Associations Between Body Weight, Hippocampal Volume, and Tissue Signal Intensity in 12- to 18-Year-Olds
Abstract
Objective
The hippocampus is a key structure in feeding behaviors and weight regulation. Obesity may lead to disruptions in hippocampal structure. In animals, obesity-related factors (e.g., high-fat/sugar foods) are associated with hippocampal insult (e.g., alterations in the blood brain barrier). In humans, individuals with obesity, relative to healthy weight, have smaller hippocampal volumes. Few studies have examined the association between body weight and the hippocampus during adolescence, a critical brain development period. This study examined hippocampal volume and tissue signal intensity in adolescents across the weight spectrum.
Methods
Structural magnetic resonance imaging and anthropomorphic data were available for 102 12- to 18-year-old adolescents (53% female; 15.07 [SD 1.84] years; standardized BMI [BMIz] scores using the Centers for Disease Control and Prevention growth charts: 0.54 [SD 1.17]) from the Pediatric Imaging, Neurocognition, and Genetics database. Linear regression models controlling for age, sex, genetic ancestry, scanner, and household income examined the relationship between BMIz, hippocampal volume, and T2-weighted hippocampal signal intensity.
Results
BMIz was negatively associated with T2-weighted hippocampal signal intensity in the left (t = −3.05; P = 0.003; r = −0.21) and right (t = −2.50; P = 0.01; r = −0.36) hippocampi. BMIz was not significantly associated with hippocampal volume.
Conclusions
BMIz is associated with hippocampal tissue characteristics during adolescence, which could impact later brain development.
Study Importance
What is already known?
- ► Obesity is associated with inflammation, gliosis, and reductions in gray matter in the hippocampus. The hippocampus is a key structure involved in feeding behaviors and weight regulation. Hippocampal tissue alterations can lead to either increased T2-weighted signal intensity (i.e., sclerosis and gliosis) or decreased T2-weighted signal intensity (i.e., increased vacuolation).
What does this study add?
- ► This study adds to the literature by examining the association between BMI and hippocampal structure in adolescents. This is the first study to date to examine changes in T2-weighted signal intensity within the hippocampus in relation to BMI in humans.
How might these results change the direction of research or the focus of clinical practice?
- ► Our results provide important information regarding the underlying mechanisms of hippocampal tissue integrity in adolescents, which will improve our understanding of the associations between weight and neural structures and contribute to prevention programs during a critical brain development period (e.g., interventions on weight control to improve hippocampal health).
Introduction
Approximately one-third of children and adolescents in the United States have overweight or obesity ((1)). This is concerning considering the comorbidities of obesity, which include cardiovascular disease, type II diabetes, osteoarthritis, cancer, and overall poor quality of life ((2)). Furthermore, obesity in childhood tracks well into adulthood, as children with obesity are six to seven times more likely to become adults with obesity, relative to children with healthy weight ((3)). Brain risk factors are an important but understudied element related to weight gain and obesity, especially in childhood. To date, most studies have compared neural measures between children with obesity and those with healthy weight ((4-7)). Yet few studies have examined neural measures in youth across the weight spectrum and even fewer during adolescence ((8-11)). Adolescence is a critical period of brain development, and changes in brain development during this period often persist into adulthood ((12)). Thus, understanding the association between weight and brain structures during adolescence could inform obesity prevention and treatment.
The hippocampus is a key structure involved in feeding behaviors and weight regulation ((13, 14)). Animal research has shown that damage to or inactivation of the hippocampus impairs perception and processing of interoceptive signals of energy states, increases food-seeking behaviors and food intake, and decreases the postprandial intermeal interval ((15, 16)). In humans, patients with bilateral hippocampal damage have difficulty identifying hunger and satiety cues and consume several meals consecutively if allowed ((17)). Thus, disruptions to the integrity of the hippocampus could lead to excessive eating, weight gain, and, ultimately, a state of obesity.
