component. Note that this is not a compositional data analysis (CoDA) because it aims to assess the effect of the intervention on the individual components (e.g., sedentary time) without adjustment for other components. CoDA is useful when assessing relationships between time-use components and health outcomes, accounting for all other time-use components. CoDA is not relevant for describing compositions (or change in compositions), which is our objective here. This analysis was also undertaken for participants who met the per-protocol requirement of at least 30 minutes difference in TST between restriction and extension weeks (n = 59). Residuals of all models were plotted and visually assessed for normality and homoscedasticity (Statistical Analysis Plan in online Supporting Information).
To describe how the time that was lost from sleep was reallocated to other movement components (sedentary behavior and physical activity) of the day, participants who did not lose sleep during the restriction week were excluded (n = 10). For each component (except sleep time), bivariate linear regression models were used to model the relationship of the component time difference between restriction and extension conditions (dependent variable) with sleep loss (independent variable). These relationships were plotted, along with 95% CIs, to illustrate how time reallocation differed with differing amounts of sleep loss. From these models, the mean changes in time of daytime components for the average sleep loss (48 minutes) were estimated along with 95% CIs using the “margins” command in Stata. These estimates were also converted into proportions of wake time by dividing the mean amount of wake time gained (i.e., from loss of sleep, time awake after sleep onset, and non-wear time, resulting in a total average of 56.5 minutes). The study was registered with the Australian New Zealand Clinical Trials Registry (ANZCTR; number ACTRN12618001671257).
RESULTS
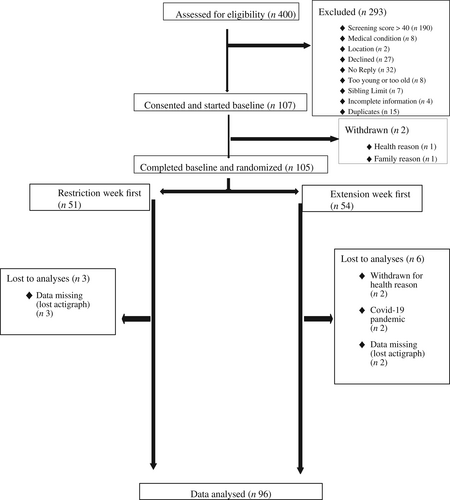
After screening 400 children between October 15, 2018, and March 23, 2020, a total of 105 children were randomly assigned to sleep restriction (n = 51) or extension (n = 54) first (Figure 1). Nine participants did not complete the study, two withdrew for health reasons, two could not complete owing to COVID-19 pandemic restrictions, and five had incomplete actigraphy data, leaving a sample of 96 for analysis. Descriptive characteristics of the study population at baseline are presented in Table 1 [(26, 27)]. The mean age of the sample was 10.2 (SD 1.4) years, the majority (77%) identified as New Zealand European, 49 (51%) were female, and 36 (38%) were above the normal-weight cutoffs. No significant differences (p > 0.05) were observed among participants included in the full sample (n = 105) compared with those who were included in this analysis (n = 96). Participants had a median of 6 days of valid time-use data from each intervention week.
| Full sample, n (%) | Sample with complete actigraphy data, n (%) | |
|---|---|---|
| Number of participants | 105 | 96 |
| Gender | ||
| Female | 53 (50) | 49 (51) |
| Age | ||
| Mean (SD) | 10.3 (1.41) | 10.2 (1.41) |
| Weight statusa | ||
| Underweight | 3 (3) | 3 (3) |
| Normal weight | 61 (58) | 57 (59) |
| Overweight | 24 (23) | 22 (23) |
| Obesity | 17 (16) | 14 (15) |
| BMI z score | ||
| Mean (SD) | 0.68 (1.24) | 0.65 (1.21) |
| Ethnicityb | ||
| European/other | 82 (78) | 74 (77) |
| Māori | 16 (15) | 15 (16) |
| Pacific | 3 (3) | 3 (3) |
| Asian | 4 (4) | 4 (4) |
| Area-level deprivationc | ||
| High (NZDep 8-10) | 22 (21) | 18 (19) |
| Medium (NZDep 4-7) | 44 (42) | 40 (42) |
| Low (NZDep 1-3) | 39 (37) | 38 (40) |
| Maternal educationd | ||
| Secondary school and below | 25 (24) | 21 (22) |
| Post-secondary education | 28 (27) | 25 (26) |
| University degree or higher | 48 (46) | 46 (48) |
| Missing | 4 (4) | 4 (4) |
- Note: Data are expressed as n (percentage) or means (SD) as indicated.
