Antibody responses to BNT162b2 mRNA vaccine: Infection-naïve individuals with abdominal obesity warrant attention
Alexis Elias Malavazos, Sara Basilico, Gianluca Iacobellis, and Massimiliano Marco Corsi Romanelli contributed equally to this work.
Funding information
This study was partially supported by Ricerca Corrente funding from Italian Ministry of Health to IRCCS San Donato Polyclinic
Abstract
Objective
The excess of visceral adipose tissue might hinder and delay immune response. How people with abdominal obesity (AO) will respond to mRNA vaccines against SARS-CoV-2 is yet to be established. SARS-CoV-2-specific antibody responses were evaluated after the first and second dose of the BNT162b2 mRNA vaccine, comparing the response of individuals with AO with the response of those without, and discerning between individuals with or without prior infection.
Methods
Immunoglobulin G (IgG)-neutralizing antibodies against the Trimeric complex (IgG-TrimericS) were measured at four time points: at baseline, at day 21 after vaccine dose 1, and at 1 and 3 months after dose 2. Nucleocapsid antibodies were assessed to detect prior SARS-CoV-2 infection. Waist circumference was measured to determine AO.
Results
Between the first and third month after vaccine dose 2, the drop in IgG-TrimericS levels was more remarkable in individuals with AO compared with those without AO (2.44-fold [95% CI: 2.22-2.63] vs. 1.82-fold [95% CI: 1.69-1.92], respectively, p < 0.001). Multivariable linear regression confirmed this result after inclusion of assessed confounders (p < 0.001).
Conclusions
The waning antibody levels in individuals with AO may further support recent recommendations to offer booster vaccines to adults with high-risk medical conditions, including obesity, and particularly to those with a more prevalent AO phenotype.
Video Abstract
Antibody responses to BNT162b2 mRNA vaccine: Infection-naïve individuals with abdominal obesity warrant attention
by Malavazos et al.Study Importance
What is already known?
- ► Individuals with obesity, and particularly those with predominant visceral adipose tissue accumulation, are at significant risk of developing a more severe case of COVID-19. The excess of visceral adipose tissue is an indicator of increased ectopic fat, which might hinder and delay the immune response.
- ► To date, the available clinical evidence demonstrates that the efficacy of mRNA vaccines against SARS-CoV-2 does not differ among individuals with obesity compared with those without obesity.
What does this study add?
- ► After receiving two doses of the BNT162b2 mRNA vaccine, infection-naïve individuals with abdominal obesity (AO) reached a lower antibody peak compared with individuals without AO.
- ► Between the first and third month after BNT162b2 mRNA vaccine dose 2, the drop in antibody levels was more remarkable in individuals with AO compared with those without. Multivariable linear regression confirmed this result after inclusion of assessed confounders. This was not evinced using BMI classes.
How might these results change the direction of clinical practice?
- ► Because obesity represents a risk factor for serious COVID-19 complications, people with obesity should be encouraged to undergo vaccination with any one of the currently available COVID-19 vaccines.
- ► The waning antibody levels in individuals with AO may further support recent recommendations to offer booster vaccines to adults with high-risk medical conditions, including obesity, and particularly to those with a more prevalent AO phenotype.
INTRODUCTION
Individuals with obesity, and particularly those with predominant visceral adipose tissue (VAT) accumulation, are at significant risk of developing a more severe case of COVID-19 ((1-3)).
mRNA vaccines against SARS-CoV-2, the causative agent of COVID-19, hold great promise for curbing the spread of infection and accelerating the timeline toward a possible level of herd immunity ((4)). Nevertheless, researchers fear that vaccines against SARS-CoV-2 might not be as effective in people with obesity ((5)) because studies on vaccines against influenza, hepatitis B, and rabies have shown a reduced immune response in these individuals ((5, 6)).
To date, the available clinical evidence has demonstrated that the efficacy of mRNA vaccines against SARS-CoV-2 does not differ among individuals with obesity compared with those without obesity ((4, 7)).
