Alterations of Serum Ser312-Phosphorylated Fetuin-A from Exercise-Induced Moderate Body Weight Loss in Individuals with Obesity
Abstract
Objective
Phosphorylated fetuin-A (pFet-A) inhibits insulin action and has been shown to be associated with obesity and insulin resistance. The objective of this cohort study was to assess the effect of incremental body weight loss on alterations in serum pFet-A and indexes of insulin sensitivity.
Methods
A total of 16 men with obesity attained a targeted weight loss of 8% to 10% of their initial body weight by achieving an energy expenditure/deficit of 2,000 to 2,500 kcal/wk. Anthropometric assessments and blood samples were obtained every 4 weeks. Weight loss was calculated and partitioned as 2% to 4%, 4% to 6%, 6% to 8%, and 8% to 10% compared with initial body weight.
Results
Targeted body weight loss of 8% to 10% decreased serum pFet-A, pFet-A:Fet-A ratio, fasting insulin, log(homeostasis model assessment of insulin resistance), quantitative insulin sensitivity check index, adipose insulin resistance, and insulin resistance index significantly. Percent changes in serum pFet-A were associated with percent changes in indexes of insulin sensitivity. Unlike insulin sensitivity indexes, which were altered starting with 6% to 8% weight loss, serum pFet-A levels were significantly decreased by 19.6% starting with 2% to 4% weight loss and decreased by 25.6%, 36.8%, and 42.3% with 4% to 6%, 6% to 8%, and 8% to 10% weight loss, respectively.
Conclusions
This study reports for the first time that the insulin-sensitizing effects of moderate weight loss are associated with a reduction in serum pFet-A levels.
Study Importance
What is already known?
- Moderate weight loss significantly improves insulin sensitivity.
- Serum fetuin-A is decreased with moderate weight loss.
- The phosphorylation status of fetuin-A is critical for its inhibitory effects on insulin action and is associated with obesity and insulin resistance.
What does this study add?
- This is the first study showing that moderate weight loss significantly decreases serum phosphorylated fetuin-A.
- With moderate weight loss, reduction of serum phosphorylated fetuin-A, but not fetuin-A, is associated with the observed improvement of insulin sensitivity.
- Phosphorylated fetuin-A levels are significantly decreased with as little as 2% to 4% of body weight lost, prior to the improvement in markers of insulin sensitivity.
How might these results change the direction of research?
- Metabolic and tissue-specific humoral responses may precede improvement in insulin sensitivity associated with moderate weight loss.
- Measurement of serum Ser312-phosphorylated fetuin-A may be important in understanding its role in insulin-resistant conditions.
Introduction
Obesity is caused by a chronic energy imbalance as a result of increased dietary intake and low physical activity. Worldwide, in 2016, more than 1.9 billion adults were overweight; of these, 650 million had obesity (1). Obesity is associated with an increased risk of type 2 diabetes (T2DM), cardiovascular diseases, and cancer (2, 3). The beneficial effects of moderate weight loss (5%-10%) on hyperglycemia, hyperinsulinemia, hyperlipidemia, and insulin resistance are well documented (4, 5). Several elegant studies, including the Diabetes Prevention Program (DPP), have demonstrated that lifestyle intervention with modest weight loss decreased the incidence of T2DM and delayed the progression from obesity to T2DM (6).
Several mechanisms have been suggested to illustrate the benefits of lifestyle intervention (both calorie restriction and physical activity) in obesity, T2DM, and cardiovascular disease, including improved insulin sensitivity and glucose homeostasis (7), alterations in fatty acid mobilization and oxidation (8), lowered intramyocellular lipid content (9), reduced inflammation (10), and improved oxidative stress (11).
An accumulating body of clinical evidence suggests that high circulating fetuin-A (Fet-A) concentrations in humans are strongly associated with insulin resistance, metabolic syndrome, atherogenic lipid profile, incident diabetes, and an increased risk of myocardial infarction and ischemic stroke (12). Interventions that produce weight loss have been shown to lower serum Fet-A concentrations (13). Gastric bypass surgery that resulted in a 35% body weight loss decreased serum Fet-A, which correlated with fasting insulin, postprandial insulin, and homeostasis model assessment of insulin resistance (HOMA-IR) (14). High-intensity exercise has led to significantly decreased serum Fet-A, which is associated with lower hepatic insulin resistance (15, 16).