- Abbreviation: NZDep, New Zealand Index of Deprivation.
- a Categories based on the WHO BMI z score cutoffs; children with underweight: ≤−2 SD, normal weight: >−2 SD to +1 SD, overweight: >+1 SD to +2 SD, and obesity: >+2 SD [(25)].
- b Uses the New Zealand census questions, allowing prioritization into specific categories: Māori/Pacific; Asian; and New Zealand European [(26)].
- c Uses the NZDep 2018, which reflects the extent of material and social deprivation and is used to construct deciles from one (least deprived) to ten (most deprived) [(27)].
- d Secondary schooling in New Zealand is from year 9 to 13 inclusive; “post-secondary education” refers to all tertiary qualifications that are not university based.
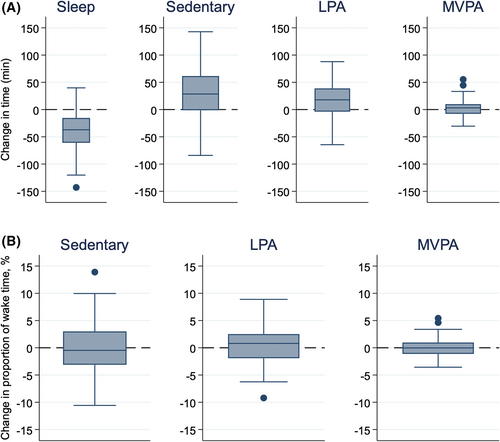
Table 2 shows that, overall, children received, on average, 40 minutes less sleep (TST) each night (95% CI: −47 to −33) during the restriction compared with the extension week. However, they also woke for less time during the night, as shown by a reduction in WASO of 8.8 minutes per night (95% CI: −13.0 to −4.4), resulting in a net average wake-time gain of 48.8 minutes per night. This time gained was reallocated mostly to sedentary time (28 minutes; 95% CI: 19 to 37), with a smaller reallocation to total physical activity (22 minutes; 95% CI: 14 to 30). Most of this increase was spent in light (LPA, 19 minutes; 95% CI: 13 to 25) rather than more intense (MVPA, 2.8 minutes; 95% CI: −0.2 to 5.7) activity. When differences were expressed as a proportion of wake time (rather than minutes), no significant differences in time use were observed between sleep restriction and extension weeks. For example, the changes in sedentary time were just −0.1% (95% CI: −1.0% to 0.9%), and that of physical activity were just 0.3% (95% CI: −0.6% to 1.2%). These minor differences are illustrated by box plots in Figure 2. We also examined whether this level of mild sleep deprivation influenced overall activity levels as indicated by mean cpm, with no evidence of a difference (−7 cpm: 95% CI: −28 to 13; Table 2).