Obesity is associated with inflammation and reductions in gray matter in the hippocampus ((5, 18)). Both adults and children with obesity show smaller hippocampal volumes, relative to those with healthy weight ((5, 7, 19)), suggesting that a smaller hippocampus in relation to greater body weight might have been present early in life. In addition to the smaller-than-normal hippocampal volumes, prior animal research has shown that obesity-related factors (i.e., a Western diet; high-fat, high-sugar foods) can lead to disruptions in the hippocampal tissue (i.e., inflammation and gliosis) ((20, 21)). In fact, a Western diet impacts the hippocampus in animals after only 10 days ((22)) through a breakdown of the blood brain barrier and reduced levels of brain-derived neurotrophic factor (BDNF), a protein involved in neurogenesis, synaptic transmission, and memory performance ((23)). However, it is unclear whether such alterations to hippocampal tissue are a direct result of weight gain and obesity, independent of diet, because of inflammation ((5, 18, 20)). Moreover, although research in humans suggests that obesity negatively impacts hippocampal volume from an early age, to date, the association between weight and hippocampal tissue morphometry has not yet been examined.
Differences in brain tissue can be assessed by examining T2-weighted signal intensity within a specific region of interest ((24, 25)). Most of the research to date has focused on the effects of obesity and diet on hypothalamic inflammation (i.e., gliosis) ((25-27)). In human adults, gliosis is associated with increased T2-weighted signal intensity ((28, 29)). Notably, adults with obesity, relative to those with healthy weight, have increased T2-weighted signal intensity in the hypothalamus, indicative of gliosis ((25)). Likewise, in children with obesity, increased T2-weighted signal intensity (suggesting hypothalamic gliosis) is positively associated with adiposity ((30)). Moreover, histological studies specifically examining hippocampal tissue have shown that different types of hippocampal tissue alterations can lead to either increased T2-weighted signal intensity (i.e., hippocampal sclerosis and gliosis) or decreased T2-weighted signal intensity (i.e., increased vacuolation) within this region ((31, 32)). However, although obesity-related factors (i.e., a Western diet; high-fat, high-sugar foods) are associated with alterations in the hippocampal tissue (i.e., inflammation and gliosis) ((20, 21)), no study to date has examined changes in T2-weighted signal intensity within the hippocampus in relation to weight or BMI in children or adults. Possible associations may demonstrate that T2 signal intensity in the hippocampus might be useful for monitoring future interventions.
To date, the majority of prior research in children has examined differences in hippocampal volume between children with obesity and those with healthy weight ((7, 33)), yet very little is known about hippocampal health in children and adolescents across the weight range. One study of 120 children and adolescents with BMI in the underweight (3% of sample; BMI < 5th percentile), healthy weight (67% of sample; BMI, 5th to 84th percentile), overweight (12% of sample; BMI, 85th to 95th percentile), and obesity (17% of sample, BMI > 95th percentile) categories showed that higher BMI percentile was associated with smaller gray matter volumes in frontal and limbic brain regions, including the hippocampus, parahippocampus, amygdala, cingulate, and cerebellum ((34)). Because of the limited number of studies available thus far, additional research on the effects of weight among youth across the weight range is warranted, particularly during crucial neurodevelopmental periods such as adolescence.
Four studies have examined the association between weight or factors related to obesity and hippocampal structure in adolescents ((8-11)). However, three of the four studies focused their analyses on insulin resistance ((10)), metabolic syndrome ((11)), and type 2 diabetes as well as their effects on the hippocampus ((8)) rather than body weight alone. Although the fourth study focused on the effects of excess weight on the hippocampus ((9)), it included only 52 adolescents, with unequal groups (69% had overweight or obesity relative to 31% with healthy weight). Adolescence is a time of significant brain maturation and reorganization in several regions, including the limbic system (e.g., the hippocampus) ((12, 35)). Indeed, adolescence is associated with higher levels of neurogenesis in the hippocampus, and disruptions of neurogenesis during this critical period could lead to long-lasting changes into adulthood ((12)). Thus, a better understanding of the association between weight and brain development during adolescence is imperative.
The current study aimed to examine whether differences in hippocampal volume and T2-weighted signal intensity (i.e., alterations in tissue properties) can be seen in a diverse sample of adolescents across the weight range. We hypothesized that a greater standardized BMI (BMIz) score would be negatively associated with total hippocampal volume. In addition, prior research has shown that hippocampal gliosis can lead to increased T2-weighted signal intensity within the hippocampus ((31, 32)). Because obesity-related factors (i.e., high-fat diet) are associated with hippocampal inflammation ((20, 21)), we hypothesized that BMIz would be positively associated with T2-weighted signal intensity in the hippocampus. Based on prior studies showing laterality of findings ((7, 33)), we chose to examine the associations between BMIz and volume and T2-weighted signal intensity in the left and right hippocampi separately.
