A Postmortem Study of Frontal and Temporal Gyri Thickness and Cell Number in Human Obesity
Funding agencies: This work was supported by Program UNAM-DGAPA-PAPIIT under grant IN200817 to JSP.
Disclosure: The authors declared no conflict of interest.
Abstract
Objective
This study aimed to compare cortex thickness and neuronal cell density in postmortem brain tissue from people with overweight or obesity and normal weight.
Methods
The cortex thickness and neuron density of eight donors with overweight or obesity (mean = 31.6 kg/m2; SD = 4.35; n = 8; 6 male) and eight donors with normal weight (mean = 21.8 kg/m2; SD = 1.5; n = 8; 5 male) were compared. All participants were Mexican and lived in Mexico City. Randomly selected thickness measures of different cortex areas from the frontal and temporal lobes were analyzed based on high-resolution real-size photographs. A histological analysis of systematic-random fields was used to quantify the number of neurons in postmortem left and right of the first, second, and third gyri of frontal and temporal lobe brain samples.
Results
No statistical difference was found in cortical thickness between donors with overweight or obesity and individuals with normal weight. A smaller number of neurons was found among the donors with overweight or obesity than the donors with normal weight at different frontal and temporal areas.
Conclusions
A lower density of neurons is associated with overweight or obesity. The morphological basis for structural brain changes in obesity requires further investigation.
Introduction
Obesity has reached epidemic proportions, becoming one of the leading causes of disability and an important risk factor for the development of certain diseases that, in turn, are major causes of death worldwide (1, 2). Excess body weight puts patients at a higher risk of hypertension, dyslipidemia, stroke, sleep apnea, and other serious comorbidities (3). In addition, obesity can lead to a series of debilitating conditions that result in reduced life expectancy and low quality of life in the presence of these comorbidities (4).
There is growing evidence that obesity is a risk factor for dementia and cognitive decline. The mechanisms underlying the relationship between excess adiposity and cognitive decline are not fully understood, and the relationship still needs to be better elucidated (5). Several imaging studies have suggested that overweight and obesity predispose one to a decline in brain volume, brain atrophy, or a decreased density of gray matter (GM) (6-10).
Brain imaging techniques such as magnetic resonance imaging (MRI) have shown brain volume deficits in populations with overweight and obesity (6, 11), particularly in the frontal lobe of young adults (12). Obesity has also been associated with axonal degeneration (8) and white matter (WM) abnormalities, which, in turn, have been postulated to result in cognitive decline (13). Voxel-based morphometry by using MRI and cortical thickness from structural MRI analysis have shown that obesity is associated with widespread reductions in GM volume and significant reductions in cortical thickness of the bilateral medial temporal lobes, anterior lobe of the cerebellum, occipital lobe, frontal lobe, precuneus, and midbrain (9, 14-16). There are reports showing that populations with obesity have low GM volumes in the middle occipital gyrus, inferior frontal gyrus, inferior and superior frontal gyri, medial temporal lobe, hippocampus, putamen, globus pallidus, thalamus, fusiform gyrus, cerebellum, precuneus, and cuneus, as well as reduced WM volume in the striatum (6, 7, 17).
High body mass index (BMI) can also result in more GM thickness. For example, Horstmann et al. (18) observed a positive correlation between BMI and GM volume in the orbitofrontal cortex, putamen, and hypothalamus. However, the increase in GM reported by Horstmann and colleagues could be the consequence of an increased number of glia cells (astrocytes) rather than nerve cells. Unfortunately, this assumption can only be clarified with histological techniques in postmortem brain donors.
There is little information about the underlying histological effects associated with high BMI. However, there is one study of particular interest to the present investigation that reported the number of neurons in postmortem brain tissue by using computerized stereology (19). Weise and collaborators (19) hypothesized that postmortem striatal samples of donors with obesity from the Harvard Brain Tissue Resource Center would exhibit lower density of neurons and glial cells (i.e., astrocytes) than donors with normal weight. In opposition to the expectations, the results showed no significant differences between groups. This unexpected finding goes in opposition with reports on structural brain alterations in humans with obesity based on in vivo imaging techniques. To better understand the relation between obesity and cerebral atrophy, the present study compares GM and WM thickness and neuronal cell density in postmortem brain tissue from deceased people with overweight or obesity and normal weight. We hypothesized smaller cortex thickness in GM and WM and a lower density of neurons among the group with overweight or obesity than the group with normal weight.