| Extension, mean (SD) | Restriction, mean (SD) | Mean difference (95% CI)a | p value | |
|---|---|---|---|---|
| Time in each component | (min) | |||
| TST (h) | 9.29 (0.65) | 8.63 (0.67) | −40 (−47 to −33) | <0.001 |
| WASO (min) | 26.3 (23.9) | 17.5 (17.9) | −8.8 (−13.0 to −4.4) | <0.001 |
| PA (h) | 5.56 (0.99) | 5.93 (1.11) | 22 (14 to 30) | <0.001 |
| LPA (h) | 4.59 (0.79) | 4.91 (0.83) | 19 (13 to 25) | <0.001 |
| MVPA (min) | 58.4 (21.8) | 61.2 (24.4) | 2.8 (−0.2 to 5.7) | 0.068 |
| Sedentary time (h) | 8.42 (1.12) | 8.88 (1.19) | 28 (19 to 37) | <0.001 |
| Non-wear time (min) | 17.2 (17.2) | 16.0 (14.3) | −1.2 (−4.2 to 1.8) | 0.436 |
| Proportion of the 24-hour day in each component | (%) | |||
| TST | 38.7 (2.7) | 36.0 (2.8) | −2.8 (−3.2 to −2.3) | <0.001 |
| WASO | 1.8 (1.7) | 1.2 (1.2) | −0.6 (−0.9 to −0.3) | <0.001 |
| PA | 23.2 (4.1) | 24.7 (4.6) | 1.5 (0.9 to 2.1) | <0.001 |
| LPA | 19.1 (3.3) | 20.4 (3.4) | 1.3 (0.9 to 1.8) | <0.001 |
| MVPA | 4.1 (1.5) | 4.2 (1.7) | 0.2 (0.0 to 0.4) | 0.068 |
| Sedentary time | 35.1 (4.7) | 37.0 (5.0) | 1.9 (1.3 to 2.6) | <0.001 |
| Non-wear time | 1.2 (1.2) | 1.1 (1.0) | −0.1 (−0.3 to 0.1) | 0.436 |
| Proportion of wake timeb in each component | (%) | |||
| PA (% of wake time)a | 39.1 (7.1) | 39.3 (7.3) | 0.3 (−0.6 to 1.2) | 0.540 |
| LPA (% of wake time)a | 32.2 (5.7) | 32.6 (5.5) | 0.4 (−0.3 to 1.1) | 0.319 |
| MVPA (% of wake time)a | 6.8 (2.6) | 6.8 (2.7) | −0.1 (−0.4 to 0.3) | 0.661 |
| Sedentary time (% of wake time)a | 59.0 (7.0) | 58.9 (7.2) | −0.1 (−1.0 to 0.9) | 0.894 |
| Counts per minute | 535 (159) | 528 (153) | −7 (−28 to 13) | 0.477 |
- Abbreviations: LPA, light PA; MVPA, moderate-to-vigorous PA; PA, physical activity; TST, total sleep time; WASO, wake after sleep onset.
- a Mean differences, 95% CIs, and p values estimated with a mixed-effects regression model with participant as a random effect.
- b Wake time defined as the time between sleep offset and sleep onset (and does not include time awake after sleep onset).
The data were also analyzed only for those children who met the per-protocol requirement of at least 30 minutes less TST during the sleep restriction compared with extension week (Table 3). This per-protocol analysis enhanced the differences observed in the intention-to-treat analysis, showing even greater mean differences in TST (−60 minutes; 95% CI: −66 to −53), sedentary time (38 minutes; 95% CI: 28 to 48), and total physical activity (25 minutes; 95% CI: 15 to 35) but smaller differences in WASO (−4.5 minutes; 95% CI: −9.8 to 0.8) and non-wear time (0.9 minutes; 95% CI: −2.7 to 4.5). As was observed in the intention-to-treat analysis, no significant differences were observed in the composition of time use when examined as a proportion of wake time.