Fet-A exists in circulation in both phosphorylated (~20%) and dephosphorylated (~80%) forms (17). Of the two phosphorylation sites on Fet-A, serine312 (Ser312) was shown to be the dominant phosphorylation site, displaying ~77% phosphorylation compared with serine120 (18). We and others have shown that phosphorylation status is critical for its inhibitory effects on insulin-stimulated insulin receptor autophosphorylation, insulin receptor tyrosine kinase activity, glucose uptake, and glycogen synthesis (19-21). Recently, we showed that serum Ser312-phosphorylated Fet-A (pFet-A) levels were elevated in mice with obesity induced by a high-fat diet, in Zucker diabetic fatty rats with insulin resistance, and in individuals with obesity and insulin resistance (21). Currently, there are no reports examining the effects of weight loss on serum pFet-A and their relationship with indexes of insulin sensitivity in individuals with obesity. In this study, we examined alterations of serum pFet-A with incremental (2%-4%, 4%-6%, 6%-8% and 8%-10%) weight loss in individuals with obesity. We hypothesized that moderate weight loss would decrease serum pFet-A levels and that these changes would be associated with improvements of surrogate markers of insulin sensitivity.
Methods
Participant recruitment and study population
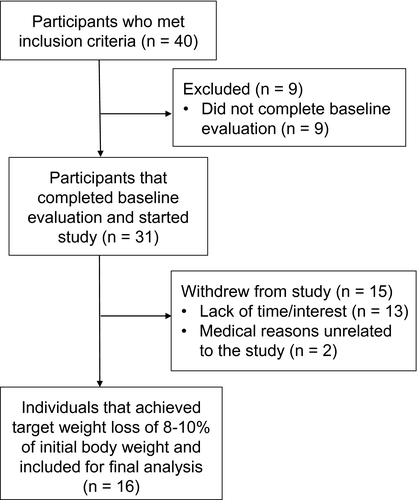
The study population described here was a subset of a larger study, reported previously (21). Briefly, 40 individuals who met the following criteria were recruited from the Auburn/Opelika, Alabama, area: male between 30 and 65 years old; BMI > 30 kg/m2 and/or body fat > 30% of total body weight and waist circumference > 40 inches; no previously diagnosed cardiovascular or metabolic disease; weight stable over the previous 6 months; no signs or symptoms of latent cardiovascular disease; sedentary lifestyle with no regular physical activity during the previous 6-month period (regular physical activity was defined as moderate or intense physical exertion [50%-80% of maximum oxygen consumption VO2max measured over 1 minute of a standard Bruce protocol–graded exercise test] for 20 minutes or more at least two times per week); nonsmoker; no current or previous history of medication known to affect lipid or glucose metabolism; and no physical impairment or condition that would prevent regular treadmill walking. Of these individuals, 31 participants completed baseline procedures (height, weight, waist and hip circumference, body fat, dual-energy x-ray absorptiometry, blood pressure, volumes of oxygen consumption and carbon dioxide production, heart rate, and an oral glucose tolerance test [OGTT] with 75 g of standard carbohydrate syrup), as described earlier, and started the weight loss protocol (21). Only 16 participants (13 Caucasian, 1 Caucasian/American Indian, and 2 of African American descent) achieved the targeted 8% to 10% body weight loss (Figure 1). This study received approval from the Auburn University Institutional Review Board (07-210 MR 0710) and was registered on ClinicalTrials.gov (NCT03478046).
Weight loss procedures
The overall targeted weight loss was 8% to 10% of the initial body weight over a 6- to 10-month period, as recommended by the National Heart, Lung, and Blood Institute (22). The weight loss intervention was designed to achieve an energy expenditure and/or energy deficit of 2,000 to 2,500 kcal/wk. Participants visited the laboratory twice a week on nonconsecutive days for measuring body weight and exercise intervention. Blood sampling and body composition analyses were performed once every 4 weeks. During this visit, participants brought with them their 3-day diet and physical activity record for review and analysis. Based on the information, participants were given diet and exercise recommendations and modifications for the next month. Average weekly body weight was used to monitor weight loss. Weight loss was categorized as 2% to 4%, 4% to 6%, 6% to 8%, and 8% to 10% of initial body weight. Participants were considered to have achieved a body weight milestone when they reached a targeted percentage of weight loss relative to their initial (preintervention) body weight and maintained the weight loss over 2 consecutive weeks. Once participants achieved the targeted weight loss goal of 8% to 10%, a second OGTT was administered.