Methods
Brain cases
Brains were acquired in compliance with the requirements of the institutional review committee of the pathology service from the Anatomical Pathology Unit at the General Hospital of Mexico in Mexico City. Relatives of the deceased authorized and signed all legal documents to perform necropsy studies at the hospital. The date of death, main causes of death, and comorbidities were recorded.
Previous to the postmortem study, anthropometric measures (height and weight) were taken on the selected individuals. The weights of the corpses were recorded while they were laid on a stretcher by using a veterinarian weighing machine (PLP 4×4 WL model; Tor-Rey Electronics, Inc., Houston, Texas). The stretcher weight was subtracted from the total weight. The brains included in the sample came from male and female donors between the ages of 16 and 61 years old (Table 1). The lower age limit was chosen based on the fact that after age 15, there is little neural plasticity, and the high age limit was chosen to avoid the brain changes associated with aging (20). Groups were divided based on the BMI calculated as body weight divided by height squared. In accordance with the World Health Organization (21), cases within 18.5 kg/m2 < BMI < 25 kg/m2 were considered the group with normal weight, cases within 25 kg/m2 < BMI < 29.9 kg/m2 were considered the group with overweight, and cases within 30 kg/m2 < BMI < 40 kg/m2 were considered the group with obesity. Two cases were excluded from the group with overweight or obesity because they had a BMI above 40 kg/m2 (morbid obesity), and two cases were excluded from the group with normal weight because they had a BMI of less than 18.5 kg/m2 (underweight). The final sample consisted of 16 brain donors (normal weight: mean BMI = 21.8 kg/m2; SD = 1.5; n = 8) (overweight or obesity: mean BMI = 31.6 kg/m2; SD = 4.35; n = 8).
| Group | Case | Age (y) | Sex | BMI (kg/m2) | Cause of death | Brain (g) |
|---|---|---|---|---|---|---|
| Normal weight | 1 | 28 | F | 20.0 | Uremic pneumonitis | 1,250 |
| 2 | 47 | M | 22.6 | Multiple foci pneumonia | 1,120 | |
| 3 | 18 | M | 19.1 | Bilateral pulmonary hemorrhage | 1,490 | |
| 4 | 61 | F | 22.5 | Sepsis | 1,250 | |
| 5 | 55 | M | 22.7 | Heart failure | 1,600 | |
| 6 | 16c | M | 21.9 | Lobar pneumonia | 1,220 | |
| 7 | 20 | F | 21.5 | Bilateral pulmonary hemorrhage | 1,260 | |
| 8 | 57 | M | 23.6 | Liver, spleen, and subarachnoid massive infiltration | 1,280 | |
| Overweight/obesity | 9 | 53 | M | 29.4 | Liver failure | 1,230 |
| 10 | 53 | M | 33.4 | Heart failure | 1,090 | |
| 11 | 59 | M | 30.1 | Total thrombosis of the aortic valve | 1,200 | |
| 12 | 56 | M | 37.9 | Bilateral pulmonary metastasis | 1,200 | |
| 13 | 50 | F | 28.6 | Bilateral pulmonary hemorrhage | 1,120 | |
| 14 | 58 | M | 37.9 | Bilateral pulmonary hemorrhage | 1,200 | |
| 15 | 49 | F | 28.9 | Bilateral pulmonary thromboembolism | 1,230 | |
| 16 | 45 | M | 26.5 | Sepsis | 1,250 |
| Mean (SD) [range] | 95% confidence interval | ||||
|---|---|---|---|---|---|
| Normal weight | Overweight/obesity | t | Inferior | Superior | |
| Sex (M/F) | 5/3 | 6/2 | 28d | ||
| Age (y) | 37.8 (19.2) [45] | 52.9 (4.8) [14] | 2.20e | −31.3 | 1.01 |
| BMI (kg/m2) | 21.8 (1.5) [4.5] | 31.6 (4.4) [11.4] | −6.03b, f | −13.5 | −6.1 |
| Brain weight (g) | 1,308.8 (156.4) [480] | 1,190 (56.1) [160] | 2.02g | −14.7 | 252.2 |
- a P < 0.05.