However, these analyses have focused on the definition of obesity assessed through BMI, but this is not the best indicator of adiposity because it does not take into account the amount and distribution of body fat, which can differ broadly among people with the same BMI ((8)). The excess of VAT is considered the main culprit in inflammatory diseases linked to obesity, and it is an indicator of increased ectopic fat, which might hinder and delay the immune response, as highlighted by COVID-19 ((1, 2, 5, 8-11)). How people with abdominal obesity (AO) will respond to mRNA vaccines against SARS-CoV-2 is yet to be established. To this end, we evaluated SARS-CoV-2-specific antibody responses after the first and second dose of the BNT162b2 mRNA vaccine in a large cohort of health care workers. We compared the response of individuals affected by AO with the response of those without AO, discerning between individuals with or without prior SARS-CoV-2 infection.
METHODS
Study design and population
The VARCO-19 study is an ongoing observational prospective cohort study started in January 2021 and lasting until March 2022. All participants provided written informed consent. The protocol was approved by the Ethics Committee of the Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS)-Lazzaro-Spallanzani (protocol code 48/2021/spall/PU/403-2021). The vaccination itself was not part of the study.
We collected blood samples from a cohort of health care workers who received two doses of the BNT162b2 mRNA vaccine at the IRCCS San Donato Polyclinic, a large academic medical center in Milan, Italy. A total of 1,060 patients met the following inclusion criteria: 1) aged >18 years; 2) received two doses of BNT162b2 mRNA vaccine. Pregnancy was an exclusion criterion. All participants provided data on medical history, pharmacotherapy (if any), smoking status, and symptoms (if they reported a prior SARS-CoV-2 infection).
Anthropometric parameters
Anthropometric parameters were assessed at baseline. Waist circumference was measured midway between the lower rib and the iliac crest to the closest 1.0 cm. The cut-off to determine AO was 94 cm in men and 80 cm in women ((8, 12)). BMI was calculated as weight (kilograms) divided by height (meters) squared ((13)).
Serological testing
Antibody levels were measured at four time points: at baseline, at day 21 after vaccine dose 1, and at 1 (within 30-40 d) and 3 months (within 90-100 d) after dose 2.
Serological testing for SARS-CoV-2 glycoprotein-specific antispike (S) immunoglobulin G (IgG) was performed using LIAISON assay (DiaSorin S.p.A., Italy), which measures IgG-neutralizing antibodies against the Trimeric complex (IgG-TrimericS), which includes the receptor-binding-domain and N-terminal-domain sites from the three S1 subunits. We used nucleocapsid (N) antibodies to detect prior infection. Given that the BNT162b2 vaccine delivers mRNA encoding only for S-protein, the expected elicited response is the production of IgG-TrimericS levels and not N antibodies, which represent a durable marker and indicator of postinfectious status ((14-16)). Antibody assay methods are discussed in more detail in the Supporting Information. Results were reported as binding antibody unit (BAU)/mL (≥33.8 BAU/mL is positive).
Statistical analyses
We expressed antibody levels as a geometric mean (95% CI). Confidence intervals of the geometric means were calculated using log-transformed data and t tables with back-transformation. Individuals were categorized according to AO and BMI classes with and without prior SARS-CoV-2 infection. For comparing between-group continuous values, the nonparametric Kruskal-Wallis test was used. We used multivariable linear regression to account for possible confounding and to evaluate the 3-month difference in absolute variation of titer levels, starting from 1 month after dose 2 in individuals with and without AO (or BMI classes). The model was specified including 1 month after dose 2 antibody levels, sex, age, smoke, prior SARS-CoV-2 infection, and the interaction between prior infection and AO (or BMI classes). Smoking status, hypertension, diabetes mellitus, cardiovascular diseases, dyslipidemia, and cancer were included if significant in univariate analysis for preserving model robustness. The null hypothesis will be refused with p < 0.05. All statistical analyses were done with SAS version 9.4 (SAS Institute, Cary, North Carolina).
