Measuring and estimating insulin resistance in clinical and research settings
Funding information: European Union H2020 Marie Skłodowska-Curie Actions, Grant/Award Number: mtFOIE GRAS No. 734719; Innovative Medicines Initiative-2, Grant/Award Number: SOPHIA No. 875534
Abstract
The article discusses how to measure insulin resistance in muscle, liver, and adipose tissue in human participants. The most frequently used methodologies to evaluate insulin resistance are described in detail starting from the gold standard, that is, the euglycemic hyperinsulinemic clamp, to the intravenous glucose tolerance test, surrogate indices based on fasting measurements, or dynamic tests (such as oral glucose or mixed meal tolerance tests). The accuracy, precision, and reproducibility of the tests as well as cutoff values are reported.
Study Importance
What is already known?
- The gold standard for measuring insulin resistance is the euglycemic hyperinsulinemic clamp; however, given its complexity, several surrogate indices have been developed and validated.
- These indices are based on insulin and glucose concentrations obtained either during fasting or in response to glucose challenges (oral glucose or meal tolerance test).
What does this study add?
- The alteration of insulin action, which is an important determinant of hyperglycemia, is usually not limited to one organ, e.g., the muscle, but is observed in many organs, the most important being the liver and adipose tissue.
- Reduced insulin clearance is an indication of insulin resistance not yet sufficiently recognized.
How might these results change the direction of research or the focus of clinical practice?
- It is important to assess insulin resistance status in all organs using validated methods.
- The measurement of insulin concentration in the fasting state and/or in response to glucose challenges, which is not common in clinical practice, is determined to evaluate the degree of insulin resistance using validated indices.
DEFINITION OF INSULIN RESISTANCE
Insulin is an essential hormone secreted by the pancreas that regulates the homeostasis of glucose, lipids, and protein. Insulin stimulates glucose uptake and glycolysis in muscle, and in the liver, it decreases endogenous glucose production (EGP) and stimulates glycogen synthesis (Figure 1). Insulin action occurs through the binding to membrane receptors and it is transmitted through the cell by a series of protein–protein interactions. Its action starts with the phosphorylation of insulin receptor substrates (IRS-1 and -2) that leads to the activation of PI3K (phosphatidylinositol 3-kinase) and phosphorylation of AKT (serine/threonine-protein kinase), which are the main signals involved in the metabolic effects of insulin [(1, 2)]. In the liver, forkhead box protein (FOXO1) stimulates gluconeogenesis (GNG) and suppresses glycolysis. Insulin mediates the phosphorylation of AKT, which then phosphorylates FOXO1, leading to its inactivation with subsequent suppression of GNG and EGP (Figure 1). In the adipose tissue, insulin suppresses lipolysis and release of nonesterified fatty acids and stimulates fatty acid re-esterification and triglyceride (TG) synthesis (Figure 1).
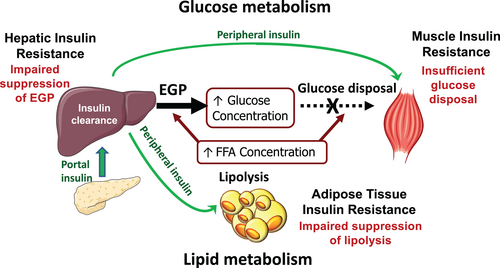
Insulin resistance (IR) is defined as an impairment in insulin action that results in reduced glucose uptake by the muscle and increased hepatic glucose production (HGP) and in the adipose tissue by increased lipolysis (Figure 1). Thus, if in animal studies, IR is evaluated by measuring the activation/suppression of the genes involved in insulin action (e.g., insulin receptors and insulin signaling cascade), in humans, the degree of IR is evaluated by measuring the metabolic effects of insulin, i.e., peripheral glucose uptake and glucose production, during fasting or insulin stimulated conditions.
THE EUGLYCEMIC HYPERINSULINEMIC CLAMP: THE GOLD STANDARD METHOD FOR MEASURING IR
However, the combined used of tracers measured throughout the clamp and mathematical modeling (e.g., using the Steele equation) will allow a more precise measurement of glucose fluxes [(12)].
The M/Icl ratio is a measure of the insulin sensitivity as the amount of glucose metabolized per unit of plasma insulin concentration [(3)]. Insulin concentration is expected to rise almost 10 times during a 40 mU/min·m2 clamp (from 6 ± 1 to 75 ± 5 mU/L and from 7 ± 1 to 82 ± 5 mU/L in healthy participants without obesity and participants with diabetes, respectively [(4)]).
However, several studies have shown that insulin doses of 40 mU/min·m2 are often not sufficient to suppress EGP in participants with severe IR, such as many participants with obesity [(4-6)], thus requiring the infusion of tracer for an accurate measurement of peripheral glucose disposal. Alternatively, higher insulin doses could be used, e.g., 50 to 160 mU/min/m2 [(4, 6, 7, 9-11)], which, in most of the participants, will allow the suppression of EGP. However, a residual EGP (2.5 μmol/min·kg FFM) was measured in insulin-resistant participants with diabetes who were studied with insulin doses of 160 mU/min/m2 [(7)].
