Differential Impact of Weight Loss and Glycemic Control on Inflammasome Signaling
Abstract
Objective
Interleukin (IL)-1β is involved in obesity-associated inflammation and in the pathogenesis of type 2 diabetes (T2D) mellitus. Our aim was to correlate serum IL-1β and caspase-1 levels with weight loss, glucose metabolism, and insulin resistance (IR) after bariatric surgery.
Methods
A total of 32 patients with morbid obesity and T2D (Ob-T2D) and 29 patients with morbid obesity and normal glucose tolerance (Ob-NGT), treated by Roux-en-Y gastric bypass, were studied before and 1 year after surgery. Sixteen healthy individuals served as a control (HC) group. IR was assessed by the oral glucose insulin sensitivity method. Plasma IL-1β levels and caspase-1 were measured.
Results
Presurgery BMI was similar in Ob-NGT and Ob-T2D. IR was progressively impaired in Ob-NGT and Ob-T2D (P < 0.0001). Fasting plasma IL-1β and caspase-1 levels were lower in HCs than in patients with Ob-NGT or Ob-T2D (P < 0.02; P = 0.05), and both were inversely correlated with IR (P = 0.01; P = 0.02). After surgery, BMI decreased and IR improved to a similar extent in Ob-NGT and Ob-T2D (P < 0.0001). Plasma caspase-1 concentrations normalized in both groups (P < 0.0001), whereas plasma IL-1β levels normalized only in Ob-NGT.
Conclusions
Plasma IL-1β and caspase-1 levels were inversely correlated with IR. Caspase-1 levels normalized after weight loss, whereas IL-1β normalized only in people without T2D, suggesting the persistence of a systemic inflammatory condition in people with T2D.
Study Importance
What is already known?
- An increase in caspase-1 activation and interleukin (IL)-1β levels has been detected in peripheral blood monocytes from patients with obesity and type 2 diabetes (T2D), as compared with healthy subjects, thus suggesting a potential role of the proinflammatory cytokine IL-1β in obesity-associated inflammation and T2D.
What does this study add?
- Caspase-1 levels normalized after surgically induced weight loss, regardless of the presence of diabetes, whereas IL-1β normalized in the group without T2D but not in patients with T2D, suggesting the persistence of a systemic inflammatory condition in the latter group.
Introduction
Over the past few decades, the prevalence of obesity has reached epidemic proportions, becoming a serious challenge for global public health (1). Beyond an excessive fat accumulation, obesity is characterized by chronic systemic inflammation, and there is evidence that such a condition contributes to the development of insulin resistance (IR) and the pathogenesis of type 2 diabetes (T2D) mellitus (2-4). In particular, it has been shown that patients with obesity are characterized by a marked unsettlement of immune cell functions, displaying an uncontrolled activation of innate immune cells (i.e., macrophages), which promote a massive release of proinflammatory cytokines (i.e., interleukin [IL]-6, tumor necrosis factor, and IL-1β), interfering in an autocrine and paracrine manner with several metabolic processes, with particular regard for insulin synthesis and signaling (5-7). Among these inflammatory mediators, IL-1β has been found to be pivotally involved in the onset of obesity-associated inflammation as well as in the pathogenesis of T2D (5-7). Of note, IL-1β production is strictly related to the transcription and subsequent storage of inactive pro–IL-1β into cells by caspase-1, a cysteine protease that converts the inactive preform of IL-1β to the active inflammatory cytokine. In turn, caspase-1 activation is mediated by a repertoire of proteins, through the formation of a multi-protein complex designated as an inflammasome (8, 9). It is also noteworthy that several studies have pointed out a critical role of purines in triggering inflammasome assembly and activation (8), as well as in orchestrating the activity of several immune cell populations involved in the inflammatory condition associated with obesity and diabetes (10-13).
