Network-Like Organization of the Circadian System Regulates Metabolic Homeostasis
Endogenous circadian clocks facilitate the body’s adaptation to an environment that is rhythmically changing because of the Earth’s rotation around its axis. In mammals, a master pacemaker residing in the hypothalamic suprachiasmatic nucleus (SCN) integrates external time cues (or zeitgebers) such as light exposure to synchronize subordinated clocks in extra-SCN brain and peripheral tissues with each other and with external time. Disruption of clock network coordination, for example, by shift work, promotes various chronic metabolic diseases, such as obesity, type 2 diabetes, or coronary heart disease ((1)). Recent studies using tissue-specific manipulations of clock function in mice have challenged the dominant role of the SCN as the primary driver of circadian rhythms throughout the body. In this perspective, we discuss the experimental evidence for a more network-like functioning of the mammalian circadian clock system and the implication of such organization for the regulation of energy metabolism as one major clock output.
The important role of the circadian clock system in the maintenance of metabolic health is well documented, and experiments involving genetic manipulations of the molecular clockwork in mice have been pivotal in uncovering this relationship. Alterations in energy metabolism and metabolic homeostasis have been reported in mice carrying mutations for many of the core clock genes. Period2 mutant mice, for example, are overweight with hyperinsulinemia and hypoglycemia. Cryptochrome1/2 double deficiency results in impaired glucose despite normal insulin tolerance and increased vulnerability to an obesogenic challenge, while mutations in circadian locomotor output cycle kaput (Clock) or brain and muscle Arnt-like protein1 (Bmal1) lead to decreased glucose tolerance, hypoinsulinemia, hyperglycemia, hyperphagy, and increased body weight at early age (reviewed in Tsang et al.) ((2)).
Bmal1 is the only single clock gene so far, the deletion of which immediately abrogates behavioral and molecular circadian rhythms in mice. Thus, for practical reasons, studies have primarily targeted Bmal1 to assess the role of specific tissue clocks in metabolic control. It should be noted, though, that Bmal1 likely has functions besides its role in clock maintenance; thus, results from these studies should be compared with other complementary approaches ((2)). In most cases, phenotypes from such tissue-specific clock ablation models are less prominent, suggesting a certain level of compensation with regard to metabolic control. Bmal1 deletion in pancreatic islets cells leads to hypoinsulinemia and impaired glucose tolerance because of reduced insulin sensitivity on organism level, but food intake and body weight regulation are unaltered ((3)). Hepatocyte- or myocyte-specific loss of Bmal1 function impairs glucose export or import in the targeted tissue, respectively, but has only minor effects on body weight regulation ((4, 5)). In contrast, though, adipose-specific Bmal1 ablation results in hyperphagy and obesity, likely through an indirect effect on central appetite regulation by reduced levels of polyunsaturated fatty acid secretion from adipose stores ((6)). Bmal1 deletion in the forebrain alters the timing of food intake and increases gluconeogenesis. The clock in the agouti-related peptide expressing neurons plays a critical role in the hepatic glucose overproduction ((7)). Genetically restoring clock function in (global) Clock mutant mice, specifically in liver, normalizes body weight but only under high-fat diet conditions ((8)). Restoration of Bmal1 expression in liver of Bmal1-deficient mice restores rhythms in local metabolic pathways but has no or little effect on body weight regulation and glucose handling, respectively ((9)).
While these data support the dominant role of the SCN, whose clock is affected in global but not tissue-specific clock gene mutants, studies using genetic ablation of SCN pacemaker function have argued for a more prominent role of peripheral clocks in metabolic control. Mice carrying mutations in Bmal1 either in the SCN or the whole forebrain show normal body weight regulation, correlating with rhythmic clock gene expression in peripheral tissues, under rhythmic light-dark conditions ((10)). Mice with surgical ablation of the SCN, however, are overweight and diabetic ((11)).
This apparent discrepancy is resolved when one assumes a less hierarchical and more network-like organization of the circadian clock system (Figure 1). Coordinated circadian synchrony with fixed phase relations between different tissue circadian clocks ensures a correct integration of interacting metabolic outputs establishing normal energy state and body weight homeostasis. In global knockouts (and in SCN lesioned mice), systemic network coordination is lost, whereas genetic ablation of the SCN (or any other tissue) alone has only very specific and, thus, ultimately less disruptive effects on metabolic homeostasis. This finding is supported by a recent study in SCN-specific Bmal1 mutant mice, which show normal body weight under light-dark and weight gain under constant darkness conditions ((12)). Interestingly, timed food restriction also restores metabolic homeostasis in these mice in constant darkness, along with peripheral clock gene coordination. Such network-level synchronization may explain the metabolic effects of time-restricted feeding reported in clock gene mutant mice. Here, food-related time cues may, at least partly, restore rhythmic network coordination of tissue metabolic outputs despite defective clock function ((13)).
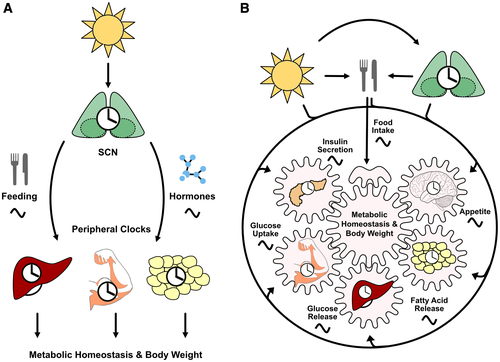
Taken together, mouse mutant data suggest that metabolic homeostasis requires network coordination of the circadian clock system. In global clock gene mutants or in shift workers, phase coherence within the clock network is lost, thus promoting disruption of energy handling and, under conditions of high energy availability, obesity and its sequelae. On the contrary, if only one tissue clock, the SCN or any other tissue of metabolic implication, is lost, specific metabolic functions may be affected, but overall energy homeostasis is, in many cases, preserved. Vice versa, stabilizing circadian network coordination by external cues such as timed feeding or other zeitgebers has restorative metabolic effects beyond its particular clocks and downstream processes in specific tissues. From an evolutionary perspective, a network-like organization of the circadian system may also allow for a much more fine-tuned adaptation to complex rhythmic environments while at the same time retaining a high level of robustness of the overall timing system.
Funding agencies
This work was supported by the German Research Foundation (GRK-1957 & OS353/7-1).
Disclosure
The authors declare no conflict of interest.