RESULTS
Baseline characteristics of patients are reported in Table 1. Vaccine recipients (n = 1,060) who provided at least three blood samples for antibody testing were aged 41.4 (12.9) years, 62% were female, and 93% were White; 825 vaccine recipients (186 with a prior infection) provided blood samples once after dose 1 and twice after dose 2, and 235 vaccine recipients (54 with a prior infection) also provided baseline (pre-vaccine) samples.
| Total (n = 1,060) | AO (n = 492) | p value | No AO (n = 568) | p value | |||
|---|---|---|---|---|---|---|---|
| No prior SARS-CoV-2 infection (n = 380) | Prior SARS-CoV-2 infection (n = 112) | No prior SARS-CoV-2 infection (n = 440) | Prior SARS-CoV-2 infection (n = 128) | ||||
| Age (y) | 41.42 ± 12.95 | 47.50 ± 12.25 | 44.54±10.99 | 0.022 | 36.75 ± 11.88 | 36.67 ± 11.80 | 0.949 |
| Ethnicity | 0.001* | 0.356 a | |||||
| White | 991 (93.49%) | 361 (95.00%) | 94 (83.93%) | 416 (94.55%) | 120 (93.75%) | ||
| Latin-American | 44 (4.15%) | 12 (3.16%) | 11 (9.82%) | 15 (3.41%) | 6 (4.69%) | ||
| Asian | 1 (0.09%) | 1 (0.78%) | |||||
| African | 9 (0.85%) | 2 (0.53%) | 3 (2.68%) | 4 (0.91%) | |||
| Arabic | 15 (1.42%) | 5 (1.32%) | 4 (3.57%) | 5 (1.14%) | 1 (0.78%) | ||
| Sex | 0.058 | 0.407 | |||||
| Male | 400 (37.74%) | 153 (40.26%) | 34 (30.36%) | 161 (36.59%) | 52 (40.63%) | ||
| Female | 660 (62.26%) | 227 (59.74%) | 78 (69.64%) | 279 (63.41%) | 76 (59.38%) | ||
| Smoking status | 0.059 | 0.360 | |||||
| Smoker | 167 (15.75%) | 69 (18.16%) | 10 (8.93%) | 73 (16.59%) | 15 (11.72%) | ||
| Former smoker | 25 (2.36%) | 12 (3.16%) | 3 (2.68%) | 7 (1.59%) | 3 (2.34%) | ||
| Nonsmoker | 868 (81.89%) | 299 (78.68%) | 99 (88.39%) | 360 (81.82%) | 110 (85.94%) | ||
| Comorbidities | |||||||
| Hypertension | 97 (9.15%) | 67 (17.63%) | 15 (13.39%) | 0.290 | 13 (2.95%) | 2 (1.56%) | 0.387 |
| Diabetes mellitus | 15 (1.42%) | 8 (2.11%) | 6 (5.36%) | 0.099* | 1 (0.23%) | 1.00 a | |
| Cardiovascular diseases | 32 (3.02%) | 19 (5.00%) | 3 (2.68%) | 0.296 | 9 (2.05%) | 1 (0.78%) | 0.338 |
| Dyslipidemia | 44 (5.15%) | 26 (6.84%) | 7 (6.25%) | 0.826 | 8 (1.82%) | 3 (2.34%) | 0.704 a |
| Cancer | 4 (0.38%) | 4 (1.05%) | 0.579* | ||||
| Other comorbidities | 73 (6.89%) | 34 (8.95%) | 6 (5.36%) | 0.222 | 21 (4.77%) | 12 (9.38%) | 0.050 |
| Anthropometric measurements | |||||||
| Weight (kg) | 70.40 ± 15.01 | 78.32 ± 14.46 | 80.66 ± 15.92 | 0.143 | 63.06 ± 10.39 | 64.17 ± 11.85 | 0.918 |
| Height (cm) | 168.35 ± 9.05 | 168.46 ± 9.18 | 167.06 ± 8.75 | 0.151 | 168.55 ± 8.74 | 168.47 ± 9.95 | 0.922 |
| Waist (cm) | 85.30 ± 13.55 | 94.95 ± 11.12 | 96.80 ± 12.54 | 0.135 | 76.52 ± 7.90 | 76.77 ± 8.55 | 0.753 |