- b P < 0.01.
- c A young adult with a BMI of 25 is considered at the top end of a healthy weight classification (Barlow SE. Pediatrics 2007;120:S164–S192).
- d Mann-Whitney U, P = 0.6.
- e Based on Levene's test t (7.6).
- f t (8.6).
- g t (8.9).
Brain samples from the subjects with overweight or obesity and normal weight were screened for evidence of neuropathology. Specifically, brains were screened for congenital malformations, primary neurological disease, and primary or secondary tumors. Additionally, donated brains were not included in the sample if the brain tissues showed macroscopic changes by trauma or evidence of neurological disease (e.g., infections, demyelinating, stroke, tumor). Moreover, clinical record files were reviewed to discard the presence of diabetes mellitus or hypertension. Finally, donated brains were excluded if individuals had a history of surgery or if the cause of death was a medicolegal-related case. The final sample consisted of eight brains from donors with overweight or obesity (six male) and eight brains from donors with normal weight (five male).
Brain preparation
Complete autopsies were performed with removal of the brains from skulls. The brains were fixed in formaldehyde 10% (immersed) at room temperature for 10 days and later were immersed in water for 1 day. The brains were weighed on a digital scale. The region from the rostral to caudal dura mater was removed and the supratentorial portion was separated from the infratentorial. Consecutive coronal 5-mm-thick cuts were made in the rostrocaudal direction starting at the white anterior commissure. Brain parenchyma, meninges, and the ventricular system were reviewed for neuropathology. Macroscopic photographs were taken of the selected cuts from the preselected gyri. Tissue was placed on capsules and immersed in a container with 10% formaldehyde for 24 hours and finally reviewed for a third time for any evidence of neuropathology.
Macroscopic examination: GM thickness
Six digital macroscopic photographs of a coronal cut at the level of the anterior white commissure were taken to measure GM thickness. The photographs were taken with a digital camera (12.1 megapixel, LUMIX DMC-ZR1PU-K; Panasonic Corp., Newark, New Jersey) that was mounted in a Reprovit system (Beseler CS-14 Copystand; B&H Foto & Electronics Corp., New York, New York). The images were full cut, left frontal lobe, right frontal lobe, left temporal lobe, right temporal lobe, and callosum. The photograph was taken 12.5 cm away from the Reprovit's plane by using an ISO (International Standards Organization; determines the sensitivity of the image sensor) of 1,600. The final image size had a 4:3 aspect ratio. Figure 1 shows an example of a coronal cut from the left frontal gyri indicating the area where the measures were obtained.
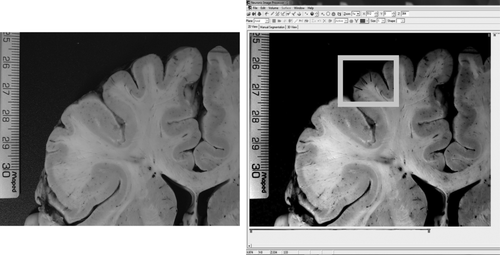
Example of cortical thickness measurements. On the left panel, a real-size photograph of a coronal cut of the left frontal gyri is shown. On the right side is the same image but by using custom software to measure size in terms of number of pixels. The three black lines within the gray frame represent an approximation of where the measures were done.
Two neuropathologists performed the macroscopic morphological brain imaging analysis by using iMagic software (Neuronic SA, Havana, Cuba). The GM thickness of each cerebral cortex was measured by taking the leptomeninges as the outer boundary and the interface cortex or WM as the internal boundary (in millimeters). Three measurements were made and the average was computed. The WM thickness of the corpus callosum was measured at the level of its junction with the depth of the sulcus, which separates it from the cingulate; three measurements were performed on the right side and three on the left side, and the average was considered for each side. The neuropathologist performed the measurements without knowing in advance which group the slides belonged to (blind evaluation).
