The Association Between the Dosage of SGLT2 Inhibitor and Weight Reduction in Type 2 Diabetes Patients: A Meta-Analysis
Funding agencies: This study was supported by AstraZeneca Ltd. (China). The funding agencies had no role in the study design, data collection or analysis, decision to publish, or preparation of the manuscript.
Disclosure: LNJ has received fees for consultations and meeting presentations from AstraZeneca, Sanofi-Aventis, Eli Lilly, Novartis, Merck, Roche, Abbott, and Takeda. The other authors declared no conflict of interest.
Author contributions: Design of this meta-analysis: LNJ and XLC; study selection and data extraction: WJY, XYG, and YFC; statistical analyses: XLC and XYH; manuscript writing: XLC, LLZ, SMZ, and LNJ. All authors contributed to the manuscript drafts and gave final approval for this manuscript.
Abstract
Objective
Sodium glucose cotransporter 2 (SGLT2) inhibitors may induce urinary glucose excretion via the inhibition of renal glucose reabsorption, improve glycemic control, and lower body weight. The aim of this meta-analysis was to evaluate weight changes in patients who received different dosages of SGLT2 inhibitors.
Methods
Overall, 55 placebo-controlled trials were included.
Results
The results indicated that treatment with 2.5 mg, 5 mg, 10 mg, and 20 mg of dapagliflozin led to significant decreases in body weight compared with a placebo (weighted mean difference [WMD], −1.30 kg, −1.51 kg, −1.79 kg, −2.24 kg, respectively; P < 0.001). Treatment with 50 mg, 100 mg, 200 mg, and 300 mg of canagliflozin also led to significant decreases in weight (WMD, −1.20 kg, −1.82 kg, −1.83 kg, −2.37 kg, respectively; P < 0.001). In the treatment with empagliflozin, ipragliflozin, tofogliflozin, and luseogliflozin, body weight also significantly decreased. The decrease in weight was associated with the dosage of dapagliflozin (P < 0.05).
Conclusions
Body weight significantly decreased in patients with type 2 diabetes who received different dosages of SGLT2 inhibitors compared with patients who received a placebo. Moreover, in patients treated with dapagliflozin, there was a statistically significant dosage-dependent trend in body weight reduction.
Introduction
Type 2 diabetes is a progressive disease characterized by declining β-cell function and insulin resistance. Weight control is an important part of type 2 diabetes management that helps to lower insulin resistance and contributes to improvements in glycemic control. Therefore, weight loss is recommended for patients with type 2 diabetes (1, 2). Moderate weight loss (5%-10%) can improve glycemic control and other cardiovascular risk factors and comorbidities (3, 4). Despite the number of antidiabetes medications currently available, achieving an optimal decrease in weight and maintaining glycemic control in patients with type 2 diabetes are difficult. Although metformin and glucagon-like peptide-1 (GLP-1) analogues provide weight reduction, most oral agents lead to weight gain, such as sulfonylureas and thiazolidinediones, or are weight neutral, such as dipeptidyl peptidase-4 (DPP-4) inhibitors, and do not meaningfully reduce body weight (1, 2).
An emerging class of antidiabetes agents, known as sodium glucose cotransporter 2 (SGLT2) inhibitors, induces urinary glucose excretion via the inhibition of renal glucose reabsorption, improves glycemic control, and lowers body weight. Recent studies of weight decrease with SGLT2 inhibitor treatment have indicated decreases of approximately 1 to 4 kg in different trials (5-9); however, the dosages and types of SGLT2 inhibitors vary among trials. Is the weight decrease associated with SGLT2 inhibitor treatment related to dosage? Previous reviews could not comprehensively answer this question. Therefore, the aim of this study is to evaluate, via a meta-analysis, the weight change for different dosages of SGLT2 inhibitors and the associated parameters.
Methods
Strategy for searching
The MEDLINE (PubMed), Embase, and Cochrane Central Register of Controlled Trials databases that covered recorded research until January 2016 were searched and then re-searched in January 2017. The following terms were used for searching and re-searching: type 2 diabetes, sodium-glucose cotransporter 2 inhibitors, dapagliflozin, canagliflozin, empagliflozin, ipragliflozin, tofogliflozin, luseogliflozin, and randomized placebo-controlled trials. The PubMed search strategy formed the basis of the strategies developed for the other electronic databases. The registration number for this meta-analysis is CRD42016033041.