| Extension, mean (SD) | Restriction, mean (SD) | Mean difference (95% CI)a | p value | |
|---|---|---|---|---|
| Time in each component | (min) | |||
| TST (h) | 9.39 (0.66) | 8.40 (0.59) | −60 (−66 to −53) | <0.001 |
| WASO (min) | 23.8 (22.6) | 19.3 (17.1) | −4.5 (−9.8 to 0.8) | 0.097 |
| PA (h) | 5.46 (0.90) | 5.87 (1.00) | 25 (15 to 35) | <0.001 |
| LPA (h) | 4.51 (0.70) | 4.87 (0.78) | 21 (13 to 29) | <0.001 |
| MVPA (min) | 56.5 (20.2) | 60.1 (21.8) | 3.6 (0.5 to 6.7) | 0.022 |
| Sedentary time (h) | 8.50 (1.11) | 9.13 (1.17) | 38 (28 to 48) | <0.001 |
| Non-wear time (min) | 15.5 (16.6) | 16.4 (14.6) | 0.9 (−2.7 to 4.5) | 0.623 |
| Proportion of the 24-hour day in each component | (%) | |||
| TST | 39.1 (2.7) | 35.0 (2.4) | −4.1 (−4.6 to −3.7) | <0.001 |
| WASO | 1.7 (1.6) | 1.3 (1.2) | −0.3 (−0.7 to 0.1) | 0.097 |
| PA | 22.7 (3.7) | 24.5 (4.2) | 1.7 (1.0 to 2.4) | <0.001 |
| LPA | 18.8 (2.9) | 20.3 (3.3) | 1.5 (0.9 to 2.0) | <0.001 |
| MVPA | 3.9 (1.4) | 4.2 (1.5) | 0.3 (0.0 to 0.5) | 0.022 |
| Sedentary time | 35.4 (4.6) | 38.1 (4.9) | 2.7 (1.9 to 3.4) | <0.001 |
| Non-wear time | 1.1 (1.2) | 1.1 (1.0) | 0.1 (−0.2 to 0.3) | 0.623 |
| Proportion of wake timeb in each component | (%) | |||
| PA (% of wake time)b | 38.5 (6.7) | 38.5 (6.8) | 0.0 (−1.0 to 1.0) | 0.999 |
| LPA (% of wake time)b | 31.8 (5.2) | 31.9 (5.3) | 0.1 (−0.7 to 0.9) | 0.842 |
| MVPA (% of wake time)b | 6.7 (2.4) | 6.6 (2.4) | −0.1 (−0.4 to 0.3) | 0.632 |
| Sedentary time (% of wake time)b | 59.7 (6.5) | 59.7 (6.7) | 0.0 (−1.1 to 1.1) | 0.987 |
| Counts per minute | 519 (140) | 516 (142) | −3 (−26 to 20) | 0.778 |
- Abbreviations: LPA, light PA; MVPA, moderate-to-vigorous PA; PA, physical activity; TST, total sleep time; WASO, wake after sleep onset.
- a Mean differences, 95% CIs, and p values estimated with a mixed-effects regression model with participant as a random effect.
- b Wake time defined as the time between sleep offset and sleep onset (and does not include time awake after sleep onset).
Table 4 reports estimates of the average reallocation of time gained from sleep loss to other components of the waking day in those children who achieved sleep loss (n = 84). For these children, average sleep loss during restriction was 47.8 minutes each night, with WASO time reducing by 8.4 minutes, resulting in a total amount of time gained during the daytime of 56.3 min/d. This wake-time gain was reallocated, on average, to sedentary time (32.6 minutes; 95% CI: 23.2 to 42.1), LPA (19.8 minutes; 95% CI: 12.7 to 26.9), and MVPA (3.9 minutes; 95% CI: 0.7 to 7.1).
| Mean change (95% CI) in time spent in component for the average sleep lostb (min) | Mean change (95% CI) in time spent in component for the average wake time gainedb (% of reallocated time) | |
|---|---|---|
| WASO | −8.4 (−12.6 to −4.2) | N/A |
| Sedentary time | 32.6 (23.2 to 42.1) | 57.3 (40.5 to 74.3) |
| LPA | 19.8 (12.7 to 26.9) | 35.4 (22.8 to 48.1) |
| MVPA | 3.9 (0.7 to 7.1) | 7.3 (1.6 to 12.9) |
| Non-wear time | −0.1 (−3.1 to 2.8) | N/A |
- Note: Wake time refers to time spent in sedentary time, LPA, or MVPA.
- Abbreviations: LPA, light physical activity; MVPA, moderate-to-vigorous physical activity; N/A, not applicable; WASO, wake after sleep onset.
- a Only participants who had met the criteria for sleep loss in the restriction week included here.