Cutoff value for M value
The cutoff for IR as M value (whole-body glucose disposal) was defined in a large cohort that included 2,321 participants (2,138 nondiabetic) from 17 centers among Europe (the EGIR-RISC study [n = 1,436], the Pima Indian Study [n = 597], and studies performed in San Antonio, Texas (n = 288, of whom 99 were Mexican American) all studied with a 40 mU/min·m2 EHC [(13)]. The distribution of the M value appeared to be bimodal with an optimal cut point of 5 mg/min·kgFFM (28 μmol/min·kgFFM) [(13)]. Tam et al. studied a smaller number of participants (116 without and 51 with diabetes) with a 120 mU/min·m2 EHC and defined IR as those with a glucose disposal rate less than 4.9 mg/min·kg (212.2 mg/min·m2 or 7.3 mg/min·kgFFM) [(11)].
The EHC with tracers
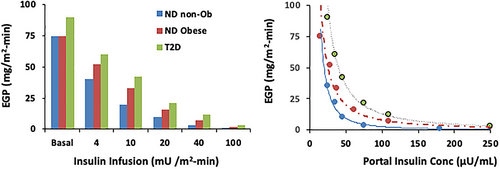
One of the hypotheses of the EHC is that the insulin dose completely suppresses EGP. With the dose of 40 mU/min·m2, this is not often true, especially in insulin-resistant participants [(4-8)], i.e., participants with obesity or type 2 diabetes (Figure 3 shows the fasting and insulin suppressed EGP during EHC with different doses of insulin).
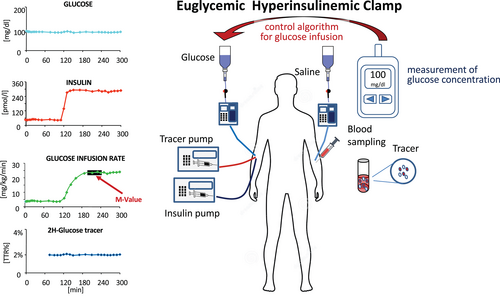
EGP is measured by glucose tracer infusion that starts at least 2 hours before starting the clamp to reach an isotopic steady state. For a 6,6-2H-glucose tracer, an optimal tracer infusion rate (TIR) for nondiabetic participants is (0.22 μmol/min·kg of body weight) preceded by a priming dose (22 μmol/kg) that should achieve an enrichment of 2% as the tracer to tracee ratio (TTR) at the end of the equilibration period (usually 120 minutes) (Figure 4). A few studies [(14, 15)] have shown that the M value measured at 120 minutes is lower than during prolonged insulin infusion (up to 8 hours), indicating that hyperinsulinemia over one or several hours may in itself enhance insulin action.
The ratio of Rd, calculated from Equation (4), and insulin, i.e., Rd/Icl, is a measure of the quantity of glucose metabolized per unit of plasma insulin concentration [(3)].
Advantages, disadvantages, and potential risks
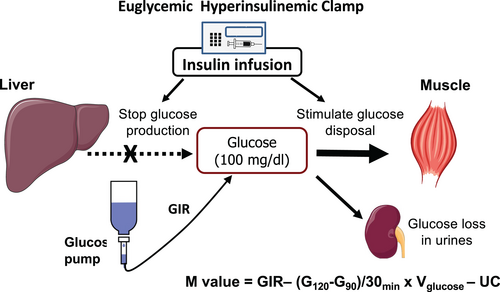
The euglycemic hyperinsulinemic test is the gold standard for the measurement of IR. It allows the amount of glucose metabolized per unit of plasma insulin concentration, which is the best way to assess muscle insulin sensitivity in humans. However, only trained operators should perform this test, which is very complex and which requires the use of at least three pumps (two for insulin and glucose infusion and one for saline) and a glucometer at the bedside (Figure 4).
The protocol is explained in detail in the online Supporting Information. It requires several blood samples from arterialized blood (the preferred sampling site) to monitor the possible changes in glycemia. Bedside plasma glucose is sampled every 5 to 10 minutes (a few microliters, depending on the glucometer) only for immediate plasma glucose determination; possible changes in GIR should be done quickly after blood sampling to avoid large variation in glucose concentrations (i.e., they should not exceed ± 10% from the desired goal level of 5.5 mmoL/L or 100 mg/dL). This is important especially in insulin-sensitive participants because changes in glucose concentrations are much slower.
Only if EGP is completely suppressed is the M value calculated using Equation (1) an accurate measure of peripheral glucose uptake (Rd). Otherwise, the M value underestimates Rd by the residual EGP. However, if a tracer is infused, the test will be 2 to 3 hours longer because it is necessary to wait for tracer equilibration before starting the EHC (Figure 4).
There is a potential risk of hypoglycemic events after discharge in nondiabetic participants who are very sensitive, especially if, after stopping insulin infusion, they will discharged before glucose concentrations reached a stable value.
Post-clamp glycemic profile
After cessation of insulin infusion, at the end of the test, GIR is gradually reduced while measuring glucose concentrations [(3)]. Owei et al. suggested decreasing infusion rates by 25% if two consecutive bedside plasma glucose values remained higher than 120 mg/dL [(17)]. If the plasma glucose value is around 100 mg/dL or lower, dextrose infusion should be increased by 25% to 50%, depending on the exact value. The iterative process should continue until dextrose infusion could be tapered and discontinued. The time for discontinuation of dextrose infusion can vary between 30 and 120 minutes depending on age, glucose tolerance, and insulin sensitivity, being lower in participants who are more insulin resistant [(17)].