Current clinical evidence has documented increased caspase-1 activity and IL-1β secretion in adipose tissue macrophages from patients with obesity and T2D, and these patterns are tightly correlated with the condition of IR (14, 15). Interestingly, an increase in caspase-1 activation and IL-1β levels has been detected in peripheral blood monocytes from patients with obesity and T2D, as compared with healthy subjects, thus confirming a critical role of the proinflammatory cytokine IL-1β in obesity-associated inflammation and T2D (14, 15).
At present, people with morbid obesity, with or without T2D, often undergo bariatric surgery, with particular regard for Roux-en-Y gastric bypass (RYGB), which has been shown to result in significant body weight loss and glycemic control improvement, without relevant malabsorption symptoms (16). Observational studies on bariatric surgery have shown a complete T2D remission rate of 44% after RYGB at 12 months, a rate of about 41% at 2 years (17), and a rate of 31% at 5 years (18). The mechanism underlying such an improvement of glycemic control appears to be complex and to involve an improvement in β-cell function (16) and insulin sensitivity, with a marked decrease in insulin levels, which may be linked to the attenuation of chronic inflammation, as suggested by the significant reduction of high-sensitivity C-reactive protein in patients with bariatric surgery (19).
Based on this background, a crucial role in the improvement of insulin sensitivity might be played by the reduction of chronic inflammation. Indeed, surgically induced weight loss is known to improve the systemic inflammatory status, and inflammatory mediators have been found to be normalized in patients with morbid obesity after bariatric surgery (20-23). However, whether, and to what extent, the improvement of the clinical condition in patients with obesity and T2D following bariatric surgery could, at least in part, depend on an improvement of inflammation remains unclear. Therefore, the present study was specifically designed to correlate plasma caspase-1 and IL-1β levels with weight loss, as well as with changes in glucose metabolism and IR, in people with morbid obesity, with or without T2D, after bariatric surgery.
Methods
Participants
The study group included 32 patients with morbid obesity and normal glucose tolerance (Ob-NGT); 29 patients with morbid obesity and T2D (Ob-T2D), who were wait-listed for laparoscopic RYGB; and 16 healthy people as a control (HC) group. Diabetes was diagnosed according to the American Diabetes Association (ADA) criteria (24). Insulin-taking patients whose age of diabetes onset was ≥ 40 years, whose BMI was > 30 kg/m2, and whose results were negative for the presence of islet autoantibodies were also considered to have T2D. Diabetes duration ranged from 2 to 14 years. The antidiabetic treatment was based on insulin (basal bolus) in 9 patients, oral antidiabetic agents in 15 patients (sulfonylurea plus metformin), and diet alone in 5 patients. The exclusion criteria included the following: (a) medical conditions requiring acute hospitalization; (b) blindness; (c) severe medical conditions (liver cirrhosis, end-stage renal failure, malignancy, connective tissue diseases, or endocrine diseases such as hypo- or hyperthyroidism) or diseases such as chronic congestive heart failure, recent myocardial infarction or stroke, or unstable angina pectoris. The study protocol was approved by the local ethics committee (number 2,360) and all patients signed a written consent form prior to the study.
Study design
After screening, patients with morbid obesity were requested to attend our clinical research unit for the baseline study 2 weeks before surgery; healthy participants were asked to take part in the baseline study as the HC group. Twelve months later, the metabolic study was repeated at the clinical research unit in all participants who underwent bariatric surgery. After surgery, complete diabetes remission was defined as glycated hemoglobin (HbA1c) < 6.0% and fasting glucose < 5.6 mmol/L without antidiabetic treatment for 1 year, and partial diabetes remission was defined as HbA1c < 6.0% and fasting glucose < 5.6 mmol/L without antidiabetic treatment for 1 year, according to 2009 ADA consensus statement criteria (24).