Methods
Participants
Participants were part of the Pediatric Imaging, Neurocognition, and Genetics (PING) Project. As described previously ((36)), PING participants were recruited through local postings and outreach activities in the greater metropolitan areas of Baltimore, Maryland; Boston, Massachusetts; Honolulu, Hawaii; New Haven, Connecticut; New York, New York; and Los Angeles, Sacramento and San Diego, California. Inclusion criteria for the entire PING sample included being between the ages of 3 and 20 years and fluent in English. Exclusion criteria included neurological disorders, history of head trauma, preterm birth < 36 weeks, diagnosis of an autism spectrum disorder, bipolar disorder, or schizophrenia, mental retardation, pregnancy, prenatal substance exposure with daily illicit drug use by the mother for more than one trimester, and contraindications for magnetic resonance imaging (MRI).
This study utilized a subset of the PING sample for whom height and weight information was available. The following three sites were able to provide this information: Honolulu (n = 206), New Haven (n = 38), and New York (n = 41). Neuroimaging data from participants collected from the three sites were acquired from the PING website (https://chd.ucsd.edu/research/ping.html) and matched to the height and weight information. Given our goal to study adolescents and to maximize the sample size, we decided to include only children ages 12 to 18 in our sample. The final PING adolescent sample for this study included 102 12- to 18-year-old adolescents (53% [n = 54] female; 15.07 [SD 1.84] years; BMI: 23.16 [SD 5.32]; BMIz: 0.54 [SD 1.17]) (Table 1 provides full demographic information). In our study sample of 102 adolescents, 4 had BMI in the underweight category (4% of sample; BMI < 5th percentile), 66 had BMI in the healthy weight category (65% of sample; BMI, 5th to 84th percentile), 13 had BMI in the overweight category (13% of sample; BMI, 85th to 95th percentile), and 19 had BMI in the obesity category (19% of sample; BMI > 95th percentile), which matches the proportion of children with overweight and obesity in the United States ((1)).
| Mean ± SD | |
|---|---|
| Age, y | 15.07 ± 1.84 |
| Sex, female | 53% (n = 54) |
| BMI, kg/m2 | 23.16 ± 5.32 |
| BMIz | 0.54 ± 1.17 |
| Ethnicity | |
| White | 50% |
| Pacific Islander | 10% |
| Asian | 27% |
| Black | 9% |
| Native American | 4% |
| Genetic ancestry factor | |
| European | 0.57 ± 0.38 |
| African | 0.12 ± 0.26 |
| Native American | 0.01 ± 0.05 |
| East Asian | 0.27 ± 0.36 |
| Oceanic | 0.01 ± 0.03 |
| Central Asian | 0.02 ± 0.11 |
Measures
Anthropometrics
BMI was calculated for each participant using the height and weight information provided by the three sites and translated to BMIz using the Centers for Disease Control and Prevention growth charts ((37)).
Genetic ancestry factor
Ancestry and admixture proportions were calculated using a supervised clustering approach implemented in the ADMIXTURE software (https://bioinformaticshome.com/tools/descriptions/ADMIXTURE.html) ((38)). Each participant had six genetic ancestry factors (GAF) (European, African, Native American, East Asian, Central Asian, and Oceanic), which were used in our models as covariates.
Image acquisition and processing
All sites used a standardized multiple-contrast structural MRI protocol, which included a three-dimensional (3D) T1-weighted (echo time (TE) = 3.5 milliseconds, repetition time (TR) = 8.1 milliseconds, inversion time = 640 milliseconds, flip angle = 8°, receiver bandwidth = ±31.25 kHz, field of view = 24 cm, frequency = 256, phase = 192, section thickness = 1.2 mm) and 3D T2-weighted volume (TE = 69.3 milliseconds, TR = 1,500 milliseconds, echo train length = 40, field of view = 24 cm, frequency = 256, phase = 192, section thickness = 1.2 mm). In addition, two axial 2D Diffusion tensor imaging scans (30 directions, b value = 1,000 s/mm2, TE = 83 milliseconds, TR = 13,600 milliseconds, frequency = 96, phase = 96, section thickness = 2.5 mm) were acquired. Imaging data from all three sites were collected on 3-T Siemens Tim Trio scanners (Siemens Medical Solutions, Erlangen, Germany).