After the clamp, participants should be discharged from the research center only if glucose concentration is stable around 120 mg/dL to avoid post-clamp hypoglycemic events.
At the end of the clamp, participants should eat a meal rich in carbohydrate to avoid potential hypoglycemia after discharge. However, not only the amount of food consumed but also the time for gastric emptying, digestion, and absorption of ingested nutrients could vary considerably among individuals. The participant should be allowed to go only if the glycemia is stable [(17)].
Quality parameters: Accuracy, precision, and reproducibility
The accuracy and precision of the EHC within each laboratory depend on several factors, but they are mainly due to technical factors, e.g., the operator-dependent decision on the magnitude of adjustment of GIR in response to a given plasma glucose level can result in overshooting and undershooting if the operator is not an expert and this occurs especially in insulin-sensitive participants in whom the effect of insulin is more marked. The interlaboratory variability may also be high if different methods are used for measuring glucose and insulin concentrations. Glucose clamp protocols usually use a laboratory-quality glucose measurement device (Figure 4), such as the Yellow Springs Instrument or the Beckman glucose oxidase analyzer [(3, 18)]. Cohen and colleagues [(19)] used the FreeStyle Mini glucose meter and concluded that is accurate enough for glucose clamp studies, based on clamp procedures conducted on seven volunteers with type 2 diabetes. Hompesch and Rave [(20)], however, argued that the systematic bias of 6% would result in substantial underestimation or overestimation of glucose requirements that would affect the comparison of the absolute amount of glucose infused between different studies.
The use of a tracer to measure glucose turnover during the clamp allows improved accuracy.
Radioactive and stable isotope tracers provide similar estimates of EGP and glucose disposal. However, the measurement of stable isotopic enrichment (i.e., TTR) is less variable because it is directly measured by mass spectrometry, whereas for radioactive tracers, the measure that corresponds to TTR (i.e., specific activity) is given by the ratio of two concentrations (i.e., labeled to unlabeled glucose in disintegrations per minute [dpm]/mL divided by μmol/mL).
Another source of error might be attributed to pump calibration, an operation that should be performed regularly (i.e., every month).
Several factors may affect the reproducibility of the measurement of insulin sensitivity by the EHC. Besides the operator and the technical issues, several other factors may influence insulin sensitivity values measured at different times, e.g., changes in body weight, health status, heavy exercise the day before the study, lack of sleep, stress (catecholamine levels), other environmental exposures. EHC reproducibility was tested by DeFronzo et al. [(3)] in the first paper on the EHC that restudied four study participants 4 to 6 weeks apart, showing high reproducibility (M value 8.43 vs. 8.53 mg/kg min). Soop et al. [(14)] performed three EHCs 48 hours and 14 days apart in seven healthy study participants (median age of 50 years; BMI 23.1 kg/m2) using an insulin dose of 0.8 mU/kg min (i.e., around 32 mU/min m2) for 2 hours. The GIRs during the three 2-hour clamps were 7.41, 7.26, and 6.63 mg/kg min, respectively, with a median intraindividual coefficient of variation of 5.8%, indicating high reproducibility at a midphysiological range of hyperinsulinemia.
James et al. [(21)] investigated the stability of insulin sensitivity in the long term by comparing the results of EHC studies conducted at Year 1 and Year 3 in 90 healthy participants participating in the Pathobiology of Prediabetes in a Biracial Cohort (POP-ABC) study. There was a strong correlation between the repeated studies (r = 0.81, p < 0.0001), and the coefficient of variation ([SD/mean] × 100) was 13.6% and repeatability coefficient was ± 0.025. Intraparticipant differences in serial insulin sensitivity clamp were less than the repeatability coefficients and within the 95% limits of agreement.
INTRAVENOUS GLUCOSE TOLERANCE TEST
The frequently sampled intravenous glucose tolerance test (FS-IVGTT) is a highly used test to measure insulin sensitivity [(22)]. The standard protocol (STANDARD-IVGTT) consists of a single injection of glucose (0.3 g/kg) and frequent blood sampling during the first minutes after the injection until glucose returns to fasting concentration. Because in study participants who are insulin resistant and/or have diabetes, the decay in glucose concentration is slow, a modified test (MODIFIED-IVGTT) was designed [(23, 24)], and either tolbutamide or insulin is administered at 20 minutes to allow a rapid decay of glucose concentration (Figure 5).
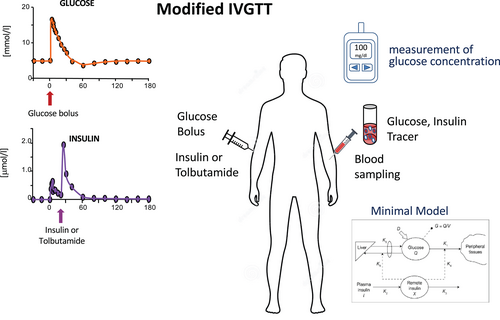
The results of the IVGTT have been tested versus the EHC in participants with different glucose tolerances (from normal [NGT] to impaired [IGT] to tdiabetes [T2D]), finding good correlation for the MODIFIED-IVGTT, whereas the standard test is not as good (STANDARD-IVGTT r = 0.55, MODIFIED-IVGTT r = 0.84, p < 0.001)) [(23-26)]. Saad et al. found that in the population with diabetes, only the modified test was correlated with the EHC (better if with insulin than tolbutamide) [(23, 26)].