Study selection and data extraction
The inclusion criteria for this meta-analysis were as follows: (1) randomized placebo-controlled trials of SGLT2 inhibitor treatment in type 2 diabetes participants as a monotherapy or add-on therapy, (2) a study length of greater than 12 weeks, (3) the change in weight from baseline was assessed in comparative groups, and (4) the doses of SGLT2 inhibitors were reported in the trial. The exclusion criteria were as follows: (1) active controlled studies, (2) nonrandomized trials, (3) trials in patients with type 1 diabetes, and (4) a study length of less than 12 weeks. The studies that reported the efficacy of nonapproved doses of each SGLT2 inhibitor were also included in this meta-analysis.
According to the above inclusion criteria, WJY and XYG evaluated the eligibility of all the studies independently. If there was a disagreement, YFC would resolve it by consultation. The quality of each study and the risk of bias were evaluated by using the Cochrane risk of bias tool (10). Then WJY and YFC extracted the details from each manuscript, including the publication data, study design, baseline characteristics such as age and BMI, study drugs and dosages, duration of the study, and changes in weight from baseline to the study end point. If there was a disagreement, XYG would resolve it by discussion.
Statistical analysis
The body weight changes from baseline to the study end point in patients who received treatment with various dosages of SGLT2 inhibitors or a placebo were evaluated by computing the weighted mean difference (WMD). The 95% CI was also calculated. Because of between-study heterogeneity, Higgins I2 statistics were used to evaluate the percentage of variance. A high level of heterogeneity was defined as an I2 > 50% in the statistical analysis; a low level of heterogeneity was defined as an I2 ≤ 50%. Both a fixed-effects model and a random-effects model were used for the analysis. Publication bias was assessed via a funnel plot and Egger's test.
In the subgroup analyses, studies were divided according to ethnicity (Asian and non-Asian) and BMI (BMI ≥ 30 kg/m2 or BMI < 30 kg/m2). In each subgroup, the body weight changes from baseline in patients treated with each SGLT2 inhibitor compared with patients treated with a placebo were calculated by WMD and 95% CI. We also performed several sensitivity analyses: short study duration or long study duration, even including or excluding the extension studies; studies in different ethnicities; and studies with different baseline BMIs, including or excluding studies with the highest or lowest baseline BMI.
A meta-regression analysis was performed to evaluate whether the prespecified covariates of baseline age, gender, baseline hemoglobin A1c (HbA1c), diabetes duration, and baseline BMI were associated with weight changes from baseline corrected by placebo for each SGLT2 inhibitor. Statistical significance was considered for P < 0.05.
The statistical analyses were primarily performed with the Review Manager statistical software package (version 5.2; The Cochrane Collaboration, London, United Kingdom). This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines for conducting and reporting meta-analyses of randomized controlled trials (RCTs) (11) (Supporting Information Table S1). The Egger's test for publication bias, the whole-body weight changes in patients treated with SGLT2 inhibitors stratified by dosage, and the meta-regression analyses were performed with the Stata statistical software package (version 11.0; StataCorp LLC, College Station, Texas).
Results
Summaries of included studies
Figure 1 summarizes the flowchart of the study selection process. A total of 487 articles were searched, and 128 full articles were read in detail. Fifty-five RCTs were considered appropriate for inclusion, including 21 studies that compared dapagliflozin with a placebo as monotherapy or add-on therapy (5-9, 12-27), 10 studies that compared canagliflozin with a placebo (28-37), 12 studies of empagliflozin compared with a placebo (38-49), five studies of ipragliflozin (50-54), two studies of tofogliflozin (55, 56), and five studies of luseogliflozin (57-61). The details are shown in Supporting Information Table S2. The range of age of the patients who received treatment with SGLT2 inhibitors was from 51.0 to 69.5 years old, with the male percentage ranging from 37.0% to 81.8%. All the baseline characteristics in this meta-analysis are shown in Supporting Information Table S3. The analyses were based on data from 4,816 individuals who received dapagliflozin, 5,628 patients who received canagliflozin, 5,448 individuals who received empagliflozin, 866 patients who received ipragliflozin, 370 individuals who received tofogliflozin treatment, and 558 individuals who received luseogliflozin.