Tissue preparation for neuronal count
The histological sample used to assess cell area density was taken from the coronal cut used to measure GM thickness. Samples of cerebral tissue were taken from 12 gyri (3 left frontal, 3 right frontal, 3 left temporal, and 3 right temporal) of approximately 1 × 1 × 0.5 cm each. Tissue was embedded in paraffin by using traditional histological techniques. Histological cuts 5 μm thick were stained by using the hematoxylin and eosin technique.
Neuronal count and data acquisition
The bidimensional counting of neurons based on the Chana et al. (22) procedure was performed by two different neuropathologists by using an Olympus microscope (model CX31; Olympus Corp., Tokyo, Japan). The counting of neuronal cell bodies was performed in 64 consecutive fields of the cortex by using 40× objective through a deliberate search of some of the following features that distinguish neurons from glia: size, shape, Nissl substance, and evident nucleolus (Figure 2). The number of neuronal cell bodies was recorded field by field in a database. A total of 12,288 fields were reviewed in “high-dry” by each neuropathologist (64 fields × 12 different cerebral gyri × 16 cases). The selection of the 64 fields depended on each reviewer's discretion, so different fields were considered by each neuropathologist. A total sum of cell bodies from 64 fields per gyrus was entered into a database.
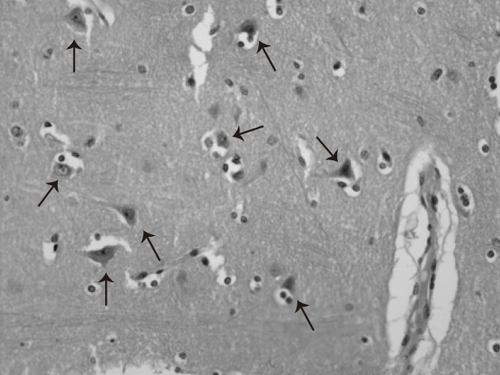
Microphotography stained with hematoxilin and eosin (40×). The picture depicts a representative cut from cerebral cortex. The arrows indicate neuronal cell bodies inside “neuropilo” with normal characteristics.
Statistical analysis
SPSS Statistics software (version 20.1; IBM Corp., Armonk, New York) was used for all statistical analyses. Two-sample t tests were used to compare groups for cortex thickness and the number of neurons on each gyrus. Cohen's d was also computed for each comparison. A series of nonparametric Mann-Whitney U tests was performed to compare both groups in cortex thickness and density of neuron measurements.
Age differences were observed because of the inclusion of two young individuals (16 < years < 20) in the normal weight group because they met the inclusion criteria. This bias was taken into consideration by performing correlation analyses between the frontal and temporal gyri and age and BMI. Nonparametric analyses were performed again, taking out the measurements of the two youngest individuals from the normal weight group.
Results
Table 1 summarizes the clinical characteristics of the donors. From the eight cases included in the group with overweight or obesity, there were four cases (9, 13, 15, and 16) that qualified as overweight (25 kg/m2 < BMI < 30 kg/m2) and four cases that qualified for obesity (30 kg/m2 < BMI < 40 kg/m2).
As shown in Table 1, subjects with overweight or obesity had a significantly greater BMI and were older than subjects with normal weight. There were no significant Pearson's correlations between both BMI and age variables and frontal and temporal gyri cortex thickness. Regarding the density of cells, both BMI and age showed a negative correlation with the left middle (ρ = −0.57; P = 0.02 and ρ = −0.65; P = 0.006, respectively) and the right inferior (ρ = −0.68; P = 0.004 and ρ = −0.73; P = 0.001, respectively) gyri of the frontal lobe. This pattern was also observed if data from the two youngest brain donors were excluded from the analysis. Significant negative correlations were also observed between both BMI and age variables and the left superior temporal gyrus (ρ = −0.67; P = 0.005) (ρ = −0.59; P = 0.015), the right superior temporal gyrus (ρ = −0.77; P < 0.001) (ρ = −0.63; P = 0.009), the left middle temporal gyrus (ρ = −0.54; P = 0.03) (ρ = −0.61; P = 0.011), and the right inferior temporal gyrus (ρ = −0.58; P = 0.019) (ρ = −0.54; P = 0.033). Analysis results excluding data from both brain donors under 20 years old showed a similar pattern, but BMI and age did not correlate with the left middle temporal gyrus and the right inferior temporal gyrus.