The test is much simpler than the EHC, but it is also quite long (3 hours), and it requires several blood samples. Typical blood draws occur at time 0, 3, 4, 5, 6, 8, 10, 14, 19, 22, 27, 30, 35, 40, 50, 70, 100, 140, and 180 minutes for the measurement of glucose and insulin concentrations (Figure 5). Moreover, a mathematical analysis of the data (i.e., by using theminimal model [(25, 27)]) is necessary for the quantification of insulin sensitivity index (SI) and glucose effectiveness, i.e., the insulin-dependent and insulin-independent processes of glucose disappearance (Figure 5).
The cutoff for IR is log(insulin sensitivity) < 0.000107 min−1·pmol/L (Table 1), and it was estimated in a population of 204 healthy study participants who underwent the insulin-modified IVGTT [(28)].
| Index | Metabolic state | Condition (units) | Formula | Cutoff for IR | Duration | Number of samples | Validation (R value) | Reference |
|---|---|---|---|---|---|---|---|---|
| EHC | Fasting | Sensitivity (μmol kgFFM−1 min−1) | M = GIR − [G120 mg/dL − G90 mg/dL]/30min × Vglucose dL/kg − UC | < 28 | 2 h | 15–20 | [(3, 11, 13)] | |
| EHC | Fasting | Sensitivity (μmol kgFFM−1 min−1 pM−1) | M/I | < 0.08 | 2 h | 15–20 | [(3, 11, 13)] | |
| IVGTT | Fasting | Sensitivity (min−1·pM) | SI (from minimal model) | < 0.000107 for log(SI) | 3 h | 20 | R = 0.89, p < 0.001 vs. EHC | [(22-24)] |
| OGIS | OGTT or MMT | Sensitivity mL/kg/min (or mL/m2/min) | f (G0, G90, G120, I0, I90, D)a | <9.8 (or <390) | 2–3 h (OGTT) or 4-6 h (MMT) | 3 | R = 0.77, p < 0.0001 vs. EHC | [(31, 32)] |
| ISI Matsuda | OGTT or MMT | Sensitivity | 104/√ [(G0 mg/dL × I0 mU/mL) × (Gmean × Imean)] | <4.3 | 2-3 h (OGTT) or 4-6 h (MMT) | 3–5 | R = 0.73, p < 0.0001 vs. EHC | [(33)] |
ISI Stumvoll |
OGTT | Sensitivity (μmol · kg−1 · min−1· pM−1) |
ISInodem = 0.157–0.00004576 × I120 pmol/L − 0.000299 × I0 pmol/L − 0.00519 × G120 mmol/L |
< 0.08 |
2 h | 3 | R = 0.707, p < 0.00005 vs. EHC | [(35)] |
| ISIdem Stumvoll | OGTT | Sensitivity (μmol · kg−1 · min−1· pM−1) | ISIdem = 0.226−0.0032 ×BMI − 0.0000645 × I120 pmol/L − 0.000375 × G90 mmol/L |
<0.08 | 2h | 2 | R = 0.79, p < 0.00005 vs. EHC | [(35)] |
| eMCR Stumvoll | OGTT | Sensitivity (mL · kg−1 · min−1) | eMCRnodem = 13–0.0042 × I120 pmol/L – 0.384 × G90 mmol/L – 0.0209 × I0 pmol/L | <9.8 | 2h | 3 | R = 0.686, P< 0.00005 vs. EHC | [(35)] |
| eMCRdem Stumvoll | OGTT | Sensitivity (mL · kg−1 · min−1) | eMCRdem = 18.8–0.271 × BMI − 0.0052 × I120 pmol/L – 0.27 × G90 mmol/L |
<9.8 | 2h | 2 | R = 0.80, P< 0.00005 vs. EHC | [(35)] |
| Gutt | OGTT | Sensitivity (mL/kg/min) | [75,000 + (G0-G120) mg/dL x 0.19 x BWkg/m2] / (120 x LOG[(I0 + I120) mU/L/2] x [(G0 + G120)/2]) | <9.8 | 2 h | 2 | R = 0.63, p < 0.001 vs. EHC | [(37)] |
| SIOGTT | OGTT | Sensitivity | 1/[LOG(G0 + G30 + G90 + G120) mg/dL + LOG(I0 + I30 + I90 + I120) mU/mL] | NA | 2 h | 4 | R = 0.65, p < 0.0001 vs. EHC | [(87)] |
| BIGTT-SI | OGTT | Sensitivity (min−1·pM) | SI = EXP (4.9 − (0.00402 × I0 pmol/L)) – (0.000556 × I30 pmol/L) – (0.00127 × I120 pmol/L) – (0.152 × G0 mmol/L) – (0.00871 × G30 mmol/L) – (0.0373 × G120 mmol/L) – (0.145 × Gender) – (0.0376 × BMI) |
< 0.000107 For Log(SI) | 2 h | 3 | R = 0.88, p < 0.0001 vs. IVGTT | [(34)] |
| Cederholm | OGTT | Sensitivity | [75,000 + (G0-G120) mg/dL × 1.15 × 180 × 0.19 × BW kg/m2]/(120 × LOG Imean mU/L × Gmean mg/dL) | NA | 2 h | 5 | R = 0.75, p < 0.001 vs. EHC | [(88)] |
| Belfiore ISIgly | OGTT or MMT | Sensitivity | 2/[(AUC-I × AUC-G) + 1] | NA | 2-3 h (OGTT) or 4-6 h (MMT) | 3–5 | R = 0.93, p < 0.05 vs. EHC | [(30)] |
- Abbreviations: BW, body weight; EHC, euglycemic hyperinsulinemic clamp; FFM, fat-free mass; OGTT, oral glucose tolerance test; MMT, mixed meal test; eMCRdem, metabolic clearance rate estimation including demographic parameters; eMCRnodem, metabolic clearance rate estimation without demographic parameters; G, glucose; GIR, glucose infusion rate; I, insulin; IR, insulin resistance; IVGTT, intravenous glucose tolerance test; ISI, insulin sensitivity index; M value, glucose disposal measured during the EHC; OGIS, oral glucose insulin sensitivity index.