As described previously ((36)), cortical and subcortical volumes of regions of interest (bilateral hippocampi in this study) were obtained by using a modified processing stream developed for PING using FreeSurfer (http://surfer.nmr.mgh.harvard.edu) which included additional software modifications developed at University of California San Diego Multimodal Imaging Laboratory. Subcortical regions of interest were then labeled using an automated, atlas-based, volumetric segmentation procedure (volumes in millimeters cubed). Although a 3D T2-weighted volume scan was part of the standardized structural MRI protocol, not all sites were able to collect this sequence. Therefore, the T2-weighted intensities were calculated from the average of the diffusion-weighted b = 0 images (averaged if multiple b = 0 images) and normalized by a scaling factor calculated as the slope of the relationship (across all brain voxels) between mean diffusivity and b = 0 values ((36)). The T2-weighted intensities were not normalized with whole-brain intensity or with cerebral spinal fluid intensity because of concerns that the normalization may be heavily influenced by partial volume artifacts in the estimate of cerebral spinal fluid T2. The T1-weighted volumetrics for bilateral hippocampi were extracted from the data set for analyses.
Raw image quality control
Following established protocols ((36)), each recruiting site uploaded Digital imaging and communications in medicine images for each scan session using a secure Web-based application. All uploaded data were then automatically checked for completeness and protocol compliance, and trained technicians reviewed images for motion artifacts, excessive distortion, operator error, or scanner malfunction. T1-weighted images were examined section by section for excessive motion (e.g., stark ribbon or criss-cross artifacts within the parenchyma, ghosting artifacts outside the head), and each volume was rated as either acceptable or recommended for rescan. Diffusion images were also examined section by section for signs of artifacts or poor image quality. Volumes with five or more sections showing significant section-to-section motion, motion artifacts, or whole-section dropout were rejected (i.e., recommended for rescan). Overall quality control ratings of good, average, or unacceptable were assigned to each data set, and only data rated good or average were used. Quality information was recorded into the quality control utility within 24 hours from time of upload to allow rescanning of participants if possible.
Processed image quality control
Following established protocols ((36)), all processed images (i.e., subcortical volumetric segmentations, cortical areal parcellations, and white and pial surface reconstructions) were examined for all participants. Quality control movies were made in Matlab (MathWorks, Natick, Massachusetts) for each participant to help with data examination. White matter texture consistency and underestimation of temporal regions were examined using movies showing coronal views in sequence and a related horizontal sequence. Pial and dural overestimation along parietal regions and signs of excessive head motion were examined using a movie showing sagittal views.
Statistical analyses
Linear regressions were run in RStudio (version 1.0.136; Boston, Massachusetts) to examine the relationship between BMIz and hippocampal signal intensity and volume, controlling for age, sex, scanner serial number, GAF, and household income. To maintain consistency with prior PING studies, we opted to retain all covariates in our models, regardless of statistical significance. We ran one linear model to assess for potential associations between BMIz and all covariates included in our models. We found no significant associations between BMIz and any covariates. We also assessed for potential differences in BMIz and demographic measures (i.e., parental income and GAF) between the three different scanning sites using their device serial numbers. In order to test the specificity of our results to the hippocampus, we also examined the relationship between BMIz and bilateral amygdala and nucleus accumbens volumes. In models examining hippocampal volume, covariates also included total intracranial volume to normalize the hippocampal volumes. The final models examining hippocampal volume included a total of 90 participants (i.e., 12 participants were removed because of the following: n = 2 missing structural T1-weighted MRI data, n = 1 missing scanner device serial number, n = 4 missing household income information, n = 5 missing GAF information). The final models examining hippocampal T2-weighted signal intensity included a total of 83 participants (i.e., 19 participants removed because of the following: n = 9 missing T2-weighted intensity data, n = 1 missing scanner device serial number, n = 4 missing household income information, n = 5 missing GAF information). To examine the potential effects of outliers, models were run with and without four outliers (< −2 BMIz). Because our results did not significantly change whether these outliers were included or not, we kept them in our models and reported on the whole sample. Effect sizes were also calculated using Pearson correlations to examine the strength of each association.
Results
Sample descriptive statistics
ANOVAs were run to assess for potential differences in BMIz and demographic measures (i.e., parental income and GAF) between the three different scanning sites. We found no differences in BMIz or parental income between sites. There were significant differences between GAF, such that there were significantly more East Asian (F = 4.16, P = 0.01), Central Asian (F = 2.76, P < 0.05), and Oceanic GAF (F = 4.21, P = 0.01) and significantly less African GAF (F = 5.89, P < 0.01) in the second scanner site relative to the other two sites. Therefore, we included the device serial number as one of our covariates in every model.