Exercise intervention
Participants were required to complete two exercise sessions per week in the laboratory under supervision and to exercise two to three times per week on their own in order to achieve their weekly energy expenditure goal. During the laboratory exercise sessions, exercise intensity, blood pressure, and body weight were documented. To achieve the energy expenditure goal of 400 to 500 kcal per session in the laboratory, respiratory gases and heart rates were monitored. All subjects were trained to use a heart rate monitor and a perceived exertion scale for monitoring exercise intensity for the remainder of their exercise sessions.
Serum Fet-A and pFet-A
Serum concentrations of Fet-A were measured using an enzyme-linked immunosorbent assay (ELISA) kit (BioVendor, LLC, Candler, North Carolina). Serum samples were assayed in duplicate, using human Fet-A standards ranging from 2 to 100 ng/mL. Standards, quality controls, and 1:10,000-diluted serum samples were added to anti-human Fet-A antibody–coated microtiter strips. After incubation, washing, and addition of conjugate solution and substrate, the absorbance was read in a microplate reader at 450 nm.
Because an ELISA to analyze pFet-A is currently not available, serum pFet-A levels were quantitated from digitized Western blot images of gels. Serum samples and quality control serum were diluted 1:100 in sterile saline, and proteins were separated on a 4% to 20% gradient sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel (Bio-Rad, Hercules, California). Proteins were transferred to a nitrocellulose membrane and blocked in 5% nonfat dry milk (Bio-Rad). Serum pFet-A was detected using a custom-generated, affinity-purified antibody (Affinity BioReagents, Golden, Colorado) against the pFet-A epitope “HTFMGVVSLGSPS(PO4)GEVSHPR” (21). Chemiluminescence was imaged with a UVP BioImaging system (UVP, Upland, California). Area densities of the bands were analyzed using the UnScan-It software package (version 6.1; Silk Scientific, Orem, Utah). To account for gel to gel variations and to assure a standardized quantitation of the samples, serum pFet-A band densities were quantitated by comparing band pixels of individual samples to a “normal body weight quality control” serum sample, which was loaded in duplicate on every gel, assuring an objective quantitation of serum levels of pFet-A. Fold changes were used for statistical analysis.
Biochemical analysis, insulin resistance, and glucose tolerance
Serum glucose was measured using a glucose hexokinase assay kit (Cliniqa Corporation, San Marcos, California). Serum insulin concentrations were measured using a human insulin-specific radioimmunoassay (Millipore Corporation, Billerica, Massachusetts). Serum nonesterified fatty acid (NEFA) concentrations were assayed using a 96-well NEFA assay kit (Wako, Richmond, Virginia). Blood chemistries, lipids, and liver enzymes were assayed by a Centers for Disease Control and Prevention–certified laboratory. HOMA-IR, which reflects hepatic insulin resistance, was calculated using the following formula: fasting insulin (microunits per milliliter) × fasting glucose (micromoles per liter) / 22.5 (23). Adipose insulin resistance (adipose-IR) was calculated as follows: fasting NEFA (microequivalents per liter) × fasting insulin (microunits per milliliter) (24). The quantitative insulin sensitivity check index (QUICKI), validated to assess insulin sensitivity, was calculated as follows: 1 / (log[fasting insulin (microunits per milliliter)] + log[fasting glucose (milligrams per deciliter)]) (25). The glucose to insulin ratio (GIR) was calculated as glucose (milligrams per deciliter) / insulin (microunits per milliliter). The area under the curve (AUC) for glucose and insulin during the OGTT was calculated using the trapezoidal method. Insulin resistance index, which primarily reflects skeletal muscle glucose uptake, was estimated by multiplying the insulinAUC and glucoseAUC and dividing by 106 (26).