- a G0, G90, and G120 are the plasma concentration of glucose measured at baseline, 90, and 120 minutes during OGTT, respectively; I0, and I90 are the plasma concentration of insulin measured at baseline and 90 minutes during OGTT, respectively. D is the oral glucose dose (g/m2 body surface area). The formula can be found at the following website: http://webmet.pd.cnr.it/ogis/.
ALTERNATIVE TESTS FOR THE ASSESSMENT OF PERIPHERAL IR
The clamp and the IVGTT are very complex and long; they require intravenous infusion and several blood samples, meaning that they cannot be applied on a large population or outside research centers. Thus, several indexes have been developed based either on dynamic data (e.g., oral glucose tolerance test [OGTT] or mixed meal tolerance test) or based on fasting plasma concentrations.
Beside the EHC and the IVGTT, other dynamic tests are used to estimate peripheral IR. Surrogate indexes of peripheral IR have been derived from the OGTT and the mixed meal tolerance test and validated either versus the EHC or the IVGTT.
Table 1 reports the most-used indexes derived from OGTT measurements with the derived cutoff. Several indexes use only fasting values (Table 2). Fasting indexes are based on glucose and insulin values such as HOMA-IR and quantitative insulin sensitivity check index (QUICKI), whereas others use TG.
| Index | Metabolic state | Condition (units) | Formula | Cutoff for IR | Validation (R value) | Reference |
|---|---|---|---|---|---|---|
| HOMA-IR | Fasting | Resistance | (I0 mU/mL × G0 mg/dL)/405 | >4.65 or >3.6 if BMI > 27.5 | R = 0.60–0.88, p < 0.0001 vs. EHC | [(13, 29, 44, 48)] |
| >2 if NAFLD | ||||||
| QUICKI | Fasting | Sensitivity | 1/(LOG10 I0 mU/mL + LOG10 G0 mg/dL) | <0.33 | R = 0.43–0.78, p < 0.0001 vs. EHC | [(48, 87)] |
| Revised QUICKI | Fasting | Sensitivity | 1/(LOG10 I0 mU/mL + LOG10 G0 mg/dL + LOG10 FFA0 mmol/L) | <0.37 | R = 0.51, p < 0.001 vs. EHC | [(49)] |
| Insulin | Fasting | Resistance | I0 mU/L | >12.2 | R = 0.59, p < 0.001 vs. EHC | [(35, 51)] |
| IGR | Fasting | Resistance | I0 mU/L/G0 mmol/L | >2.4 | R = −0.56, p < 0.001 vs. EHC | [(89)] |
| ISI Bennett | Fasting | Sensitivity | 1/(ln G0 mg/dL × ln I0 mU/L) | <0.089 | R = 0.48, p < 0.0001 vs. EHC | [(51, 90)] |
| VAI | Fasting | Resistance | Men: (Waistcm/39.68 + [1.88 × BMI]) × (TGmmol/L/ 1.03) × (1.31/HDL-Cmmol/L); |
>0.34 | R = −0.39, p < 0.0001 vs. EHC | [(53, 56)] |
| Women: (Waistcm 36.58 + [1.89 × BMI]) × (TGmmol/L 0.81) × (1.52/ HDL-Cmmol/L) | ||||||
| TG/HDL-C | Fasting | Resistance | TGmg/dL/HDL-Cmg/dL | >0.57 | R = −0.41, p < 0.0001 vs. EHC | [(52, 56)] |
| TyG | Fasting | Resistance | Ln[TGmg/dL × glucosemg/dL/2] | >9.36 | R = −0.38, p < 0.0001 vs. EHC | [(55, 56)] |
| LAP | Fasting | Resistance | Men: (Waistcm - 65) × (TGmmol/L) Women: (Waistcm - 58) × (TGmmol/L) |
>3.84 | R = −0.47, p < 0.0001 vs. EHC | [(54, 56)] |
| McAuley index | Fasting | Sensitivity | exp [2.63–0.28xln (I0 mU/L) − 0.31x ln (TGmmol/L)] |
<6.07 | R = −0.39, p < 0.0001 vs. EHC | [(51)] |
- Note: Cutoffs were either published or calculated considering the limit published by McAuley for insulin 12.2 mU/L, glucose 90 mg/dL, and TG 1.5 mmoL/L [(51)].
- Abbreviations: EHC, euglycemic hyperinsulinemic clamp; G, glucose; HDL-C, high-density lipoprotein cholesterol; HOMA, homeostasis model of assessment; I, insulin; IGR, insulin to glucose ratio; IR, insulin resistance; ISI, insulin sensitivity index; LAP, lipid accumulation product; QUICKI, quantitative insulin sensitivity check index; TG, triglycerides; TG/HDL-C, triglyceride to high-density lipoprotein cholesterol ratio; TyG, triglycerides × fasting glucose; VAI, visceral adiposity index.