Study protocol
For the metabolic study, all participants were instructed to not exercise for 48 hours prior to the study and were examined in the morning after an overnight (12- to 14-hour) fast. Patients with T2D on oral hypoglycemic agents were requested to discontinue these medications for 48 to 72 hours before the study; in those on insulin, injections were discontinued 16 hours before the metabolic study (patients on bedtime insulin glargine had been switched to NPH 2 days before the study). The metabolic study consisted of a frequently sampled oral glucose tolerance test (OGTT). After an overnight fast, blood samples were collected through an indwelling cannula. Peripheral blood samples were taken for the assessment of routine blood chemistry and plasma glucose, insulin, C-peptide, HbA1c, and cytokine (Il-1β and caspase-1) concentrations. After ingestion of 75 g of glucose in an aqueous solution, venous blood was sampled at 10, 20, 30, 45, 60, 90, 120, 150, and 180 minutes for glucose, insulin, and C-peptide assay. In people with morbid obesity, laparoscopic RYGB was performed as described elsewhere (16).
The plasma glucose concentration was measured on a Beckman Glucose Analyzer 2 (Beckman Coulter, Inc., Fullerton, California). Fasting concentrations of serum total cholesterol, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol were measured by standard techniques (Synchron CX4, Beckman Coulter, Brea, California). Plasma insulin and C-peptide were measured using a Cobas e411 analyzer (Roche Diagnostics S.p.A., Milan, Italy).
Measurements of plasma caspase-1 and IL-1β levels
Caspase-1 and IL-1β levels in plasma samples were measured by Quantikine enzyme-linked immunosorbent assay (ELISA) assay kits (R&D Systems, Zug, Switzerland), as previously described (25, 26). For this purpose, 3 mL of fasting venous blood was collected in K2-coated tubes containing 30 μL (0.5 mol/L) of EDTA plus 2,000 KIU of aprotinin. Samples were mixed and centrifuged at 2,000 g for 20 minutes. After centrifugation, 2 mL of cold acetone (4°C) was added to 1 mL of plasma, mixed, and centrifuged at 2,000 g for 20 minutes at 4°C. The supernatant was added to 4 mL of cold petroleum ether, mixed, centrifuged, and dried under a vacuum to remove any residual acetone. The samples were then stored at −20°C until use. Plasma caspase-1 and IL-1β levels were expressed as picograms per milliliter.
Modeling
Insulin sensitivity and β-cell function parameters were derived from mathematical modeling of the plasma glucose, insulin, and C-peptide concentrations measured during the frequently sampled OGTT, as previously described (16). In brief, insulin sensitivity was calculated as the oral glucose insulin sensitivity index, which estimates plasma glucose clearance rate (in mL/min/m2) at a level of hyperinsulinemia in the range of that achieved during a standard (240 pmol/min/m2) euglycemic hyperinsulinemic clamp, against which this index has been validated in participants with normal glucose tolerance, impaired glucose tolerance, or overt diabetes (16). The β-cell function model was computed as elsewhere described (16).
Statistical analysis
Results are expressed as mean ± SD or median (interquartile range [IQR]) for variables with normal or skewed distribution, respectively. Group differences were compared using the χ2 test for categorical variables, the Mann Whitney U test for continuous variables, and the Wilcoxon signed rank test for paired data. Analyses of changes over time (before, early after surgery, and late after surgery) in the two patient groups (Ob-NGT and Ob-T2D) were carried out by ANOVA for repeated measures; for this test, parameters with a skewed distribution were log-transformed. The output of this ANOVA model is a P value for the time factor (i.e., overall changes over time), a P value for the group (i.e., between-group differences), and a P value for the time by group interaction (i.e., differential changes between groups over time). P < 0.05 was considered significant.
Results
Baseline anthropometric and metabolic parameters
The degree of obesity was similar in patients with Ob-T2D and Ob-NGT, and it was significantly different as compared with the HC group (Table 1). Patients with Ob-T2D were older (P < 0.001) and had higher baseline HbA1c, fasting glucose, and mean plasma glucose concentrations than patients with Ob-NGT and patients who were in the HC group (P < 0.001) (Table 1). Baseline β-cell glucose sensitivity was significantly lower in people with Ob-T2D than in people with Ob-NGT or in people in the HC group (P = 0.0001), without differences between patients with Ob-NGT and HCs. Fasting insulin concentrations differed significantly among groups, with progressive reduction from patients with Ob-NGT to patients with Ob-T2D to HCs (P = 0.008), whereas mean plasma insulin concentrations during OGTT were higher in patients with Ob-NGT as compared with patients with Ob-T2D and HCs (P < 0.0001). Fasting and total insulin secretion rate did not differ substantially among the three groups, although total insulin secretion rate was reduced in the group with Ob-T2D as compared with the HC group (P = 0.03) (Table 1). Finally, insulin sensitivity was impaired progressively from HCs to patients with Ob-NGT to patients with Ob-T2D (P < 0.0001).