Statistical analysis
A paired Student t test was used to determine statistical differences before and after weight loss in people with obesity. Temporal changes with weight loss were analyzed using repeated-measures one-way ANOVA. The Duncan New Multiple Range test was employed to describe significant differences determined from ANOVA. Because earlier studies indicate that weight loss in the 5% to 10% category contributes to health benefits, including reduced blood pressure, indexes of glycemia, and levels of triglycerides and increased levels of high-density lipoprotein cholesterol (27), we examined the temporal response by capturing weight loss at each month and partitioning this into percentages as follows: 2% to 4%, 4% to 6%, 6% to 8%, and 8% to 10% weight loss. Categorical weight loss was employed to allow for incremental comparison based primarily on weight loss. Individual participants were expected to have a variety of physical and physiological adjustments to exercise. In addition, participants presented with a range of fitness levels and physical abilities. Because of these factors, not everyone was expected to achieve the same rate of weight loss. Therefore, milestones were established to make comparisons based on the amount of weight loss achieved rather than based on the time in the intervention. The lowest monthly weight achieved and the corresponding variables from dual-energy x-ray absorptiometry scans and fasting blood samples that fell within each range were used for statistical analyses. Pearson product moment correlation coefficients were used to examine associations, and significance was accepted as P < 0.05. Data are expressed as mean ± SD, unless noted otherwise.
Results
Anthropometric, physiological, and metabolic characteristics before and after 8% to 10% weight loss
The mean age of recruited participants was 43.3 ± 9.0 years with BMI of 33.3 ± 4.4 kg/m2. Of the 16 participants, 12 of them met the National Cholesterol Education Program Adult Treatment Panel III criteria for metabolic syndrome. Participants lost an average of 9.3 kg (ranging from 6.8 kg to 12.7 kg) or 8.8% (ranging from 7.6% to 10.9%) of their initial body weight. With 8% to 10% weight loss, participants had significantly lower BMI (30.4 ± 4.2 kg/m2; P < 0.01). Most of the participants achieved their target weight loss goal within 6 to 10 months. The anthropometric, physiological, and metabolic characteristics before and after target weight loss are shown in Table 1. As expected, body weight loss of 8% to 10% significantly decreased waist circumference (P < 0.001), percent total fat (P < 0.001), fat mass (P < 0.001), lean mass (P = 0.008), percent android fat (P < 0.001), percent gynoid fat (P < 0.001), systolic blood pressure (SBP) (P < 0.001), and diastolic blood pressure (DBP) (P = 0.002) and significantly increased VO2max (P < 0.001) (Table 1).
| Pre-WL (n = 16) | Post-WL (n = 16) | P value | |
|---|---|---|---|
| Weight (kg) | 106.3 ± 17.7 | 97.0 ± 16.5 | < 0.001 |
| Waist circumference (cm) | 112.5 ± 12.2 | 104.1 ± 12.2 | < 0.001 |
| Total fat % | 35.9 ± 4.3 | 32.4 ± 5.7 | < 0.001 |
| Fat mass (kg) | 37.5 ± 9.3 | 31.4 ± 10.0 | < 0.001 |
| Lean mass (kg) | 66.1 ± 10.0 | 64.2 ± 10.2 | 0.008 |
| Android fat % | 47.8 ± 4.6 | 43.5 ± 5.6 | < 0.001 |