Advantages and limitations
The surrogate indexes are easier to calculate and less expensive than the clamp or IVGTT. It is important to note that there is large interlaboratory variation in the measurement of insulin concentrations (25%) [(29)]; the measurements of other variables such as glucose or TG are standardized among laboratories and they have a lower range of variability (between 5% and 10%).
Cutoff values for surrogate OGTT indexes
Several indexes of peripheral IR, or insulin sensitivity, were derived from glucose and insulin concentrations measured during the OGTT (Table 1) and validated versus the glucose disposal rate, or the glucose metabolic clearance rate measured during the clamp [(30-37)]. The number of blood samples depends on the indexes, and they range from 3 to 5 samples (compared with 12–20 samples for EHC or IVGTT).
Among the most used indexes, there are the Matsuda [(33)], the oral glucose insulin sensitivity index (OGIS) [(31)], and the Stumvoll [(35)] indexes that were also validated in populations with different characteristics and ethnicities [(38-43)]. The Matsuda index and OGIS, together with the Belfiore index, can also be used for the OGTT longer than 2 hours or for the mixed meal tolerance test. A meta-analysis showed that the Stumvoll-metabolic clearance rate, OGIS, Matsuda, Stumvoll, and Gutt indexes were also those that exhibited the strongest correlations with the insulin sensitivity measured by the EHC [(42)].
Cutoff values for surrogate fasting indexes
Among the best predictors of peripheral IR measured with the clamp (M values) are fasting glucose and plasma insulin concentrations, BMI, waist circumference, and low-density lipoprotein (LDL) cholesterol [(13)]. Fasting glucose and plasma insulin are used in many surrogate fasting indexes (Table 2). The most used is the HOMA-IR index that was first proposed in 1985 and then updated in 1998 [(44, 45)]. Stern et al. analyzed a large cohort (2,138 without and 193 with diabetes) studied with a 40 mU/min·m2 EHC and defined presence of IR if any of the following conditions were met: (1) HOMA-IR > 4.65, (2) BMI > 28.9 kg/m2, or (3) HOMA-IR > 3.60 and BMI > 27.5 kg/m2 [(13)]. Tam et al. analyzed White participants (116 without and 51 with diabetes) studied with a 120 mU/min·m2 EHC and defined presence of IR if HOMA-IR >5.9 or if HOMA-IR >2.8 when high-density lipoprotein (HDL) < 51 mg/dL [(11)]. Isokuortti et al. determined that HOMA-IR >2.0 identified participants with nonalcoholic fatty liver disease (NAFLD) [(29)]. This low cutoff for HOMA-IR also defined IR in studies on general populations involving both Caucasian [(46)] and Asian [(47)] participants.
The QUICKI is very similar to the inverse of HOMA, but it takes into account the skewness of insulin because it uses log-transformed values [(48)]. The revised QUICKI also uses the concentration of nonesterified fatty acids and has a better correlation with the M value, especially in participants without type 2 diabetes [(42, 49, 50)]. The meta-analysis by Otten et al. [(42)] showed that among the fasting indexes of IR/sensitivity, the revised QUICKI had the strongest correlations with the insulin sensitivity measured by the EHC.
Other fasting indexes of peripheral IR use serum TG instead of plasma glucose as a surrogate marker of IR; among these indexes, there is the insulin-TG index by McAuley et al. [(51)], the TG to HDL cholesterol (TG/HDL-C) ratio [(52)], the visceral adiposity index [(53)], the lipid accumulation product (LAP), [(54)] and triglycerides × fasting glucose (TyG) index [(55)] (Table 2). The score for insulin sensitivity developed by McAuley et al. with fasting insulin and TG showed good sensitivity and specificity versus the M/I measured during the EHC with insulin infused at 40 mU/min m2 [(51)]; of interest is that the addition of BMI to the formula did not improve sensitivity and specificity. Other indexes such as as LAP, TyG, TG/HDL-C, and visceral adiposity index were tested versus the EHC by Fiorentino et al. in a cohort of 631 study participants with different degrees of glucose tolerance [(56)]; all indexes were well correlated with the insulin sensitivity measured by the EHC, and the cutoff for IR was derived (Table 2). The area under the receiver operating characteristic curve (AUROC) to detect individuals with IR (defined as the bottom quartile of M value) was ≥ 0.688 for all indexes, with LAP showing the highest AUROC (0.728).
ASSESSMENT OF EGP AND HEPATIC IR
Another main action of insulin is the modulation of EGP. Hepatic insulin resistance (Hep-IR) is defined as a reduced capacity of insulin to suppress EGP.
Glucose is produced mainly by the liver (90%) and only in minimal part by the kidney (maximum 10%) [(57)]. EGP is in part due to glycogenolysis and in part to GNG (which was shown to be around 47% in lean control participants but was significantly increased in association with both obesity and diabetes [(58)]). The kidney contributes to EGP with GNG as renal glycogen stores are minimal (except in people with severe hyperglycemia) [(59)].