| Ob-NGT | Ob-T2D | HC | P value | |
|---|---|---|---|---|
| Participants | 32 | 29 | 16 | |
| Age (y) | 40 ± 8 | 52 ± 9* | 39 ± 10 | 0.0001 |
| Diabetes duration (y) | — | 8 ± 6 | — | — |
| Gender (F/M) | 25/7 | 22/7 | 12/4 | ns |
| BMI (kg/m2) | 47.2 ± 7.2 | 43.2 ± 6.3 | 22.3 ± 4.1** | 0.0001 |
| Fasting glucose (mmol/L) | 5.5 ± 0.7 | 8.1 ± 2.0* | 4.9 ± 0.6 | 0.0001 |
| HbA1c (%) | 5.5 ± 0.3 | 8.2 ± 2.1* | 5.5 ± 0.2 | 0.0001 |
| Mean glucose (mmol/L) | 6.8 ± 1.1 | 8.9 ± 2.2* | 6.1 ± 0.8 | 0.0001 |
| Fasting insulin (pmol/L) | 179 ± 163 | 143 ± 85 | 77 ± 16** | 0.008 |
| Mean insulin (pmol/L) | 415 ± 226*** | 176 ± 93 | 198 ± 85 | 0.0001 |
| Fasting insulin secretion (pmol/min/m2) | 102 ± 69 | 101 ± 54 | 71 ± 24 | ns |
| Total insulin output (nmol/m2) | 50 ± 28 | 41 ± 23 | 56 ± 22**** | ns |
| β-cell glucose sensitivity (pmol/min/m2/mM) | 59 ± 35 | 28 ± 24 | 75 ± 53 | 0.0001 |
| Insulin sensitivity (mL/min/m2) | 345 ± 54 | 284 ± 50* | 439 ± 52 | 0.0001 |
- Data given as n (participants) or mean ± SD.
- * P values, Ob-T2D vs. other groups.
- ** P values, HC vs. other groups.
- *** P values, Ob-NGT vs. other groups.
- **** P = 0.03, HC vs. Ob-T2D.
- F, female; HbA1c, glycated hemoglobin; HC, healthy control; ns, not significant; M, male; Ob-NGT, morbid obesity and normal glucose tolerance; Ob-T2D, morbid obesity and type 2 diabetes; P1, before vs. after surgery; P2, Ob-T2D vs. Ob-NGT; P3, time by group interaction.
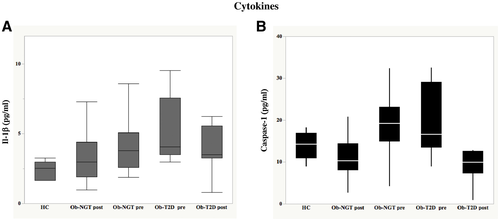
Baseline plasma IL-1β concentrations were significantly and progressively higher in patients with Ob-T2D as compared with patients with Ob-NGT (P = 0.03) and HCs (P < 0.0001), being significantly higher in the group with Ob-NGT than in HCs (P = 0.017) (median [IQR]: 2.52 [1.99-2.85] vs. 3.78 [3.53-5.63] vs. 4.07 [4.08-7.35] pg/mL, P = 0.004, respectively, in HCs vs. Ob-NGT vs. Ob-T2D) (Figure 1A). Similarly, plasma caspase-1 concentrations were lower in HCs than in the groups with Ob-NGT or Ob-T2D (mean ± SD: 13.8 ± 3.2 vs. 19.2 ± 7.7 vs. 20.5 ± 8.8 pg/mL, P = 0.042, respectively), but there were no differences in patients with morbid obesity according to the glucose tolerance status (Figure 1B).