| Gynoid fat % | 37.4 ± 5.5 | 34.2 ± 5.8 | < 0.001 |
| SBP (mm Hg) | 131 ± 10 | 121 ± 11 | < 0.001 |
| DBP (mm Hg) | 83 ± 8 | 75 ± 7 | 0.002 |
| VO2max (mL/kg/min) | 30.3 ± 4.4 | 35.7 ± 5.8 | < 0.001 |
| VO2max absolute (L/min) | 3.18 ± 0.43 | 3.49 ± 0.47 | 0.031 |
| Fasting glucose (mg/dL) | 107 ± 17 | 109 ± 15 | 0.640 |
| Fasting insulin (μU/mL) | 27.9 ± 11.0 | 20.3 ± 6.9 | 0.001 |
| Fasting Fet-A (μg/mL) | 374.3 ± 70.9 | 306.4 ± 41.3 | 0.002 |
| Fasting pFet-A (scan units) | 2.31 ± 1.20 | 1.22 ± 0.64 | < 0.001 |
| Fasting pFet-A: Fet-A ratio | 0.59 ± 0.35 | 0.42 ± 0.27 | 0.004 |
| Fasting NEFA (mEq/L) | 0.72 ± 0.23 | 0.76 ± 0.23 | 0.696 |
| 2-h glucose (mg/dL) | 118 ± 41 | 103 ± 39 | 0.048 |
| 2-h insulin (μU/mL) | 90.0 ± 47.9 | 60.0 ± 37.4 | 0.001 |
| 2-h Fet-A (μg/mL) | 322.8 ± 58.4 | 292.1 ± 50.3 | 0.043 |
| 2-h pFet-A (scan units) | 2.85 ± 1.48 | 2.16 ± 1.95 | 0.067 |
| GlucoseAUC | 18,260 ± 4,821 | 16,916 ± 3,791 | 0.095 |
| InsulinAUC | 15,632 ± 4,999 | 10,637 ± 4,856 | < 0.001 |
| IR index | 292.1 ± 128.5 | 182.9 ± 102.1 | < 0.001 |
| Adipose-IR | 19.9 ± 9.2 | 15.1 ± 5.6 | 0.025 |
| HOMA-IR | 7.6 ± 3.7 | 5.5 ± 2.2 | 0.005 |
| log(HOMA-IR) | 0.83 ± 0.22 | 0.72 ± 0.15 | 0.017 |
| QUICKI | 0.29 ± 0.02 | 0.30 ± 0.01 | 0.031 |
| G:I ratio | 4.3 ± 1.5 | 5.9 ± 1.8 | 0.001 |
- Data are expressed as mean ± SD.
- 2-h, 2-hour time point during an oral glucose tolerance test; adipose-IR, adipose insulin resistance; AUC, area under the curve; DBP, diastolic blood pressure; Fet-A, fetuin-A; G:I ratio, glucose to insulin ratio; HOMA-IR, homeostasis model assessment of insulin resistance; IR index, insulin resistance index; NEFA, nonesterified fatty acid; pFet-A, Ser312 phosphorylated fetuin-A; QUICKI, quantitative insulin sensitivity check index; SBP, systolic blood pressure; VO2max, maximum oxygen consumption measured over 1 minute of a standard Bruce protocol–graded exercise test; WL, weight loss.
Alterations in serum insulin, glucose, and indexes of insulin sensitivity with 8% to 10% body weight loss
Fasting serum insulin, HOMA-IR, log(HOMA-IR), adipose-IR, QUICKI, GIR, insulinAUC, insulin resistance index, 2-hour glucose, and 2-hour insulin significantly improved with 8% to 10% body weight loss (Table 1). On the other hand, fasting glucose and NEFA concentrations were unaltered with 8% to 10% weight loss (P > 0.05).
Alterations in Fet-A and pFet-A with 8% to 10% body weight loss
Serum Fet-A, pFet-A, and pFet-A:Fet-A ratio were significantly decreased with target weight loss of 8% to 10% (Table 1; Supporting Information Figure S1). Serum Fet-A concentrations ranged from 262.6 to 535.6 μg/mL in individuals with obesity before weight loss and from 221.9 to 377.2 μg/mL after 8% to 10% weight loss. Serum Fet-A concentrations were decreased by 24.0% and pFet-A concentrations were decreased by 42.3% with 8% to 10% weight loss. In individuals with obesity, serum Fet-A was correlated with fasting NEFA (r = 0.6, P = 0.01, n = 16), and serum pFet-A was correlated with adipose-IR (r = 0.56, P = 0.02, n = 16). Following weight loss, serum pFet-A was correlated with fasting insulin (r = 0.56, P = 0.02, n = 16), QUICKI (r = −0.58, P = 0.02, n = 16), and GIR (r =−0.54, P = 0.03, n = 16) (Supporting Information Table S1).