| Waist male (cm) | 93.22 ± 12.11 | 103.02 ± 8.71 | 103.90 ± 11.29 | 0.618 | 84.36 ± 5.99 | 84.87 ± 6.47 | 0.602 |
| Waist female (cm) | 80.50 ± 12.05 | 89.52 ± 9.09 | 93.71 ± 11.84 | 0.001 | 72.00 ± 4.67 | 71.24 ± 4.36 | 0.203 |
| WHtR | 0.51 ± 0.08 | 0.56 ± 0.06 | 0.58 ± 0.07 | 0.018 | 0.45 ± 0.04 | 0.46 ± 0.04 | 0.718 |
| BMI (kg/m2) | 24.75 ± 4.41 | 27.50 ± 3.96 | 28.81 ± 4.74 | 0.003 | 22.10 ± 2.44 | 22.09 ± 2.46 | 0.983 |
| BMI classes | 0.065 | 0.257 | |||||
| Underweight | 37 (3.49%) | 32 (7.27%) | 5 (3.91%) | ||||
| Normal weight | 594 (56.04%) | 106 (27.89%) | 22 (19.64%) | 361 (82.05%) | 105 (82.03%) | ||
| Overweight | 311 (29.34%) | 191 (50.26%) | 55 (49.11%) | 47 (10.68%) | 18 (14.06%) | ||
| Obesity | 118 (11.13%) | 83 (21.84%) | 35 (31.25%) | ||||
| Prior SARS-CoV-2 infection symptoms | |||||||
| Mild or asymptomatic | 120 (50.0%) | 52 (46.85%) | 68 (53.54%) | ||||
| Symptomatic | 118 (49.12%) | 59 (53.15%) | 59 (46.46%) | ||||
| IgG-TrimericS antibody levels, BAU/mL, mean (95% CI) | |||||||
| Baseline b | 10.7 (8.9-13.0) | 5.2 (4.7-5.8) | 164.1 (74.2-363.0) | <0.001 | 6.0 (5.3-6.9) | 53.4 (39.9-88.4) | <0.001 |
| 21 days after dose 1 c | 536.4 (501.7-573.46) | 270.4 (243.4-300.4) | 1,910.6 (1,768.1-2,064.6) | <0.001 | 484.6 (449.2-522.7) | 1,912.2 (1,805.4-2,025.2) | <0.001 |
| 1 month (within 30-40 days) after dose 2 c | 1,693.4 (1,656.7-1,730.9) | 1,434.6 (1,365.7-1,507.0) | 1,992.4 (1,937.1-2,049.2) | <0.001 | 1,780.0 (1,739.7-1,821.2) | 2,024.2 (1,977.2-2,072.2) | <0.001 |
| 3 months (within 90-100 days) after dose 2 c | 929.7 (889.75-971.51) | 591.5 (548.1-638.3) | 1,691.5 (1,564.3-1,829.2) | <0.001 | 983.1 (931.5-1,037.6) | 1,740.5 (1,646.5-1,840.0) | <0.001 |
Note
- Data are presented as n (%) or mean ± SD, unless otherwise indicated. AO = waist circumference ≥ 94 cm for men, ≥80 cm for women; no AO = waist circumference < 94 cm for men, <80 cm for women; underweight = BMI <18.5 kg/m2; normal weight = BMI from 18.5 to <25 kg/m2; overweight = BMI from 25 to <30 kg/m2; obesity = BMI ≥ 30 kg/m2.
- Other comorbidities (thyroid diseases, thyroid nodules, glaucoma, alopecia, depression, osteoporosis, kidney stones, gastritis, gastroesophageal reflux disease, irritable bowel syndrome, hallux valgus, carpal tunnel, diverticulosis, celiac disease, allergic asthma, and anemia). To compare variables between different groups, the χ2 or aFisher’s exact test was used for categorical variables. A t test or Wilcoxon rank sum test was used to compare unpaired means in normally or nonnormally continuous distributed variables, respectively.
- Abbreviations: AO, abdominal obesity; BAU, binding antibody units; WhtR, waist to height ratio.
- a Fisher exact test was used for categorical variables.
- b n total = 235.
- c n total = 1,060.