Regression analysis
In the linear regression analysis of the data from the overall population, mean plasma glucose levels were correlated positively with plasma IL-1β concentrations (r = 0.35, P < 0.0001), whereas insulin sensitivity was correlated negatively with plasma IL-1β concentrations (r = 0.30, P = 0.0009). IL-1β plasma concentrations were slightly correlated with caspase-1 (r = 0.22, P = 0.05). In a multivariate model analysis including age, BMI, fasting insulin secretion, insulin sensitivity, mean plasma glucose, HbA1c, only the latter parameter was a significant predictor of plasma IL-1β concentrations (P = 0.0063) (Figure 2A).
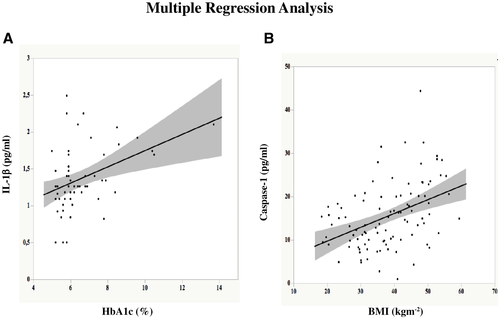
Plasma caspase-1 concentrations were correlated positively with mean plasma glucose levels (r = 0.27, P = 0.0045) and BMI (r = 0.45, P < 0.0001) and negatively correlated with insulin sensitivity (r = 0.35, P < 0.0001). In a multivariate model analysis including age, BMI, insulin sensitivity, fasting insulin secretion, HbA1c, and mean plasma glucose, only BMI was a significant predictor of plasma caspase-1 concentrations (P = 0.0035) (Figure 2B).
Effects of RYGB in patients with Ob-T2D and Ob-NGT
As shown in Table 2, 1 year after surgery, BMI decreased similarly in all patients with morbid obesity; the respective values were −13.9 kg/m2 in the Ob-NGT group and −12.1 kg/m2 in the Ob-T2D group (P < 0.0001). Fasting and mean plasma insulin concentrations decreased at a similar extent in both groups of patients (P < 0.0001 and P < 0.0009, respectively). Glycemic control (as indexed by HbA1c), fasting, and mean plasma glucose levels improved more significantly in patients with Ob-T2D than in patients with Ob-NGT (P = 0.035, P = 0.0009, and P = 0.004, respectively) (Table 2).
| Ob-NGT before | Ob-NGT after | Ob-T2D before | Ob-T2D after | P1 | P2 | P3 | |
|---|---|---|---|---|---|---|---|
| Participants | 32 | 32 | 29 | 29 | |||
| BMI (kg/m2) | 47.2 ± 7.2 | 33.3 ± 2.0 | 43.2 ± 6.3 | 31.1 ± 2.2 | 0.0001 | 0.003 | ns |
| Fasting glucose (mmol/L) | 5.5 ± 0.7 | 5.9 ± 0.4 | 8.1 ± 2.0 | 5.9 ± 1.0 | 0.0001 | 0.0001 | 0.0009 |
| HbA1c (%) | 5.7 ± 0.3 | 5.3 ± 0.3 | 8.2 ± 22 | 6.4 ± 0.9 | 0.001 | 0.0005 | 0.035 |
| Mean glucose (mmol/L) | 6.8 ± 1.1 | 5.9 ± 0.83 | 8.9 ± 2.2 | 6.52 ± 1.6 | 0.0001 | 0.004 | 0.004 |
| Fasting insulin (pmol/L) | 179 ± 163 | 94 ± 75 | 143 ± 85 | 62 ± 41 | 0.0001 | ns | ns |
| Mean insulin (pmol/L) | 415 ± 226 | 227 ± 98 | 176 ± 93 | 115 ± 56 | 0.0009 | 0.0001 | ns |
| Fasting insulin secretion (pmol/min/m2) | 102 ± 69 | 63 ± 21 | 101 ± 54 | 79 ± 52 | 0.0008 | ns | ns |
| Total insulin output (nmol/m) | 50 ± 28 | 50 ± 20 | 41 ± 23 | 36 ± 18 | ns | 0.015 | ns |
| β-cell glucose sensitivity (pmol/min/ m2/mM) | 59 ± 35 | 81 ± 38 | 28 ± 24 | 52 ± 30 | 0.001 | 0.002 | ns |
| Insulin sensitivity (mL/min/m2) | 345 ± 54 | 440 ± 60 | 284 ± 50 | 381 ± 57 | 0.0001 | 0.0001 | ns |
- Data given as n (participants) or mean ± SD.