Tables 2 and 3 show the statistical comparisons between the groups in GM cortical thickness by using parametric and nonparametric statistics, respectively. Although there was no significantly thinner GM in people with overweight or obesity than in subjects with normal weight, Cohen's d values ranged from medium to strong at different regions, mainly at the superior frontal middle temporal gyri of the right hemisphere. Comparisons made between groups by using Mann-Whitney U tests revealed a similar pattern, but the right superior frontal gyrus of the group with overweight or obesity was significantly thinner compared with the group with normal weight when the two younger individuals were taken out of the analyses.
| Mean (SD) in millimeters | 95% confidence interval | ||||||
|---|---|---|---|---|---|---|---|
| Gyrus | H | Normal weight | Overweight/obesity | t (14) | Inferior | Superior | Cohen's d |
| Frontal | |||||||
| 1 | R | 3.5 (0.23) | 3.4 (0.19) | 1.53 | −0.064 | 0.385 | 0.817 |
| 1 | L | 3.4 (0.22) | 3.5 (0.29) | −0.92 | −0.388 | 0.154 | 0.494 |
| 2 | R | 3.5 (0.34) | 3.4 (0.23) | 0.76 | −0.201 | 0.423 | 0.408 |
| 2 | L | 3.3 (0.16) | 3.2 (0.28) | 0.50a | −0.187 | 0.303 | 0.271 |
| 3 | R | 3.4 (0.32) | 3.3 (0.17) | 1.28 | −0.109 | 0.437 | 0.689 |
| 3 | L | 3.3 (0.41) | 3.4 (0.23) | −0.21 | −0.390 | 0.319 | 0.113 |
| Temporal | |||||||
| 1 | R | 3.6 (0.32) | 3.5 (0.20) | 0.89 | −0.164 | 0.400 | 0.479 |
| 1 | L | 3.4 (0.22) | 3.5 (0.18) | −1.0 | −0.311 | 0.111 | 0.544 |
| 2 | R | 3.5 (0.23) | 3.4 (0.21) | 1.41 | −0.080 | 0.392 | 0.758 |
| 2 | L | 3.3 (0.18) | 3.4 (0.26) | −0.43 | −0.289 | 0.191 | 0.234 |
| 3 | R | 3.4 (0.16) | 3.3 (0.21) | 0.62 | −0.143 | 0.259 | 0.330 |
| 3 | L | 3.5 (0.18) | 3.4 (0.36) | 0.97b | −0.164 | 0.440 | 0.523 |
| Corpus callosum | 6.0 (0.44) | 5.9 (0.81) | 0.23 | −0.626 | 0.778 | 0.124 | |
| Global thickness GM | 3.4 (0.18) | 3.4 (0.11) | 0.72c | −0.108 | 0.218 | 0.388 | |
- Bold Cohen's d values show medium to strong effect sizes. Thickness of corpus callosum and total thickness included.
- aDifferences between variances assumed, Levene test, P = 0.007, t (11.02).
- bLevene test, P = .04, t (10.1).
- cLevene test, P = 0.03, t (11.8).
- H, hemisphere; L, left; R, right.
| Normal weight, n = 8 | Overweight/ obesity, n = 8 | Mann-Whitney | |||||
|---|---|---|---|---|---|---|---|
| Gyrus | H | Mdn | Average range | Mdn | Average range | U | Ua |
| Frontal | |||||||
| 1 | R | 3.6 | 10.1 | 3.3 | 6.9 | 19 | 5b |
| 1 | L | 3.4 | 7.5 | 3.4 | 9.5 | 24 | 19 |
| 2 | R | 3.5 | 9.1 | 3.3 | 7.9 | 27 | 14 |
| 2 | L | 3.2 | 9.1 | 3.2 | 7.9 | 27.5 | 19.5 |
| 3 | R | 3.4 | 9.6 | 3.3 | 7.4 | 23.5 | 13.5 |
| 3 | L | 3.2 | 8.1 | 3.4 | 8.9 | 28.5 | 21.5 |
| Temporal | |||||||
| 1 | R | 3.6 | 9.3 | 3.4 | 7.8 | 26 | 17 |
| 1 | L | 3.4 | 7.7 | 3.4 | 9.3 | 25.5 | 21.5 |
| 2 | R | 3.5 | 10.1 | 3.3 | 6.9 | 19 | 11 |
| 2 | L | 3.4 | 9.0 | 3.3 | 8.0 | 28 | 18 |
| 3 | R | 3.3 | 9.3 | 3.3 | 7.8 | 26 | 16 |
| 3 | L | 3.5 | 9.3 | 3.3 | 7.8 | 26 | 20 |
| Corpus callosum | 6.1 | 9.1 | 5.9 | 7.9 | 27 | 16 | |
| Global thickness GM | 3.3 | 8.6 | 3.3 | 8.4 | 31.5 | 15.5 | |
- Thickness of corpus callosum and total thickness included.