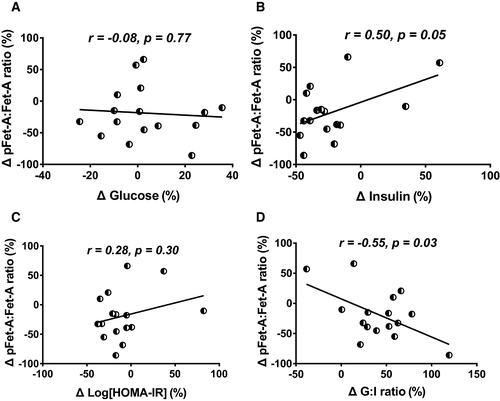
Association of percent change of Fet-A and pFet-A with indexes of insulin sensitivity
Following 8% to 10% body weight loss, the percent change of pFet-A was correlated with the percent change of insulin, log(HOMA-IR), and GIR, but not with glucose (Supporting Information Figure S2E-S2H). Further, with 8% to 10% body weight loss, the changes in the ratio of pFet-A to Fet-A were correlated with percent changes in serum insulin and GIR (Figure 2). However, the percent change of Fet-A was not correlated with the percent change of glucose, insulin, log(HOMA-IR), or GIR (Supporting Information Figure S2A-S2D). Additionally, the percent change of Fet-A or pFet-A was not associated with the percent change in body weight, waist circumference, total fat, fat mass, lean mass, or blood pressure (data not shown).
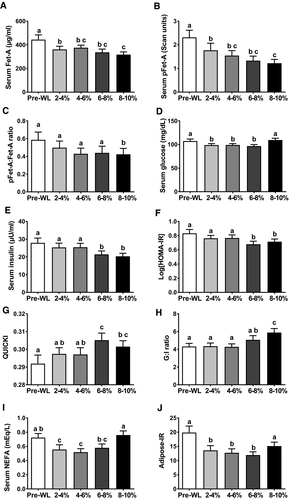
Alterations of Fet-A, pFet-A, and indexes of insulin sensitivity with incremental weight loss
We observed a temporal decrease in waist circumference, percent total fat, fat mass, android fat, gynoid fat, and SBP and DBP starting with 2% to 4% weight loss and continuing through 4% to 6%, 6% to 8%, and 8% to 10% weight loss (Table 2). Similarly, Fet-A, pFet-A, and log(HOMA-IR) demonstrated temporal reductions with 2% to 4%, 4% to 6%, 6% to 8%, and 8% to 10% weight loss (Figure 3A, 3B, 3F; Supporting Information Figure S1). Serum Fet-A concentrations were decreased significantly by 15.1%, 10.3%, 19.7%, and 24% with 2% to 4%, 4% to 6%, 6% to 8%, and 8% to 10% body weight loss compared with before weight loss, respectively. In comparison, pFet-A levels were decreased significantly by 19.6%, 25.6%, 36.8%, and 42.3% with 2% to 4%, 4% to 6%, 6% to 8%, and 8% to 10% body weight loss compared with before weight loss, respectively. Although we observed a trend in the reduction of pFet-A:Fet-A ratio with 2% to 4%, 4% to 6%, and 8% to 10%, this was significant only with 8% to 10% weight loss (Figure 3C). Further, log(HOMA-IR) decreased 1.7%, 4.1%, 16.5%, and 8.6% compared with before weight loss with 2% to 4%, 4% to 6%, 6% to 8%, and 8% to 10% body weight loss, respectively. On the other hand, we observed a significant decrease in serum insulin only with 6% to 8% and 8% to 10% weight loss (Figure 3E). QUICKI and GIR showed significant improvement only with 6% to 8% and 8% to 10% body weight loss, respectively (Figure 3G-3H). Serum glucose, NEFA, and adipose-IR showed temporal decreases at 2% to 4%, 4% to 6%, and 6% to 8% body weight loss (Figure 3D, 3I, 3J). However, this was not sustained with 8% to 10% weight loss.