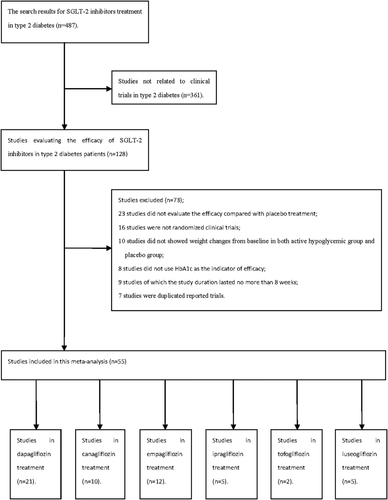
Flowchart of included studies.
Methodological quality
All of the studies included treatment with an active hypoglycemic agent compared with a placebo in a double-blind model. The eligibility criteria were clearly reported in all of the trials. The risk of bias was evaluated with the Cochrane instrument. Overall, the risk of bias was low; random sequence generation, allocation concealment, the blinding of participants and personnel, the blinding of outcome assessments, and incomplete outcome data were all well reported, and selective reporting was low (Supporting Information Figure S1). Funnel plot results indicated that there was an even distribution of the mean values for the parameters that were studied (data not shown).
Weight changes by SGLT2 inhibitor treatment stratified by dosage
When dapagliflozin was compared with a placebo, dapagliflozin 2.5 mg/d resulted in a significantly greater change in weight (WMD, −1.30 kg; P < 0.001); dapagliflozin 5 mg, 10 mg, and 20 mg also led to significantly greater changes in weight (WMD, −1.51 kg, −1.79 kg, and−2.24 kg, respectively) (Figure 2). Compared with a placebo, treatment with canagliflozin 50 mg led to a significant decrease in weight (WMD, −1.20 kg; P < 0.001), and treatment with canagliflozin 100 mg, 200 mg, and 300 mg also led to significant decreases in weight (WMD, −1.82 kg, −1.83 kg, and −2.37 kg, respectively) (Figure 3). Compared with a placebo, treatment with empagliflozin 10 and 25 mg/d was associated with significant decreases in weight (WMD, −1.84 kg and −1.93 kg, respectively; both P < 0.001) (Figure 4). Compared with a placebo, treatment with ipragliflozin 12.5 mg/d led to a significant decrease in weight (WMD, −0.44 kg; P < 0.001), and ipragliflozin 50 mg, 150 mg, and 300 mg/d also led to significant decreases in weight (WMD, −1.40 kg, −1.49 kg, and −1.73 kg, respectively) (Figure 5). Compared with a placebo, treatment with tofogliflozin 10 mg, 20 mg, and 40 mg/d was associated with significant decreases in weight (WMD, −1.68 kg, −2.15 kg, and −2.35 kg, respectively; all P < 0.001) (Figure 6). Compared with a placebo, treatment with luseogliflozin 2.5 and 5 mg/d was associated with significant decreases in weight (WMD, −1.54 kg and −1.92 kg, respectively; both P < 0.001) (Figure 7). Details are shown in Table 1.
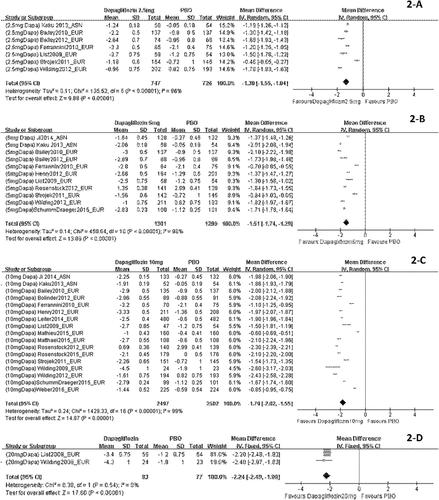
Body weight changes stratified by dosage and corrected by a placebo in patients who received dapagliflozin. The sequence of the study name is <Dosage > <Drug > <Author > <Year > _ < Ethnicity>. EUR represented studies mainly performed in Caucasian individuals, while ASN represented studies mainly performed in Asian individuals. (A) Body weight changes corrected by a placebo in patients who received dapagliflozin 2.5 mg/d. (B) Body weight changes corrected by a placebo in patients who received dapagliflozin 5 mg/d. (C) Body weight changes corrected by a placebo in patients who received dapagliflozin 10 mg/d. (D) Body weight changes corrected by placebo in patients who received dapagliflozin 20 mg/d.