- a Comparison of six with normal weight (without two younger subjects) versus eight with overweight/obesity.
- b P < 0.05, two-tailed.
- H, hemisphere; L, left; R, right; Mdn, median.
The results of the comparison between groups in terms of the number of neurons are displayed in Table 4. There was a significantly larger number of neurons in the group with normal weight when compared with the group with overweight or obesity for the middle and inferior frontal gyri of the right hemisphere, the superior temporal gyri of both hemispheres, the left middle temporal gyrus, and the right inferior temporal gyrus. Cohen's d values corresponding to those differences ranged from strong to very strong.
| Mean (SD) | 95% confidence interval | ||||||
|---|---|---|---|---|---|---|---|
| Gyrus | H | Normal weight | Overweight/obesity | t (14) | Inferior | Superior | Cohen's d |
| Frontal | |||||||
| 1 | R | 1,909.6 (199.9) | 1,675.1 (366.9) | 1.58 | −82.4 | 551.4 | 0.849 |
| 1 | L | 1,880.9 (290.3) | 1,745.3 (196.1) | 1.09a | −133.5 | 404.8 | 0.585 |
| 2 | R | 1,895.9 (154.3) | 1,663.4 (202.9) | 2.58b | 39.2 | 425.8 | 1.38 |
| 2 | L | 1,855.1 (213.2) | 1,694.3 (256.4) | 1.36 | −92.0 | 413.8 | 0.729 |
| 3 | R | 1,887.3 (215.9) | 1,610.8 (174.3) | 2.81b | 66.1 | 486.1 | 1.51 |
| 3 | L | 1,789.9 (180.3) | 1,674.1 (195.7) | 1.23 | −86.0 | 317.5 | 0.658 |
| Temporal | |||||||
| 1 | R | 1,873.6 (262.4) | 1,497.3 (196.0) | 3.25c | 128.0 | 624.7 | 1.74 |
| 1 | L | 1,828.5 (156.0) | 1,525.4 (163.7) | 3.79c | 131.6 | 474.6 | 2.03 |
| 2 | R | 1,719.0 (309.5) | 1,516.9 (165.6) | 1.62 | −64.1 | 468.0 | 0.870 |
| 2 | L | 1,753.3 (258.2) | 1,517.8 (121.5) | 2.33b | 19.1 | 451.9 | 1.25 |
| 3 | R | 1,793.5 (218.1) | 1,520.5 (135.9) | 3.00c | 78.1 | 467.9 | 1.61 |
| 3 | L | 1,749.0 (201.1) | 1,592.8 (162.4) | 1.71 | −39.7 | 352.2 | 0.914 |
- Bold Cohen's d values show strong and very strong effect sizes.
- a Differences between variances assumed, Levene's test t (12.2).
- b P < 0.05.
- c P < 0.01, two-tailed. H, hemisphere; L, left; R, right.