| Pre-WL | 2%-4% | 4%-6% | 6%-8% | 8%-10% | |
|---|---|---|---|---|---|
| Weight (kg) | 106.3 ± 17.7a | 103.3 ± 17.2b | 100.8 ± 16.9c | 98.8 ± 16.8d | 97.0 ± 16.5e |
| Waist circumference (cm) | 112.5 ± 12.2a | 108.0 ± 11.7b | 104.4 ± 12.2c | 103.4 ± 11.7c | 104.1 ± 12.2c |
| Total fat % | 35.9 ± 4.3a | 34.7 ± 5.0b | 33.5 ± 5.2c | 32.5 ± 5.5d | 32.4 ± 5.7d |
| Fat mass (kg) | 37.5 ± 9.3a | 35.4 ± 10.2b | 34.0 ± 10.0c | 31.8 ± 10.1d | 31.4 ± 10.0d |
| Lean mass (kg) | 66.1 ± 9.9a,b | 65.4 ± 10.0b,c | 66.4 ± 9.1a | 64.8 ± 10.4c,d | 64.2 ± 10.2d |
| Android fat % | 47.8 ± 4.6a | 46.5 ± 5.3b | 44.7 ± 5.8c | 43.6 ± 6.0d | 43.5 ± 5.6d |
| Gynoid fat % | 37.4 ± 5.5a | 36.3 ± 5.7b | 35.2 ± 5.5c | 34.5 ± 5.0c,d | 34.2 ± 5.8d |
| SBP (mm Hg) | 131 ± 10a | 121 ± 8b | 122 ± 11b | 118 ± 8b | 121 ± 11b |
| DBP (mm Hg) | 83 ± 8a | 77 ± 5b | 77 ± 7a,b | 76 ± 6b | 75 ± 7b |
- Data are expressed as mean ±SD. Different letters in superscript following values indicate statistical significance, P < 0.05.
- DBP, diastolic blood pressure; SBP, systolic blood pressure; WL, weight loss.
Discussion
The benefits of moderate weight loss (5% to 10%) have been well established, with 5% of body weight loss reported to improve the insulin sensitivity of several organs, improve β-cell function, and decrease cardiometabolic risks (4, 28). However, the mechanisms mediating such beneficial changes are unclear. Evidence suggests that such changes are independent of changes in systemic or subcutaneous adipose tissue markers of inflammation (4).
In the present study, we report novel findings regarding pFet-A and markers of insulin sensitivity with moderate weight loss in individuals with obesity, and we demonstrate that 8% to 10% weight loss induced significant reductions in serum pFet-A and pFet-A:Fet-A ratio. The DPP Research Group demonstrated that weight loss and physical activity lowered the incidence of T2DM in individuals with impaired glucose tolerance (29). In the current study, there was a remarkable decrease in pFet-A (42.3%) with 8% to 10% weight loss. In comparison, fasting insulin concentrations were decreased by 21.4%, log(HOMA-IR) was lower by 8.6%, and adipose-IR was lower by 14%. The improvement of insulin sensitivity after moderate weight loss was confirmed through a decrease in insulinAUC (30.3%) and insulin resistance index (33.8%). This suggested that the moderate weight loss observed in our study resulted in the improvement of hepatic insulin sensitivity (30), adipose insulin sensitivity (31), and skeletal muscle insulin sensitivity (9).
These findings are consistent with previous findings demonstrating improved insulin sensitivity with exercise and calorie restriction in adults (32) and adolescents (33). Several studies have also shown that exercise alone (34), hypocaloric diets (35), gastric banding or bypass surgery (36), and medication (37) exert beneficial effects and alter insulin sensitivity. Among older adults, a 12-week supervised exercise training was shown to lower serum Fet-A by 8%, which was correlated with hepatic insulin resistance and high-molecular-weight adiponectin (15). Further, a 16-month longitudinal study of 75 patients with morbid obesity after gastric bypass demonstrated that changes of serum Fet-A were significantly correlated with changes in fasting insulin, 2-hour insulin, and HOMA-IR (14). We demonstrate that, with 8% to 10% weight loss, serum pFet-A was decreased (42.3%) considerably more than Fet-A (24.0%) in our study subjects. Importantly, our findings show that percent changes in pFet-A with 8% to 10% weight loss, but not Fet-A, were associated with the observed improvement of insulin sensitivity. This is consistent with findings that pFet-A but not the dephosphorylated form of Fet-A inhibited glucose transporter type 4 translocation, glucose uptake, and glycogen synthesis in skeletal muscle cells (21).
Our study shows that with as little as 2% to 4% weight loss, serum NEFA and adipose-IR were decreased. Similarly, Fet-A and pFet-A levels were significantly decreased with 2% to 4% weight loss and they continued to decrease with incremental weight loss. Further, we observed significant associations between serum Fet-A and pFet-A with NEFA after 2% to 4% weight loss. This is consistent with the findings of Lee et al., who showed that serum Fet-A and free fatty acids were lowered following 12 weeks of combined endurance and strength exercise (38). Previous studies have shown that in subjects with elevated free fatty acids, serum Fet-A was associated with insulin resistance (39). In addition, Kahraman et al. reported that NEFA upregulated Fet-A’s mRNA expression in human primary hepatocytes (40). Fet-A was identified as an adapter protein linking free fatty acids to toll-like receptor 4, resulting in inflammation and insulin resistance (41).