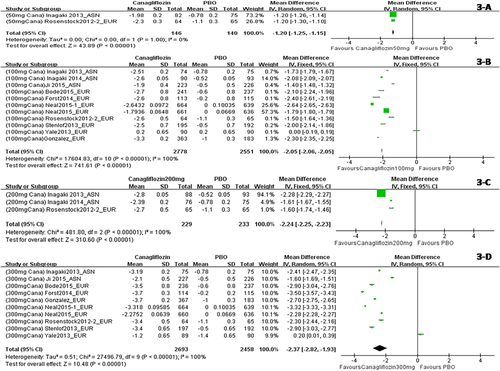
Body weight changes corrected by a placebo in patients who received canagliflozin stratified by dosage. The sequence of the study name is <Dosage > <Drug > <Author > <Year > _ < Ethnicity>. EUR represented studies mainly performed in Caucasian individuals, while ASN represented studies mainly performed in Asian individuals. (A) Body weight changes corrected by a placebo in patients who received canagliflozin 50 mg/d. (B) Body weight changes corrected by a placebo in patients who received canagliflozin 100 mg/d. (C) Body weight changes corrected by a placebo in patients who received canagliflozin 200 mg/d. (D) Body weight changes corrected by a placebo in patients who received canagliflozin 300 mg/d. [Color figure can be viewed at wileyonlinelibrary.com]

Body weight changes corrected by a placebo in patients who received empagliflozin stratified by dosage. The sequence of the study name is <Dosage > <Drug > <Author > <Year > _ < Ethnicity>. EUR represented studies mainly performed in Caucasian individuals, while ASN represented studies mainly performed in Asian individuals. (A) Body weight changes corrected by a placebo in patients who received empagliflozin 10 mg/d. (B) Body weight changes corrected by a placebo in patients who received empagliflozin 25 mg/d. [Color figure can be viewed at wileyonlinelibrary.com]
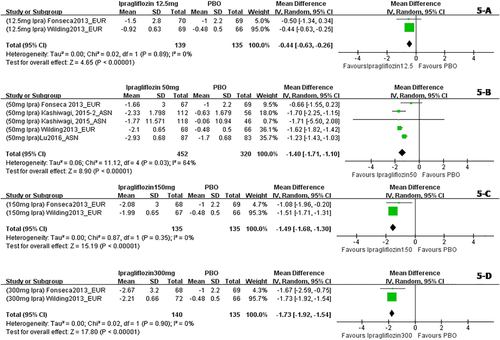
Body weight changes corrected by a placebo in patients who received ipragliflozin stratified by dosage. The sequence of the study name is <Dosage > <Drug > <Author > <Year > _ < Ethnicity>. EUR represented studies mainly performed in Caucasian individuals, while ASN represented studies mainly performed in Asian individuals. (A) Body weight changes corrected by a placebo in patients who received ipragliflozin 12.5 mg/d. (B) Body weight changes corrected by a placebo in patients who received ipragliflozin 50 mg/d. (C) Body weight changes corrected by a placebo in patients who received ipragliflozin 150mg/d. (D) Body weight changes corrected by a placebo in patients who received ipragliflozin 300 mg/d. [Color figure can be viewed at wileyonlinelibrary.com]
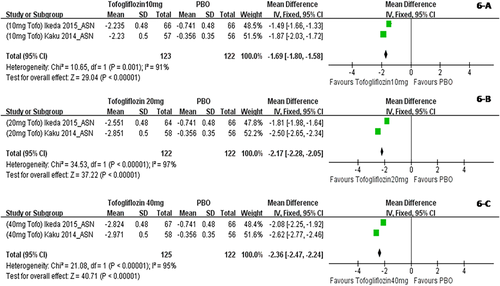
Body weight changes corrected by a placebo in patients who received tofogliflozin stratified by dosage. The sequence of the study name is <Dosage > <Drug > <Author > <Year > _ < Ethnicity>. ASN represented studies mainly performed in Asian individuals. (A) Body weight changes corrected by a placebo in patients who received tofogliflozin 10 mg/d. (B) Body weight changes corrected by a placebo in patients who received tofogliflozin 20 mg/d. (C) Body weight changes corrected by a placebo in patients who received tofogliflozin 40 mg/d. [Color figure can be viewed at wileyonlinelibrary.com]