Table 5 displays the nonparametric statistical analysis results, which showed the same pattern as parametric analyses. Mann-Whitney U test analyses without the data from the youngest individuals showed significant differences regarding the same brain gyri with the exception of the right inferior frontal gyrus and left middle temporal gyrus.
| Normal weight, n = 8 | Overweight/obesity, n = 8 | Mann-Whitney | |||||
|---|---|---|---|---|---|---|---|
| Gyrus | H | Mdn | Average range | Mdn | Average range | U | Ua |
| Frontal | |||||||
| 1 | R | 1,889.5 | 10.1 | 1,572.5 | 6.9 | 19 | 15 |
| 1 | L | 1,880.4 | 9.1 | 1,648.5 | 7.9 | 27 | 19 |
| 2 | R | 1,936.0 | 10.9 | 1,635.5 | 6.1 | 12.5b | 8b |
| 2 | L | 1,830.5 | 9.6 | 1,696.5 | 7.4 | 23 | 19 |
| 3 | R | 1,922.0 | 11.3 | 1,585.5 | 5.8 | 10b | 9 |
| 3 | L | 1,750.0 | 9.5 | 1,684.0 | 7.5 | 24 | 20 |
| Temporal | |||||||
| 1 | R | 1,794.0 | 11.8 | 1,505.5 | 5.3 | 6c | 4c |
| 1 | L | 1,784.0 | 12.0 | 1,516.0 | 5.0 | 4c | 3c |
| 2 | R | 1,629.0 | 10.5 | 1,506.5 | 6.5 | 16 | 12 |
| 2 | L | 1,761.5 | 10.9 | 1,486.0 | 6.1 | 13b | 13 |
| 3 | R | 1,835.5 | 11.4 | 1,479.0 | 5.6 | 9b | 8b |
| 3 | L | 1,730.0 | 10.4 | 1,533.0 | 6.6 | 17 | 14 |
- a Comparison of six with normal weight (without two younger subjects) versus eight with overweight/obesity.
- b P < 0.05.
- c P < 0.01, two-tailed. H, hemisphere; L, left; R, right; Mdn, median.
Figure 3 shows a scatterplot between BMI and the average number of neurons in all the brain regions studied. The significant negative trend shows that higher BMI is associated with a lower number of neurons. Data from the youngest donors are represented by asterisks.
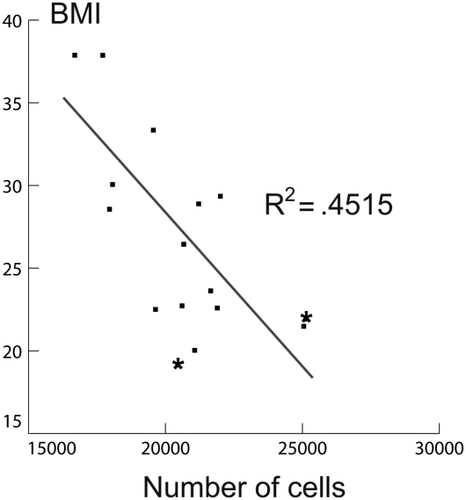
Scatterplot of BMI and number of neurons. Two data from the youngest donors are represented by asterisks (< 20 years).
Discussion
The purpose of the present study was to explore the relationship between brain volume deficits and obesity. We compared the thickness of the GM, WM, and neuronal density through a collection of brain donors who were identified as having either overweight/obesity or normal weight. It was hypothesized that brain tissue samples from the necropsies of brain donors with BMI ≥ 25 kg/m2 would have a reduction in GM and WM thickness and a reduction in neuronal density in the frontal and temporal lobes. Our results partially confirmed our hypothesis because subjects with overweight or obesity had less neuronal density in the right middle and inferior frontal gyri, in the right superior and inferior temporal gyri, and in the left superior and middle temporal gyri compared with people who had normal weight, but we found no evidence of cortex thickness reduction in the group with overweight or obesity. Because neuronal density significantly correlated with age and BMI, it could be argued that age is a confounding variable. However, we found different neuronal densities between groups at the right middle frontal gyrus independent of age. Similar results were found when excluding the two youngest donors from the analyses, but in this case, neuronal density from left middle and right inferior temporal gyri did not correlate with BMI and age. Nevertheless, both brain regions were significantly different when the number of neurons was compared among groups.
Thus, our results could be in accordance with brain imaging studies that have shown brain GM volume deficits and axonal degeneration in populations with overweight or obesity (6, 8, 11, 12), which have been postulated to result in cognitive decline (13). Unfortunately, there is not much research comparing the microstructural differences between brain organ donors with obesity and normal weight. The one study exploring the microstructural changes in brain donors with high and normal BMI showed no histological differences (19). A possible explanation for this result is that brain donors with a BMI above 40 were included in the study. A BMI above 40 is considered a rare pathological condition (morbid obesity), and the biological mechanisms behind obesity and morbid obesity are not comparable (23).