- HbA1c, glycated hemoglobin; ns, not significant; Ob-NGT, obesity and normal glucose tolerance; Ob-T2D, morbid obesity and type 2 diabetes; P1, before vs. after surgery; P2, Ob-T2D vs. Ob-NGT; P3, time by group interaction.
Insulin sensitivity improved both in patients with Ob-NGT and in patients with Ob-T2D (P = 0.0001); in the latter group, however, it remained lower than in patients with Ob-NGT (P < 0.0001) (Table 2). β-cell glucose sensitivity showed a marked improvement in both groups (P = 0.001); in patients with Ob-T2D, it remained significantly lower than in patients with Ob-NGT (P = 0.002). Fasting insulin secretion declined after surgery in both groups (P = 0.0008), whereas total insulin output did not change (Table 2).
Fasting plasma IL-1β concentrations decreased after surgery in both groups (median [IQR]: 2.97 [1.65-3.22] vs. 3.66 [1.67-6.58] pg/mL, P = 0.008, respectively, in patients with Ob-NGT and Ob-T2D) and normalized in patients with Ob-NGT, but it still remained significantly higher in patients with Ob-T2D compared with patients with Ob-NGT and HCs (P = 0.03 and P = 0.0058, respectively) (Figure 1A). After surgery, fasting plasma caspase-1 levels fell similarly in both groups, reaching plasma concentrations similar to those in HC participants (12.08 ± 6.01 vs. 11.34 ± 4.13 pg/mL, P < 0.0001, effect of surgery, in patients with Ob-NGT and Ob-T2D, respectively) (Figure 1B).
Diabetes remission was observed in 15 of the 29 patients 1 year after surgery. With regard to glucose-lowering agents, all participants on diet treatment and 10 of the 15 patients on oral agents had diabetes remission, whereas none of the patients on insulin were in remission, although their metabolic control improved, and insulin was stopped. HbA1c was significantly reduced after surgery in all patients with Ob-T2D (people with remission vs. no remission: 5.8 ± 0.4 vs. 7.0 ± 0.95%, P = 0.0001). However, during the OGTT, within the group of patients with partial diabetes remission, plasma glucose concentrations were above 11.1 mmol/L at 30 minutes after glucose load in 30% of patients, at 60 minutes after glucose load in 58% of patients, and at 90 minutes after glucose load in 34% of patients. Moreover, within patients with complete remission, plasma glucose concentrations were above 11.1 mmol/L at the same times after glucose load in 23%, 24%, and 26% of patients, respectively. All patients with partial or complete diabetes remission had plasma glucose concentrations lower than 11 mmol/L at 120 minutes after glucose load.
No differences were observed for plasma IL-1β and caspase-1 concentrations when comparing participants with remission and nonremission (IL-1β median [IQR]: 3.52 [1.7-9.5] vs. 4.30 [0.8-5.7] pg/mL, P = not significant; caspase-1: 112.73 ± 7.48 vs. 10.35 ± 2.25 pg/mL, P = ns, respectively). Finally, a positive correlation was found between plasma glucose concentrations at 60 minutes (after glucose load) and IL-1β levels after surgery (r = 0.33, P = 0.026).