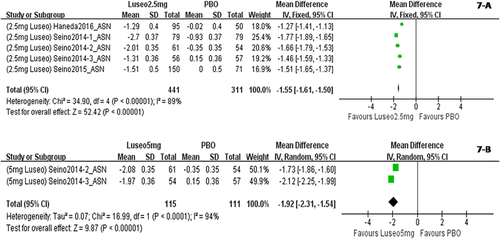
Body weight changes corrected by a placebo in patients who received luseogliflozin stratified by dosage. The sequence of the study name is <Dosage > <Drug > <Author > <Year > _ < Ethnicity>. ASN represented studies mainly performed in Asian individuals. (A) Body weight changes corrected by a placebo in patients who received luseogliflozin 2.5 mg/d. (B) Body weight changes corrected by a placebo in patients who received luseogliflozin 5 mg/d. [Color figure can be viewed at wileyonlinelibrary.com]
| SGLT2 inhibitors (once-daily dosing) | WMD from baseline in weight (kg) | 95% CI (kg) | Asian | Non-Asian | BMI < 30 kg/m2 | BMI ≥ 30 kg/m2 |
|---|---|---|---|---|---|---|
| WMD (95%CI) (kg) | WMD (95%CI) (kg) | WMD (95% CI) (kg) | WMD (95% CI) (kg) | |||
| Dapagliflozin | ||||||
| 2.5 mg | −1.30* | −1.55 to −1.04 | −1.19 (−1.26 to −1.12) | −1.32 (−1.67 to −0.96) | −1.19 (−1.26 to −1.12) | −1.32 (−1.67 to −0.96) |
| 5 mg | −1.51* | −1.74 to −1.29 | −1.69 (−2.32 to −1.06) | −1.47 (−1.73 to −1.21) | −1.69 (−2.32 to −1.06) | −1.47 (−1.73 to −1.21) |
| 10 mg | −1.79* | −2.02 to −1.55 | −1.92 (−2.04 to −1.80) | −1.77 (−2.05 to −1.49) | −1.92 (−2.04 to −1.80) | −1.77 (−2.05 to −1.49) |
| 20 mg | −2.24* | −2.49 to −1.99 | / | −2.24 (−2.49 to −1.99) | / | −2.24 (−2.49 to −1.99) |
| Canagliflozin | ||||||
| 50 mg | −1.20* | −1.25 to −1.15 | / | −1.20 (−1.25 to −1.15) | / | −1.20 (−1.25 to −1.15) |
| 100 mg | −1.82* | −2.10 to −1.53 | −1.74 (−2.14 to −1.34) | −1.85 (−2.26 to −1.44) | −1.74 (−2.14 to −1.34) | −1.85 (−2.26 to −1.44) |
| 200 mg | −1.83* | −2.37 to −1.30 | −1.95 (−2.60 to −1.29) | −1.60 (−1.74 to −1.46) | −1.95 (−2.60 to −1.29) | −1.60 (−1.74 to −1.46) |
| 300 mg | −2.37* | −2.82 to −1.93 | −2.01 (−2.80 to −1.21) | −2.47 (−2.97 to −1.96) | −2.01 (−2.80 to −1.21) | −2.47 (−2.97 to −1.96) |
| Empagliflozin | ||||||
| 10 mg | −1.84* | −1.98 to −1.69 | −2.79 (−2.83 to −2.75) | −1.84 (−1.98 to −1.69) | −1.89 (−2.29 to −1.48) | −2.03 (−2.34 to −1.72) |
| 25 mg | −1.93* | −2.08 to −1.77 | −2.15 (−2.21 to −2.09) | −1.90 (−2.07 to −1.73) | −1.93 (−2.04 to −1.82) | −1.94 (−2.34 to −1.55) |
| Ipragliflozin | ||||||
| 12.5 mg | −0.44* | −0.63 to −0.26 | / | −0.44 (−0.63 to −0.26) | / | −0.44 (−0.63 to −0.26) |
| 50 mg | −1.40* | −1.71 to −1.10 | −1.35 (−1.66 to −1.04) | −1.58 (−1.77 to −1.38) | −1.35 (−1.66 to −1.04) | −1.58 (−1.77 to −1.38) |