EGP is measured noninvasively by infusing glucose labeled with either a radioactive (3H or 14C) or a stable (2H or 13C) isotope (i.e., glucose tracer). As mentioned previously, the glucose tracer is infused using a primed continuous infusion for at least 2 hours in normoglycemic participants and 3 hours in diabetic participants (see online Supporting Information for more details on the protocol). Insulin exerts its main effect by suppressing mainly glycogenolysis rather than GNG and, during a 40 mU/min·m2 hyperinsulinemia, suppresses GNG by approximately 20% while completely blocking glycogenolysis [(60, 61)].
The gold standard for the measurement of Hep-IR is the insulin-mediated EGP suppression during the EHC with the infusion of tracers (to measure EGP). Insulin suppresses EGP following a hyperbolic relationship as shown by Groop et al. [(4)] who measured the dose response insulin-EGP during the EHC with five different insulin infusion rates, i.e., first study, three-step insulin infusion of 4, 20, and 100 mU/min·m2 ; second study, two-step insulin infusion of 10 and 40 mU/min·m2; each step lasted about 2 hours) in study participants either with or without diabetes and BMI equal to 23.8 kg/m2 and 22.7 kg/m2, respectively.
Hep-IR is measured during the EHC by low insulin doses (e.g., 10 or 20 mU/min·m2). Because the kidney contributes minimally to EGP during the clamp, the changes in EGP mainly reflect changes in HGP. The liver “senses” portal rather than peripheral insulin. During the five insulin steps (4, 10, 20, 40, and 100 mU/min·m2), peripheral plasma insulin concentrations ranged from 10 to 240 mU/L at the end of each step, whereas the estimated portal insulin concentrations, i.e., the prehepatic insulin, were 30% higher (Figure 3). Groop et al. estimated that in study participants without diabetes, an increment in portal insulin level of only 5 mU/L (e.g., from 20 to 25 mU/L) was associated with a 50% reduction in HGP, and that at a portal insulinemia of 45 mU/L, HGP was suppressed by more than 90%; in contrast, for study participants with diabetes, insulin suppression required higher portal insulin levels, and for complete suppression of HGP, portal insulin > 100 mU/L was required [(4)]. Mean portal insulinemia of 17 ± 2 mU/L was required to achieve half-maximal suppression of HGP in study participants without diabetes compared with those with diabetes [(4)].
Several factors can induce Hep-IR such as the excess free fatty acids (FFA, e.g., from adipose tissue lipolysis) that impair insulin action in the liver [(61)]. Moreover, hepatic and visceral fat accumulation is associated to impaired insulin-mediated suppression of EGP [(62, 63)], but only visceral fat is related to increased GNG [(62)].
Indirect indexes of Hep-IR
| Index | Metabolic state | Condition | Tissue | Formula | Reference |
|---|---|---|---|---|---|
| Hep-IR | Fasting | Resistance | Liver | EGP × I0 mU/L | [(4, 62, 64)] |
| TG/HDL-C | Fasting | Resistance | Liver | TG/HDL-C | [(91)] |
| Hep-IR OGTT (HIRI) | OGTT | Resistance | Liver | (G0 mg/dL + G30 mg/dL)/100/2 × (I0 mU/mL + I30 mU/mL)/2 | [(64)] |
| LIRI | OGTT | Resistance | Liver | − 0.091 + LOG10 (Imean × 6) × 0.4 + LOG10 (FM kg/weightkg × 100) × 0.346 – LOG10 HDL-C mg/dL × 0.408 + LOG10 BMI × 0.435 | [(65)] |
| Adipo-IR | Fasting | Resistance | Adipose | FFA × I0 mU/L | [(4, 74)] |
| Lipo-IR | Fasting | Resistance | Adipose | RaGly × I0 mU/L | [(75)] |
| ATIRI | Fasting | Resistance | Adipose | RaPalmitate × I0 mU/L | [(76)] |
| Belfiore ISIFFA | OGTT | Sensitivity | Adipose | 2/[(AUC-I × AUC-FFA) + 1] | [(30)] |
- Abbreviations: EGP, endogenous glucose production; FFA, free fatty acid; FM, fat mass; G, glucose ; HDL-C, high-density lipoprotein cholesterol; Hep-IR, hepatic insulin resistance; HIRI, hepatic insulin resistance index; I, insulin; ISI, insulin sensitivity index; LIRI, liver insulin resistance index; OGTT, oral glucose tolerance test; RaGly, rate of appearance of glycerol measured by tracer infusion; RaPalmitate, rate of appearance of palmitate measured by tracer infusion; TG, triglycerides.
Both indexes were validated, with good correlations with fasting Hep-IR measured by tracer infusion (r = 0.64, p < 0.0001) [(64)] and (r = 0.65, p < 0.001) [(65)] for HIRI and Vangipurapu index, respectively. The Vangipurapu index performed better in participants with abnormal glucose tolerance (r = 0.69, p < 0.001) than in those with normal glucose tolerance (r = 0.62, p < 0.001). Fasting Hep-IR estimated with tracers and with indexes were tested in a population of IR study participants with NAFLD and mean BMI of 27.4 kg/m2 and it was found that they had increased Hep-IR compared with study participants without NAFLD (p = 0.008 for Hep-IRtracer and HIRIOGTT and p = 0.012 for Hep-IROGTT-VA) [(66)].