At baseline, among infection-naïve individuals, as expected, there was no difference in IgG-TrimericS levels between individuals with or without AO (Figure 1A). At other times, among infection-naïve individuals, IgG-TrimericS levels were significantly lower in individuals with AO than in those without AO (Figure 1B). Two aspects are noteworthy: 1) at 1 month after dose 2, individuals with AO reached a lower peak of IgG-TrimericS levels than individuals without AO (geometric mean BAU/mL: 1,434.6 BAU/mL [95% CI: 1,365.7-1,507.0] vs. 1,780.0 BAU/mL [95% CI: 1,739.7-1,821.2], p < 0.001; Figure 1B); 2) between the first and third month after vaccine dose 2, the drop in IgG-TrimericS levels was more remarkable in individuals with AO compared with those without AO (2.44-fold [95% CI: 2.22-2.63] vs. 1.82-fold [95% CI: 1.69-1.92], respectively, p < 0.001; Figure 1B).
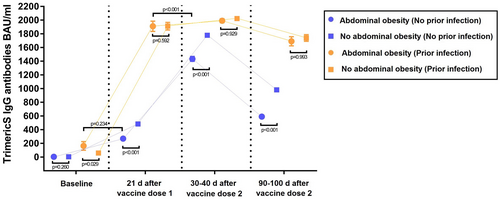
At baseline, among individuals with a prior infection, those with AO had higher IgG-TrimericS levels than those without AO (164.1 BAU/mL [95% CI: 74.2-363.0] vs. 59.4 BAU/mL [95% CI: 39.9-88.4], p = 0.029; Figure 1C). There was no difference between the two groups at other times (Figure 1D).
Among individuals with AO, IgG-TrimericS levels were slightly lower in individuals with prior infection at baseline than in infection-naïve individuals after dose 1 (164.1 BAU/mL [95% CI: 74.2-363.0] vs. 270.4 BAU/mL [95% CI: 243.4-300.4], p = 0.234; Figure 1E).
Among individuals with AO, IgG-TrimericS levels were significantly lower in infection-naïve individuals after dose 2 than in previously infected individuals after dose 1 (1,434.6 BAU/mL [95% CI: 1,365.7-1,507.0] vs. 1,910.6 BAU/mL [95% CI: 1,768.1-2,064.6], p < 0.001; Figure 1F).
Analysis of these data by multivariable linear regression showed evidence of interaction between AO and SARS-CoV-2 infection (p = 0.002; Table 2). Specifically, AO is associated with a drop in absolute IgG-TrimericS levels in infection-naïve individuals at 3 months after dose 2, regardless of sex, age, or smoking, and IgG-TrimericS levels reached at 1 month after dose 2 (159.2 BAU/mL [95% CI: 96.9-221.4], p < 0.001). No interaction was evinced using BMI classes in the same regression model (p = 0.267; Table 2).
| p value | |||
|---|---|---|---|
| Univariate | Multivariable considering AO | Multivariable considering BMI class | |
| Sex | 0.1553 | 0.0582 | 0.1255 |
| Age | 0.0002 | 0.0008 | <0.0001 |
| IgG-TrimericS antibody levels 1 month (within 30-40 days) after dose 2 | 0.7499 | <0.0001 | <0.0001 |
| Prior SARS-CoV-2 infection | <0.0001 | <0.0001 | <0.0001 |
| AO | 0.0016 | 0.0427 | − |
| Interaction with prior SARS-CoV-2 infection × AO | − | 0.0021 | − |
| BMI classes | 0.1723 | − | 0.2854 |
| Interaction with prior SARS-CoV-2 infection × BMI classes | − | − | 0.2673 |
| Smoking status | 0.0320 | 0.0388 | 0.0382 |
| Hypertension | 0.0665 | − | − |
| Diabetes mellitus | 0.5016 | − | − |
| Cardiovascular diseases | 0.0865 | − | − |
| Dyslipidemia | 0.7023 | − | − |
| Cancer | 0.7425 | − | − |
Note
- AO = waist circumference ≥ 94 cm for men, ≥80 cm for women; no AO = waist circumference < 94 cm for men, <80 cm for women; underweight = BMI < 18.5 kg/m2; normal weight = BMI from 18.5 to <25 kg/m2; overweight = BMI from 25 to <30 kg/m2; obesity = BMI ≥ 30 kg/m2. For prior SARS-CoV-2 infection, AO, smoking status, hypertension, diabetes mellitus, cardiovascular diseases, dyslipidemia, and cancer the reference was no; for sex the reference was male; and for BMI class the reference was normal weight.