Discussion
The major findings of the present study are as follows. (a) Subjects with morbid obesity display higher plasma caspase-1 concentrations than HCs, independently of the glucose tolerance status, whereas plasma IL-1β levels were progressively higher when moving from healthy participants to patients with Ob-NGT and from the latter to patients with Ob-T2D (Figure 1). (b) Interestingly, in a multivariate model adjusted for age, BMI, insulin sensitivity, and HbA1c, only the BMI correlated with the changes in caspase-1 concentrations; at variance, in a similar model analysis, HbA1c was the only variable correlated with IL-1β (Figure 2). (c) After significant weight loss by surgery, plasma caspase-1 levels fell down in all patients with morbid obesity, independently of the glucose tolerance status, reaching plasma concentrations similar to those of healthy individuals; by contrast, IL-1β plasma concentrations, although decreasing after weight loss in both groups, normalized only in patients with Ob-NGT (Figure 1) and remained significantly higher in patients with Ob-T2D as compared with the healthy group.
It is well known that obesity is linked to a variety of disorders including T2D (27). Recent studies have suggested that the persistent low-grade inflammation found in people with obesity is a major contributor to the progression toward IR and T2D, and several proinflammatory cytokines, including IL-1β, have been strongly associated with this progression (4, 28, 29). In particular, enhanced IL-1β production and release in the presence of hyperglycemia has been reported in different cell types (30), including β cells, suggesting a role of IL-1β in β-cell dysfunction (30, 31).
Because there is evidence that IL-1β production is regulated mainly by cytosolic molecular complexes, designated as the NLRP3 inflammasome (nucleotide-binding domain, leucine-rich–containing family, pyrin domain–containing-3 or Nod-like receptor protein 3), some studies have shown that the activation of the NLRP3 inflammasome could play a relevant role in obesity (32, 33), IR, and progression to T2DM (34). However, conflicting results have been reported in regard to the circulating concentrations of IL-1β in humans; it has been found to be increased or unchanged in people with obesity without T2D versus HCs (35-37). In patients with T2D, elevated plasma IL-1β levels were found in the presence of hyperglycemia and IR (15), but in a large prospective study (the European Prospective Investigation into Cancer and Nutrition Study), it has been reported that plasma IL-1β, per se, did not differ between participants with incident T2D and participants without T2D; by contrast, a combined elevation of IL-6 and IL-1β predicted the risk of T2D (38).
In the present study, circulating IL-1β concentrations were significantly higher in patients with morbid obesity as compared with HCs, in agreement with previous data reported by Verheggen R (35), and they increased progressively, passing through Ob-NGT to Ob-T2D and being inversely correlated with insulin sensitivity and positively associated with mean plasma glucose. In particular, in a multivariate model analysis, only HbA1c was associated with IL-1β concentrations, suggesting a major role of hyperglycemia in determining the circulating IL-1β concentrations, independently of BMI. These data are in line with findings reported by Ruscitti et al., who showed that hyperglycemia can lead to an increased secretion of IL-1β in monocytes from patients with T2D (39). Furthermore, other authors have shown an enhanced release of IL-1β from CD14+ macrophages freshly isolated from adipose tissue biopsies of patients with obesity and diabetes, as compared with HCs, and a positive correlation between IL-1β and HbA1c values (40). Based on these data, it is not surprising that, in the present study, after the weight loss induced by bariatric surgery, circulating IL-1β levels normalized in patients with Ob-NGT but not in patients with Ob-T2D. Indeed, after RYGB, a high glucose variability (GV), as a consequence of the anatomical changes induced by surgery, has been reported (41), even though some patients fulfilled the agreed-on criteria of diabetes remission. In our study, of the patients who fulfilled the ADA criteria for diabetes remission (complete or partial together) (21), 50% had plasma glucose concentrations at 90 minutes after a glucose load ≥ 200 mg/dL and 82% had plasma glucose concentrations at 60 minutes after a glucose load ≥ 200 mg/dL. To the best of our knowledge, the present study is the first one to describe how patients who underwent diabetes remission after surgery but retained a high GV (30 to 60 to 90 minutes after oral glucose load) showed persistent plasma IL-1β concentrations