| 150 mg | −1.49* | −1.68 to −1.30 | / | −1.49 (−1.68 to −1.30) | / | −1.49 (−1.68 to −1.30) |
| 300 mg | −1.73* | −1.92 to −1.54 | / | −1.73 (−1.92 to −1.54) | / | −1.73 (−1.92 to −1.54) |
| Tofogliflozin | ||||||
| 10 mg | −1.68* | −2.06 to −1.31 | −1.68 (−2.06 to −1.31) | / | −1.87 (−2.03 to −1.72) | −1.49 (−1.66 to −1.30) |
| 20 mg | −2.15* | −2.82 to −1.48 | −2.15 (−2.82 to −1.48) | / | −2.50 (−2.65 to −2.34) | −1.81 (−1.98 to −1.64) |
| 40 mg | −2.35* | −2.87 to −1.83 | −2.35 (−2.87 to −1.83) | / | −2.62 (−2.77 to −2.46) | −2.08 (−2.25 to −1.92) |
| Luseogliflozin | ||||||
| 2.5 mg | −1.54* | −1.71 to −1.36 | −1.54 (−1.71 to −1.36) | / | −1.54 (−1.71 to −1.36) | / |
| 5 mg | −1.92* | −2.31 to −1.54 | −1.92 (−2.31 to −1.54) | / | −1.92 (−2.31 to −1.54) | / |
- *P < 0.01.
In the subgroup analysis, studies were divided on the basis of ethnicity (Asian and non-Asian), and the weight changes in the Asian population for each SGLT2 inhibitor stratified by dosage are presented in Table 1. In another subgroup analysis, studies were divided according to BMI (BMI ≥ 30 kg/m2 or BMI < 30 kg/m2). Subgroup analysis results indicated that body weight changes stratified by dosage in patients with a BMI < 30 kg/m2 were comparable with those in patients with a BMI ≥ 30 kg/m2. The details are also shown in Table 1. Moreover, by using the fixed-effects model and the random-effects model, the results indicated that body weight changes stratified by dosage in the two kinds of analysis were comparable. Details are shown in Supporting Information Table S4.
Associations between the dosage of SGLT2 inhibitors and weight change
Linear trend testing for dosage and weight change for the six types of SGLT2 inhibitors indicated the following: with dapagliflozin, the trend of dosage and weight change was statistically significant (P < 0.05). However, the results for treatment with canagliflozin, empagliflozin, ipragliflozin, tofogliflozin, and luseogliflozin were not statistically significant (Supporting Information Figure S2).
A meta-regression analysis indicated that baseline age, gender, baseline HbA1c, diabetes duration, and baseline BMI were not associated with weight changes from baseline corrected by a placebo in each SGLT2 inhibitor treatment group. The details are also shown in Supporting Information Table S5.
Discussion
Results from this meta-analysis indicated that all four SGLT2 inhibitors resulted in significant decreases in body weight from baseline. In addition, different dosages of each SGLT2 inhibitor were associated with a statistically significant body weight decrease. Weight changes were dose dependent for the dapagliflozin treatment.