Our study demonstrates that serum insulin significantly decreased during 6% to 8% weight loss, QUICKI significantly increased during 6% to 8% weight loss, and GIR increased in 8% to 10% weight loss, indicating insulin sensitivity enhancement from 6% to 8% weight loss. Our findings are fairly consistent with those of the DPP study, which showed that 7% weight loss can significantly improve insulin sensitivity and further decrease the risks of T2DM (29). Accordingly, numerous other clinical studies have documented that slight weight loss, as little as 4% to 6%, begins to improve insulin sensitivity and decrease insulin-resistant clinical markers (11, 28, 42-45). A major finding from our study is that both Fet-A and pFet-A levels decreased with as little as 2% to 4% weight loss, prior to an improvement of markers of insulin sensitivity, tempting us to speculate that alterations of this hepatokine may “predict” improvements in insulin sensitivity with modest weight loss. Concomitantly with this decrease, we observed a significant decrease in waist circumference, percent body fat, and SBP and DBP with 2% to 4% weight loss. These findings suggest that a 2% to 4% weight loss may set into motion the therapeutic benefits of improvement of metabolic function.
There are several limitations to this study. Although we recruited 31 individuals with obesity into this study, only 16 participants achieved the targeted 8% to 10% weight loss. Participants achieved the targeted weight loss over a period of 6 to 10 months. The duration of the study and the required energy expenditure and/or energy deficit may have contributed to the increased attrition rate. The small number of participants is a limitation of this study. Likewise, because the sample size was low, we were unable to adjust for the time it took participants to achieve the targeted weight loss. Currently, we are limited in our understanding of potential structural and/or biological differences in adipose- versus liver-secreted serum Fet-A or pFet-A. If such differences exist, then tissue-specific antibodies will be required to identify the origin of circulating Fet-A and pFet-A. Additionally, this study was conducted only in men and therefore may not be generalized to the wider population. Furthermore, our study is limited to examining changes in pFet-A with incremental weight loss and with changes in indexes of insulin resistance/sensitivity. Finally, approximately 20% of our study population did not show a decrease in Fet-A, pFet-A, or pFet-A:Fet-A ratio. Whether this can be attributed to an erosion of the impact of weight loss after a certain period of time, potential dietary influences, or other factors is speculation. It is possible that a larger study may contribute to a better understanding of Fet-A and pFet-A in weight loss and of mechanisms leading to improvement in insulin sensitivity.
Conclusion
In conclusion, we present novel findings that moderate body weight loss of 8% to 10% significantly decreased serum pFet-A, which was associated with improvement of insulin sensitivity indexes. Additionally, serum pFet-A levels were significantly decreased with as little as 2% to 4% weight loss, which is suggestive of metabolic and tissue-specific humoral responses that may precede the improvement in insulin sensitivity associated with moderate weight loss. Furthermore, these studies suggest that measurement of serum pFet-A may be important in understanding its role in insulin-resistant conditions.
Acknowledgments
The authors would like to thank the volunteers for their time and commitment to our study. The excellent technical assistance of Jennifer Dennis is gratefully acknowledged. All data relevant to the study are included in the article or uploaded as Supporting Information.
Clinical trial registration
ClinicalTrials.gov identifier NCT03478046.
Funding agencies
This work was supported by the American Diabetes Association (ADA 7-04-JF-36), the Alabama Agricultural Experiment Station (ALA080-052), and the Malone Zallen Graduate Research Fellowship (MZRF10-01).
Disclosure
The authors declared no conflicts of interest.
Author contributions
PWJ and STM designed the study. GR, RLB, TK, FA-R, AJM, and DMD conducted the study. GR and STM drafted the manuscript, and GR, PWG, and STM analyzed the data, helped with interpretation of data, edited the manuscript, and had primary responsibility for final content. All authors read and approved the final manuscript.