- b Average sleep lost = 47.8 minutes; average wake time gained = 56.3 minutes.
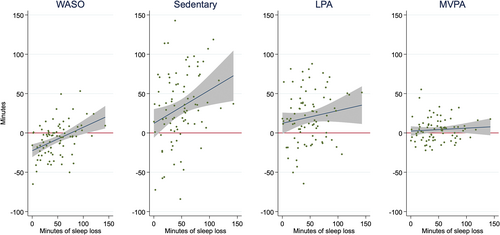
Whereas the results from Table 4 report estimated reallocations for the average sleep loss, Figure 3 illustrates how reallocations differed with greater amounts of lost sleep time. This figure shows that the more sleep children lost, the more time was reallocated to sedentary time and to LPA to a lesser degree. By contrast, the smaller amount of additional time spent in MVPA was consistent regardless of how much sleep was lost. Those with smaller amounts of sleep loss decreased their waking in the night, whereas those with higher amounts of sleep loss had greater amounts of night waking.
DISCUSSION
Our results indicate that children experiencing levels of mild sleep deprivation that are thought to be common are not less physically active compared with when they sleep more. Over the 24-hour day, any wake time gained from sleeping less was distributed proportionally, not preferentially, to sedentary time and physical activity. An alternative indicator of overall activity rates (mean cpm) also did not change with mild sleep deprivation. Collectively, our findings suggest that reductions in physical activity are unlikely to be the major mechanism explaining the strong link between short sleep and increased obesity risk observed in children [(1, 2)].
Related literature for comparison is scarce. Our results align with previous 24-hour accelerometry research showing that more physically active children have shorter sleep times than less-active children [(28)]. Whereas some observational literature has suggested that being tired may reduce physical activity [(10, 29)], such research cannot answer how time is reallocated from sleep when sleep changes, particularly cross-sectional research [(30)]. The only previous experimental study, to our knowledge, reported that, whereas total activity counts were higher during sleep restriction as a function of having more time awake (we saw no difference), mean activity cpm were lower (we saw no difference), suggesting that intensity of activity was reduced with mild sleep deprivation (intensity remained proportionately the same in our study) [(31)]. However, these authors [(31)] did not analyze their data in terms of the 24-hour day, despite using 24-hour accelerometry. They also had a bigger difference in sleep with their more restrictive protocol (difference in time in bed of 3 compared with 2 hours per night), which may have influenced outcomes. We purposely chose a shorter sleep restriction to more appropriately reflect the mild but chronic level of sleep deprivation many modern children are likely to experience across the week [(17)].
To our knowledge, DREAM is the first experimental sleep study to assess what children do with the additional wake time gained when experiencing mild sleep loss. We show here that time gained when sleep is reduced is reallocated proportionally (not preferentially) to time spent in each of the other behaviors (e.g., sedentary time, LPA, MVPA). Whereas the present study investigates causal effects of sleep loss, previous cross-sectional studies have shown similar results, with the composition of waking behaviors being similar in short compared with adequate sleepers [(12)], although such data are not entirely consistent [(13)]. Although ours was not a CoDA, the experimental design and use of objectively measured 24-hour movement data provides insight into true time reallocation in a free-living situation, which can be used to inform CoDA. In the past, cross-sectional research using CoDA has referred to how a “reallocation” of time from one component to another is related to health [(30, 32, 33)]. However, such research is simply estimating how different compositions of time in different individuals are related to health, rather than assessing true reallocation of time within individuals. Until now, it has been unknown how time is “actually” reallocated from sleep to other components when sleep time is altered. Our experimental findings suggest that CoDA associations between sleep and health should be reported relative to all components proportionally, rather than as one-to-one substitutions, as often occurs [(30)].