The mechanisms by which SGLT2 inhibitors affect weight loss may include the following. First, these mechanisms are likely driven by the loss of calories associated with increased urine glucose excretion (UGE). The SGLT2 inhibitors inhibit renal glucose reabsorption and induce UGE. As previously reported, exposure to dapagliflozin has resulted in a dose-dependent increase in UGE, and a maximal increase in UGE was generally observed at doses greater than 20 mg/d in subjects with type 2 diabetes (62, 63). Moreover, in patients who received canagliflozin, canagliflozin increased UGE dose dependently, and approximately 80 to 120 g/d of UGE was observed with doses of canagliflozin of 100 mg and higher, which might be associated with a caloric loss of 320 to 480 kcal/d (64-66). Second, the weight loss observed with SGLT2 inhibitor treatment might be associated with fat loss. It was reported that approximately two-thirds of the weight loss with canagliflozin treatment was attributed to fat loss (67). In addition, body weight loss with dapagliflozin treatment may be explained by reduced total body fat mass, visceral adipose tissue, and subcutaneous adipose tissue volume (12). Third, it was also reported that the initial weight loss with SGLT2 inhibitors may be attributable to fluid loss associated with mild osmotic diuresis (66); however, weight loss continues even after indicators of volume depletion attenuate.
Although the above factors may contribute to weight changes associated with SGLT2 inhibitor treatment in patients with type 2 diabetes, some of the reasons for weight loss with SGLT2 treatment are not completely understood. For example, assuming no dietary changes, it was reported that canagliflozin treatment led to an average excretion of 80 to 120 g/d of glucose and, therefore, weight loss (35, 65). Based on this, a mathematical model associated with weight loss (68) predicted that a caloric deficit of 320 to 480 kcal/d should provide approximately 5.5 to 7.0 kg of weight loss. However, canagliflozin-associated weight loss was less than the amount predicted based on UGE. Moreover, the possible reason for the SGLT2-induced weight loss that was lower than expected by calorie calculations might be the role of SGLT1 upregulation in renal reabsorption of glucose, which can decrease weight loss but was not inhibited by SGLT2 inhibitors (69, 70). Therefore, the reasons for weight loss associated with SGLT2 inhibitor treatment might be more complicated than we currently believe.
It has been suggested that obesity is associated with diabetes and insulin resistance (1, 2), and weight loss has been demonstrated to be associated with an improvement in glycemic control and cardiovascular risk factors (3, 4); therefore, weight loss is an additional treatment goal for most patients with type 2 diabetes and might improve their adherence to treatment (1, 2). Currently, the management of type 2 diabetes mellitus in terms of weight remains complex and challenging. Treatments with dipeptidyl-peptidase 4 inhibitors or alpha glucosidase inhibitors are weight neutral in patients with type 2 diabetes; treatments associated with weight gain are insulin secretagogues (predominantly sulfonylureas), insulin, and thiazolidinediones. Metformin treatment is associated with a small weight reduction, and GLP-1 agonists were associated with weight loss. SGLT2 inhibitor treatment of type 2 diabetes maintained glucose homeostasis and led to significant weight reduction and appears to be promising based on this meta-analysis. And, in fact, the use of at least empaglifozin and canagliflozin have been shown to have an impact on reducing cardiovascular risk in patients with type 2 diabetes (71, 72).
According to the results of this meta-analysis, body weight decreased more with higher doses of SGLT2 inhibitors, and this result is especially significant with dapagliflozin, for which there is a statistically significant dose-dependent trend. We might hypothesize that with an increase in dosage, there might be more significant weight loss in patients treated with dapagliflozin, and it would benefit patients with type 2 diabetes and obesity. We might also hypothesize that in nondiabetic people with obesity, this kind of drug with higher dosages might be approved as a diet pill. This hypothesis is based on experience with another antidiabetes drug, liraglutide, a GLP-1 analogue with 97% homology to human GLP-1. Liraglutide was first approved for the treatment of type 2 diabetes at a dosage of approximately 0.6 to 1.8 mg once daily (73). Weight loss with liraglutide was found to be dose dependent and resulted in more than a 5% decrease in body weight at dosages up to 3.0 mg once daily in the treatment of people with obesity (74, 75). Based on these findings, liraglutide was approved in the United States and the European Union for chronic weight management at a dose of 3.0 mg. If this postulation of weight loss with dosages of uptitrated dapagliflozin is confirmed at more than a 5% decrease in body weight, perhaps this type of antidiabetes drug can be approved to treat people with obesity without diabetes in the near future. Therefore, more trials with higher doses of dapagliflozin should be carried out to explore the promising weight decrease in people with obesity.