Hippocampal volume
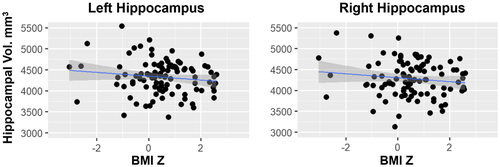
Linear models including all covariates (i.e., age, sex, site, GAF, parental income, and total intracranial volume) showed that BMIz was not significantly associated with left (t = −0.94, P = 0.35) or right (t = −0.57, P = 0.57) hippocampal volume, although the relationship was in the predicted direction (Figure 1). Total intracranial volume was significantly associated with left (t = 4.32, P < 0.001) and right (t = 4.32, P < 0.001) hippocampal volume.
Amygdala and nucleus accumbens volumes
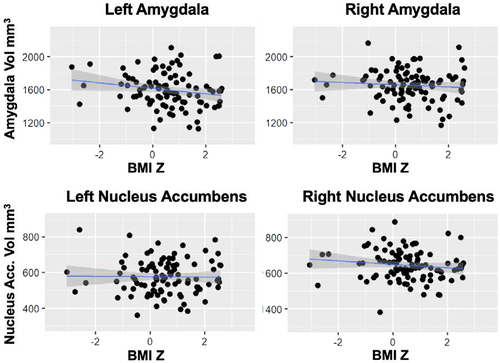
Linear models including all covariates (i.e., age, sex, site, GAF, parental income, and total intracranial volume) showed that BMIz was not significantly associated with left (t = −1.15, P = 0.25) or right (t = −0.50, P = 0.62) amygdala volume or left (t = −0.83, P = 0.41) or right (t = −1.99, P = 0.05) nucleus accumbens volume (Figure 2 and online Supporting Information).
T2-weighted signal intensity
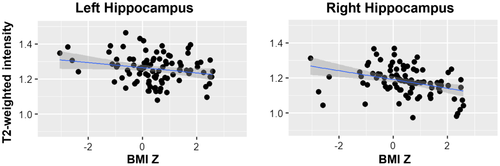
Linear models including all covariates (i.e., age, sex, site, GAF, and parental income) showed that BMIz was negatively associated with left (t = −3.26, P = 0.002) and right (t = −2.57, P = 0.01) hippocampal signal intensity (Figure 3). These associations remained significant after removing four potential outliers from the models (i.e., left [t = −2.63, P = 0.01] and right [t = −3.59, P = 0.001]). Effect sizes were r = −0.2 in the left hippocampus and r = −0.36 in the right hippocampus.
Discussion
This study examined the association between whole-body weight and hippocampal volume and tissue signal intensity in a diverse sample of adolescents across the weight range. Although prior research in children showed that higher BMIz was associated with smaller hippocampal volume ((7, 33)), this study found only a trend for this relationship. However, this study is the first to examine the relationship between body weight and T2-weighted signal intensity within the hippocampus to assess for potential alterations in hippocampal tissue properties (e.g., gliosis). In this study, higher BMIz was associated with lower bilateral hippocampal T2-weighted signal intensities. Most of the prior literature that examined the relationship between weight and T2-weighted signal intensity focused on the hypothalamus and showed a positive association between BMI and T2-weighted signal intensity ((25)). Thus, it is possible that this association manisfests itself differently in the hippocampus. Overall, these results suggest that in adolescents, greater body weight is associated with altered hippocampal tissue integrity but not altered volumes.
Our results did not show that heavier body weight was associated with smaller hippocampal volume. A prior study in adolescents also found no differences in hippocampal gray matter volumes in adolescents with excess weight relative to healthy weight ((9)). It is possible that being overweight in adolescence does not negatively impact hippocampal volume as seen in younger children. Alternatively, the association between excess body weight and hippocampal volume may happen earlier in life, whereas weight-related alterations in hippocampal tissue integrity is more apparent during adolescence. In fact, adolescence is associated with significant brain development, with increases in hippocampal volume ((39)). Therefore, it is possible that the negative association between total body weight and hippocampal volume is compensated by the higher levels of neurogenesis in the hippocampus ((12)) during this time period. Future research should examine the association between total body weight and hippocampal structure over time using a longitudinal design to examine whether changes in hippocampal volume vary at different ages during development in these children. In addition, our results revealed a trend between body weight and right nucleus accumbens volume. Based on prior research showing that children with obesity show hyperactivation to food cues in this region ((6)), future research should further examine the potential association between total body weight and nucleus accumbens structure.