- Abbreviations: AO, abdominal obesity.
DISCUSSION
Our results showed that infection-naïve individuals with AO had lower antibody development over time compared with individuals without AO. They reached a lower antibody peak, and they had a more significant drop in antibody levels at 3 months after dose 2 after inclusion of assessed confounders.
It is worth noting that infection-naïve individuals with AO reached a lower antibody peak after dose 2 than individuals with AO and prior infection after a single vaccine dose. Recent studies have shown that individuals with previous SARS-CoV-2 infection may have a superior humoral response after the first dose of BNT162b2 compared with uninfected individuals who were fully vaccinated with two doses of BNT162b2 ((17, 18)). Furthermore, we have found that, among individuals with prior infection, those with AO had higher IgG-TrimericS levels than those without AO at baseline. These data are consistent with a recent paper in which it was observed that individuals with severe obesity and prior infection exhibited a higher neutralizing antibody titer, likely due to a more severe case of COVID-19 ((11)).
A recent small study, conducted over a period of only 4 weeks after vaccine dose 2, hypothesized that central obesity is associated with lower antibody titers following mRNA vaccines against SARS-CoV-2 ((19)).
The lower antibody response and its more significant drop over time, highlighted by classifying the population by AO phenotype rather than BMI-based obesity, can be explained in several ways, the most important being the greater chronic inflammatory status due to an excess of abdominal VAT, which could compromise the immune system ((1, 5, 6)).
Remarkably, the available clinical evidence and a recent position statement showed that vaccines are not less effective for individuals with obesity and that they are an important protection against COVID-19 ((4, 7)). Antibody titer following SARS-CoV-2 vaccination cannot alone predict the risk of developing COVID-19, and even low antibody levels could be equally protective against infection. Moreover, in a recent study, it was shown that BNT162b2 mRNA vaccine efficacy tends to gradually decrease after 6 months while maintaining a high safety profile ((20)).
Limitations of our study include lack of measurement of virus-specific T cells. Anti-N antibodies were assessed only once to determine a prior SARS-CoV-2 infection. In addition, we did not evaluate proinflammatory markers of inflammation.
CONCLUSION
Obesity represents a risk factor for serious COVID-19 complications. Therefore, people with obesity should be encouraged to undergo vaccination with any one of the currently available vaccines. As of today, the efficacy of COVID-19 vaccines is reported not to be significantly different in people with and without obesity. Our findings warrant future studies to assess the effectiveness of COVID-19 vaccines as a function of AO. The waning antibody levels in individuals with AO may further support recent recommendations to offer “booster” vaccines to adults with high-risk medical conditions, including obesity, and, particularly, to those with a more prevalent AO phenotype. The clinical significance and relevance to extend serological monitoring of antibody levels in individuals with AO is still unclear and should be interpreted with caution.O
ACKNOWLEDGMENTS
We acknowledge the sample collection effort of Matteo Maiocchi, Michele Iovane, Mirco Bonaccorso, and Sofia Sichel from the Endocrinology Unit of the Clinical Nutrition and Cardiovascular Prevention Service at the Institute of IRCCS San Donato Polyclinic, in Milan, Italy, as well as that of Ketty Miani, Ganiyat Adenike, Ralitsa Adebanjo, and Mariapia Zagaria from the residency program in Clinical Pathology and Clinical Biochemistry at University of Milano, Milan, Italy and that of Maria Luisa Trovato from the Operative Unit of Laboratory Medicine1-Clinical Pathology in the Department of Pathology and Laboratory Medicine at IRCCS San Donato Polyclinic.
CONFLICT OF INTEREST
The authors declared no conflict of interest.