Although dose-dependent weight loss associated with dapagliflozin treatment was uncovered in this study, weight loss as a result of treatment with the other three SGLT2 inhibitors did not show a dose-dependent trend. According to previous RCTs, an ipragliflozin treatment study (50) suggested that dose-dependent decreases in body weight from baseline compared with the placebo at week 12 were observed, but in other studies (51-53), dose-dependent weight loss was not found. In some canagliflozin treatment studies (29, 30), significant dose-related reductions in body weight from baseline were observed with canagliflozin 100 and 300 mg compared with a placebo, while in other studies (33, 35, 36), dose-related weight loss was not shown. In empagliflozin treatment studies (39, 42, 43), decreases in mean body weight did not show clear dose dependency. In dapagliflozin treatment studies, weight loss was reported to be dose dependent (24, 26) and non-dose dependent (5, 8, 15). Some of the results of our meta-analysis are consistent with previous RCT results, but some are different. We suggest that more clinical trials be performed to evaluate higher doses of each SGLT2 inhibitor to determine which SGLT2 treatments are actually dose dependent.
This meta-analysis compared placebo-corrected weight changes from baseline in a large sample of individuals with type 2 diabetes who underwent SGLT2 inhibitor treatment. However, as a meta-analysis, the study has several limitations. Data from separate studies were combined to determine the treatment effects on body weight. The inclusion criteria and the baseline characteristics, such as age, BMI, duration of diabetes, and ethnicity, may be different across studies, which may cause a high level of heterogeneity. We used the random-effects model for analysis when the level of heterogeneity was high and performed a sensitivity analysis. We performed several sensitivity analyses: short study duration or long study duration, including or excluding the extension studies; studies in different ethnicities; and studies in different baseline BMIs, including or excluding studies with the highest or lowest baseline BMI. Moreover, the add-on therapy studies were included or excluded. We have also made the analysis of the body weight changes in the placebo control group add on different groups of antidiabetes drugs, such as metformin, sulfonylurea, pioglitazone, insulin, etc. (Supporting Information Table S6). The sensitivity analysis indicated that there was no significant difference in the results when including or excluding the above types of studies. In addition, data on weight changes from baseline in each dosage group were used as the parameters in this meta-analysis, but not the pooled, patient-level data, which should be more useful to draw a conclusion. However, these data are seldom available because most trials are sponsored by industry. Therefore, we used the parameters in each trial as surrogates. Of course, another limitation might be publication bias because positive results had a greater chance of being selected for publication than negative results. We used the funnel plot assessment to minimize this limitation, but our results should be interpreted cautiously. Another limitation of this study was that when using meta-regression analysis to evaluate whether weight change caused by SGLT2 inhibitors might be affected by the baseline characteristics besides dosage, for the limited studies we collected, it may not be feasible to fit a model when the number of parameters (such as baseline age, male percent, duration of diabetes, BMI, HbA1c, etc.) to estimate is larger than the number of observations (four studies for Ipragliflozin, two studies for tofogliflozin, and five studies for luseogliflozin). Finally, we should also mention the data extraction. For example, in the Schumm-Draeger 2015 study, dapagliflozin 2.5 mg was taken twice daily, and we categorized this study in the group of dapagliflozin 5 mg/d but not twice daily. In the Ferrannini 2010 study, dapagliflozin 2.5 mg was taken in the morning in one group and in the evening in another group. It was recommended in the majority of the trials that the SGLT2 inhibitors should be taken after fasting; therefore, we did not include the group that received dapagliflozin 2.5 mg in the evening. All of the above were limitations of this meta-analysis.
In conclusion, our meta-analysis showed that a body weight decrease from baseline was significantly related to dapagliflozin, canagliflozin, empagliflozin, and ipragliflozin treatment in type 2 diabetes patients when compared with a placebo. Moreover, the weight changes were dose dependent in patients who received dapagliflozin treatment.
Acknowledgments
Many thanks should be given to the people working in the Endocrinology and Metabolism Department at Peking University People's Hospital. We also thank Aiyun Song, Yunguang Li, and Xia Li at AstraZeneca Ltd. in China.