The strengths of this study include the use of an experimental crossover design, free-living measures to simulate real life, and restricting the intervention to the school term, as lifestyle patterns can change considerably in school holidays. We compared sleep restriction against sleep extension rather than “usual sleep” to assess the true effects of mild sleep deprivation. This was because we could not be sure that none of our children were receiving insufficient sleep [(16)] despite meeting sleep recommendations, as these recommendations are based on average requirements rather than individual sleep need [(19)]. The fact that many of our children were able to extend their sleep when given a simple opportunity to do so confirms our choice of sleep extension rather than usual sleep as the most appropriate study design. Furthermore, by using 24-hour actigraphy, we were able to objectively assess all components of the 24-hour day using the same method of assessment, which should reduce any errors from “missing” or overlapping data experienced when a combination of actigraphy and questionnaires are used [(31)]. With these data, we were able to calculate the reallocation of time to each individual movement component of the waking day.
There are also limitations to consider. We had some missing actigraphy data due to non-wear, although this was just 16 to 17 min/d from a possible total of 1440 minutes. We attempted to reduce missing data and improve 24-hour wear-time compliance by using a waterproof option, but not all participants tolerated this for the full 7 days. If any non-wear time was during water-based activities, it could have been time in MVPA (e.g., swimming) or sedentary (e.g., a bath) in either intervention week and, therefore, potentially influenced the outcomes to a small degree. It is interesting to note that children generally experienced more consolidated and, thus, better-quality sleep, as shown by a reduction in WASO (time awake during the night), when under mild sleep restriction conditions compared with sleep extension. However, we did not explore whether improvement in sleep quality had an impact on movement behavior, as this was not an objective of our intervention. Furthermore, we noted that those with the most sleep loss tended to have more WASO in the restriction week, indicating that sleep quality may have varied considerably with sleep loss. Future research should explore the influence of sleep quality on movement behaviors in more detail. The use of actigraphy means that we do not have contextual information about activities, such as exploring whether sedentary time changed from reading to more screen time, which may allow for increased opportunities for eating [(31)]. Furthermore, our study was not powered and likely too short to detect whether change in movement behaviors differs in relation to weight status, which may provide additional insight into mechanisms linking insufficient sleep with excessive weight gain.
In summary, our DREAM study provides novel insight into the effect of sleep loss on 24-hour time use, indicating that children were not less physically active with mild sleep deprivation. The time children gained from sleeping less was reallocated to sedentary time and physical activity proportionally, not preferentially, to certain movement behaviors, compared with sleeping more. The fact that our study did not find evidence of reduced physical activity with mild sleep deprivation indicates that energy intake behaviors may be the more likely intermediary factor to explain the association between short sleep duration and obesity in children.
AUTHOR CONTRIBUTIONS
Rachael W. Taylor, Barbara C. Galland, Jillian J. Haszard, and Kim A. Meredith-Jones conceived and designed the study, and Rachael W. Taylor and Barbara C. Galland secured funding. Silke Morrison and Rosie Jackson conducted the research; Jillian J. Haszard and Silke Morrison analyzed the data; Dawn E. Elder was responsible for clinical review of screening questionnaires; Silke Morrison, Jillian J. Haszard, and Rachael W. Taylor wrote the manuscript; Rachael W. Taylor had final responsibility for the final content of this paper; and all authors read and approved the final manuscript.
ACKNOWLEDGMENTS
The authors wish to thank the participants and their families for their participation in this study. Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval. Open access publishing facilitated by University of Otago, as part of the Wiley - University of Otago agreement via the Council of Australian University Librarians.
FUNDING INFORMATION
The Daily, Rest, Eating, and Activity Monitoring (DREAM) study was funded by the Marsden fund through the Royal Society of New Zealand (19-UOO-225) and from a University of Otago Research Grant. Rachael W. Taylor is supported by the Karitane Fellowship. Silke Morrison and Rosie Jackson were supported by postgraduate scholarships funded by the University of Otago. The funding bodies played no role in the study design, data collection, analysis, and interpretation of data or the writing of the manuscript.
CONFLICT OF INTEREST
The authors declared no conflict of interest.
CLINICAL TRIAL REGISTRATION
Australian New Zealand Clinical Trials Registry (ANZCTR) number ACTRN12618001671257.