compared with patients with Ob-NGT; further, it is the first to show that plasma IL-1β levels did not differ among patients with or without diabetes remission after surgery, suggesting a high risk of vascular damage. On the other hand, as suggested by other authors (41), HbA1c levels in the normal range are not able to predict a wide GV, and silent GV could be responsible for a persistent detrimental inflammatory stimulus. Indeed, pathophysiological and clinical studies have shown that high GV can be involved in the pathogenesis of diabetic vascular complications via activation of inflammatory pathways, increased oxidative stress, and endothelial dysfunction (42, 43). Therefore, these data support the findings that several patients, fulfilling ADA diabetes remission criteria 1 year after surgery (21), still retain a substantial risk of diabetes relapse, supporting the need for redefining diabetes remission. As far as we know, circulating caspase-1 has not been investigated in patients after bariatric surgery, and data on tissue NLRP3 expression are quite conflicting. Indeed, although some authors described a decrease in NLRP3 expression in the abdominal subcutaneous adipose tissue after weight loss in patients with obesity and T2D, in concomitance with an improved insulin sensitivity (44, 45), others (31) did not find any variation of NLRP3 expression in subcutaneous adipose tissue, visceral adipose tissue, or liver from patients with severe obesity 6 months after adjustable gastric banding surgery.
In order to better evaluate the pathophysiological significance of the NLRP3 inflammasome, we also measured caspase-1 concentrations, which were higher in plasma from patients with obesity, independently of the glucose tolerance status, and were independently correlated with BMI. After surgery, in concomitance with the weight loss, we observed a significant reduction and normalization of caspase-1 concentrations, independently of glucose tolerance status, whereas BMI remained in the range of first-degree obesity. Therefore, in the present study, different patterns have emerged for circulating caspase-1 and IL-1β, highlighting major roles for body weight in caspase-1 concentrations and for hyperglycemia in circulating IL-1β levels. These different patterns could be explained by evidence in the literature that the activation of IL-1β precursor depends not only on inflammasome-mediated caspase-1 activity but also on extracellular serine proteases (i.e., proteinase-3, elastase and cathepsin G) related to the presence of activated neutrophils (8).
Potential limitations of the present findings include the small number of study patients and the lack of continuous glucose monitoring, even if variations in plasma glucose were detected during OGTT. Furthermore, another limitation of the study is the lack of a control group who achieved diet-induced weight loss, in order to exclude the effects of altered gastrointestinal anatomy on cytokine concentrations.
Conclusions
Plasma IL-1β and caspase-1 concentrations are elevated in people with morbid obesity and are inversely related to insulin sensitivity. However, although caspase-1 was found to be linked to body weight, independently of glucose tolerance status, IL-1β was associated with plasma hyperglycemia. In addition, caspase-1 levels normalized after surgically induced weight loss, regardless of the presence of diabetes, whereas IL-1β normalized in people without T2D, but not in those with T2D, suggesting the persistence of a systemic inflammatory condition in the latter group. On the basis of the present findings, an accurate evaluation of early postprandial glucose control should also be suggested in patients with diabetes remission after surgery, in order to more incisively modify the lifestyle and undertake appropriate pharmaceutical therapy by virtue of a high vascular risk.
Overall, our findings support the need for further studies aimed at assessing the impact of GV on inflammatory status and the need for critical redefinition of the condition of diabetes remission.
Funding agencies
The study was supported by a European Medical Information Framework (EMIF) grant (IMI JU GA 115372-2).
Disclosure
The authors declared no conflicts of interest.
Author contributions
LA made substantial contributions to the conception of the study and interpretation of data, and he was involved in drafting the manuscript. DM, DB, SM, CP, MF, and MA carried out the experiments and made substantial contributions to data collection. EF, CB, and ST were involved in revising the article critically for important intellectual content. MN made substantial contributions to the conception and design of the study and data interpretation, and she was involved in writing the manuscript.