Contrary to our prediction and prior findings ((31, 32)), our results found that greater weight was associated with lower T2-weighted signal intensity within the hippocampus. However, our findings may be explained by a prior study that showed abnormalities in tissue composition (i.e., tissue viscosity; accumulation of macromolecules and lipid in the tissue) were associated with decreased T2-signal intensity ((40)). In that study, the authors postulated that an accumulation of macromolecules and lipids would lead to an increase in tissue viscosity and a shortening of T2 relaxation times, which would appear as a decreased signal intensity on T2-weighted images. Therefore, our results could indicate that adolescents with greater body weight also had more accumulation of lipids in the hippocampal tissue, resulting in an increase in tissue viscosity and decrease in T2-weighted signal intensity. The greater lipid accumulation could have resulted from their consumption of a high-fat diet, which has been associated with alterations in hippocampal tissue in animals (e.g., breakdown of blood brain barrier and a reduction in levels of brain-derived neurotrophic factor) ((41, 42)). Unfortunately, this study did not include dietary information, so we were unable to explore this hypothesis. Future research should include dietary intake assessments to examine whether a high-fat diet (i.e., Western diet; high saturated fats and sugar) also might contribute to the inverse relationship between body weight and T2-weighted signal intensity in the hippocampal regions. In addition, hypertension and associated microhemorrhages are associated with decreases in T2-weighted signal intensity ((43)). Therefore, it is possible that teenagers with higher BMIz also have higher blood pressure, which could result in microlesions and differences in T2-weighted signal. Future research should include blood pressure measurements to assess for their possible relationships with T2-weighted signal intensities in adolescents.
Overall, this study suggests a negative association between body weight and hippocampal tissue property in adolescents. Negative influences on the hippocampus during adolescence, which is a critical time period for brain development, could have long-lasting effects and might predispose these adolescents to a lifetime of overeating and overweight. However, the hippocampus is known for its capability for neurogenesis ((44)). Thus, additional research to determine possible mechanisms related to the negative association between body weight on the hippocampus in youth could help to guide interventions to promote hippocampal health.
This study has several strengths. First, this study included a diverse sample of adolescents from various backgrounds and environments (i.e., Honolulu, New Haven, and New York), which increases the generalizability of the study findings. In addition, all data included in the study were analyzed by the same team at University of California San Diego using an automated and robust processing stream, limiting the possibility for variability in data processing and human error. However, this study also has some limitations. Because of the cross-sectional nature of this study, we were unable to examine causal implications for the reported associations. The sample size in our study was smaller than originally planned because of missing imaging data or other relevant information in some of the participants. In addition, although all models included variables that accounted for the different scanners, it is still possible that unmeasured variables associated with the scanner could impact these outcomes. Future studies should strive to use data collected under similar, if not exactly the same, conditions (i.e., same site and same scanner). Furthermore, although we sought to account for the effects of pubertal development by including only children between the ages of 12 and 18, we do not have a formal measure of puberty and therefore could not truly account for this confound. Future studies should include a formal measure of pubertal development (e.g., pubertal hormones), which can be used as a covariate in models examining brain development. Finally, because of the lack of dietary information, we were unable to examine whether a high-fat diet also might have contributed to the lower signal intensity in those with higher body weight. It is crucial that future studies examine the effects of diet on hippocampal strucuture and tissue integrity.
In conclusion, this is the first study to show that greater body weight in adolescents is associated with lower T2-weighted signal intensity, suggesting lipid accumulation in the tissue. In addition, although our results did not show a significant correlation between heavier body weight and smaller hippocampal volume, the expected inverse correlation was observed. Our results provide important information that will improve our understanding of the association between weight and neural structures, and they will contribute to prevention programs during a critical brain development period (e.g., evaluate outcomes on brain images after interventions on weight control to improve hippocampal health). Future research should further examine the underlying mechanisms for the hippocampal tissue integrity in children with overweight.
Funding agencies
The PING Project was supported by the National Institute on Drug Abuse and the Eunice Kennedy Shriver National Institute of Child Health and Human Development with the following awards: RC2DA029475 and R01HD061414. This work was supported by grants F31DK117556 and K23DK114480 from the University of California San Diego (UCSD). The funding did not influence reported results. The views discussed may not reflect the views of the National Institutes of Health.
Disclosure
The authors declared no conflict of interest.