Because Hep-IR is estimated as the product of EGP x I0 and increased fasting glucose is highly correlated to increased EGP [(67)], then the HOMA-IR, which is the product of fasting glucose and insulin, also can be considered a surrogate index of Hep-IR. It is interesting to note that diabetic hyperglycemia and increased EGP are due mainly to increased GNG rather than glycogenolysis [(67)].
ASSESSMENT OF LIPOLYSIS AND ADIPOSE TISSUE IR
Lipolysis, i.e., the rate of adipose tissue TG hydrolysis, is measured by infusing labeled glycerol and calculating the rate of appearance (Ra-glycerol) because the FFA can be retained and re-esterified to TG [(12, 68, 69)]. On the other hand, glycerol cannot be reused within the adipocytes for TG synthesis because they lack the enzyme glycerol kinase [(12, 68, 69)]. The hydrolysis of one mole of TG results in the release of a mole of glycerol into the systemic circulation while the rate of release of FFA may vary because of intracellular re-esterification [(69)]. Studies by Wolfe and colleagues using glycerol and palmitate as tracers [(69)] and by Frayn and colleagues [(70, 71)] using the abdominal vein catheterization technique have shown that during fasting conditions, the ratio of FFA to glycerol released from abdominal adipose is close to 3:1, suggesting minimal re-esterification, but during other conditions, such as epinephrine infusion, TG cycling is increased [(69)]. Thus, the rate of FFA release (measured by the infusion of labeled fatty acids, e.g., palmitate or oleate) may reflect lipolysis only in part. Table 3 reports the most common indices for adipose tissue IR.
All indexes using fasting values of insulin have been validated externally in participants with different BMI, with or without type 2 diabetes, although data on participants with type 1 diabetes are lacking. The fasting indexes are widely used [(77-79)], especially Adipo-IR because it does not require tracer infusion. The indexes that use OGTT values (Table 3) should be employed with caution especially in participants with diabetes, because during the OGTT, the curve FFA-insulin does not necessarily follow a hyperbolic relationship [(72, 74)]. It is of note that the contribution of plasma FFA spillover and hydrolysis of very LDL-TG to the plasma pool of FFA and glycerol might contribute to the overestimation of adipose tissue lipolysis and of the indexes of adipose tissue IR in participants with high TG. Lipoprotein lipase-induced hydrolysis of circulating very LDL-TG releases FFA, which are mainly taken up by the adipose tissue, whereas the glycerol remains in the circulation [(70, 71)]; however, the contribution becomes relevant compared with tissue lipolysis mainly in the postprandial state [(70)]. Another limitation is associated with the fact that the measurement of insulin concentration is not standardized, as discussed earlier.
REDUCED INSULIN CLEARANCE, ANOTHER MARKER OF IR
Peripheral insulin concentrations are the balance between pancreatic insulin secretion and insulin degradation. Insulin is secreted into the portal vein and in great part (~60%–70%) taken up by hepatocytes (Figure 1) by endocytosis, binding to specific receptors; the rest appears in the systemic circulation and it is cleared by extrasplanchnic organs (mainly kidney ~80%, muscle ~6%, adipose tissue and other organs 7-14% [(80-82)]).
In conditions of IR, the need for more insulin in peripheral tissues is associated with increased insulin secretion and reduced insulin clearance mainly in the liver [(26, 83, 84)]. IR in muscle, liver, and adipose tissue is strongly correlated with whole-body insulin clearance, although there is a debate on which dysfunction is driving the other [(85, 86)].
It is now established that postprandial insulin clearance is not constant throughout the day, but it decreases in response to a meal both during the rise and the decay in insulin secretion and glucose concentration [(83, 84)], more in participants with obesity than in nonobese participants but similarly in participants with normal and impaired glucose tolerance [(83)].
Similarly, insulin infusion during the clamp is associated with a reduction in insulin clearance [(83)] and higher steady-state plasma insulin in conditions of IR [(26)].
Thus, reduced insulin clearance rate is an indication of IR, although not yet sufficiently recognized.
CONCLUSION
The EHC remains the gold standard for the measurement of IR in muscle, liver, and adipose tissue, although it requires trained operators and several blood samples and, for very insulin-resistant participants, the dose of 40 mU/min·m2 does not completely suppress the HGP, thus underestimating the total glucose disposal. Several surrogate indexes have been derived and validated and they use either fasting insulin, glucose, fatty acids, and TG levels or insulin and glucose concentrations during the OGTT.
ACKNOWLEDGMENTS
AG gratefully acknowledge the financial support from the European Union's Horizon 2020 Research and Innovation Programme for the projects “Non-invasive Profiling of Mitochondrial Function in Non-Alcoholic Fatty Liver Disease” (mtFOIE GRAS) and “Stratification of Obesity Phenotypes to Optimize Future Obesity Therapy” (SOPHIA).
mtFOIE GRAS has received funding from the H2020-MSCA-RISE (Horizon 2020 Marie Skłodowska-Curie Research and Innovation Staff Exchange [RISE]) program under grant agreement No. 734719. SOPHIA has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No. 875534. This Joint Undertaking received support from the European Union's Horizon 2020 Research and Innovation program, EFPIA, T1D Exchange, JDRF, and Obesity Action Coalition.
The communication reflects the author's view. Neither the Innovative Medicines Initiative nor the European Union, EFPIA, or any Associated Partners are responsible for any use that may be made of the information contained herein.
CONFLICT OF INTEREST
The author declares no conflict of interest for this paper.