Although the present study does not provide evidence of a direct association between cortical thickness and neuronal density, neuroimaging studies with large samples have shown a reduction in volume and cortical thickness that could be associated with a minor number of neurons. This density reduction may represent an adverse direct consequence on the subject's cognition, as some reports have shown that obesity is associated with cognitive decline throughout one's lifetime (24). The relationship between obesity and cognitive decline is based on epidemiologic and prospective reports (25). Several studies have shown that having overweight not only predisposes the individual to a lower brain volume, but it also seems that obesity, by itself, is a risk factor for Alzheimer's disease (26). Obesity may have a negative effect on attention, intellectual coefficient, executive, and global functioning (27), which is evident throughout childhood or adolescence (28). Deficits on executive functioning may play an important role in the development and maintenance of obesity, especially when a poor inhibitory control of food overconsumption is present (28, 29). Our results showing lesser neuronal density in frontal lobe regions of individuals with overweight or obesity may suggest the presence of inhibitory control disturbance. People with obesity display deficiencies in learning, memory, and executive control when compared with individuals with normal weight (30), which seems to reflect the effect of obesity per se (31).
Currently, it is widely accepted that cytokine expression can be induced in brain cells, therefore promoting neural apoptosis and cognitive decline (32). However, the biological mechanisms by which neuronal density is associated with obesity are largely unknown. Our findings are based on association and not causation; therefore, we cannot offer a mechanistic explanation of our results. Nevertheless, we have considered two possible mechanistic explanations: low-grade systemic inflammation and vascular damage.
Individuals with obesity who suffer from systemic inflammation show a similar clinical picture of that found in diabetes mellitus. For example, human studies (33) and animal models (34) have shown that obesity is associated with increased brain oxidative stress and neuroinflammation, which are both implicated in the pathogenesis of neurodegenerative diseases (for a brain effect of obesity review, see reference 24). Therefore, in the long run, the chronic exposure to cytokines in the brain because of low-grade systemic inflammation can result in brain damage.
The other possible mechanism that contributes to lower neural density in people with overweight or obesity is the vascular damage (35). In humans, one cause of microvascular disease (microangiopathy) is long-term diabetes mellitus (36), in which neural dysfunction correlates closely with the development of blood vessel abnormalities that contribute to diminished oxygen tension and hypoxia (34, 37). Similarly, subjects with obesity have narrower arteriolar and wider venular calibers when compared with subjects with normal weight, independent of conventional cardiovascular risk factors (38). Animal models show sustained contraction (increased myogenic tone) in the wall structure of vessels (inward remodeling) and the endothelial cells, which can result in a significant reduction in blood flow (39). Therefore, it is very likely that microvascular damage in the central nervous system can cause chronic ischemia.
This study had limitations. We consider that the small sample size was an important factor that could have blurred the differences in cortical thickness between groups. This effect was not evident regarding the number of neurons because the data resolution of cortex thickness could have been lower because of fewer measurements.
Another major limitation is the wide age range of the sample, as age is highly associated with brain morphological measures and neuronal dysfunction and death (20). For this reason, we performed our analyses with and without the two brain donors under 20 years of age, and our findings were found to be similar.
It is pertinent to consider that several factors other than adiposity can influence brain neuronal density in the cerebral cortex (e.g., cerebral hypoxia/ischemia without being related to trauma, neoplasms, infections, vascular malformations, vasculitis, inflammatory processes, microvascular damage), and that when working with organ donors, it is difficult to discard factors that could have contributed to the cause of death.
The results of the present investigation are correlational, and therefore, the structural and substructure basis behind brain volume reduction in the group with overweight or obesity is beyond the scope of this study.
Conclusion
To our knowledge, this is the first postmortem study in which the thickness of the corpus callosum, the thickness of the cortex, and the number of neurons of frontal and temporal gyri were compared in the brains of otherwise healthy subjects with overweight or obesity and normal weight. A significantly smaller mean neuronal density, mainly at the right gyri of the frontal lobe and at both superior gyri of the temporal lobe, was observed in the brain donors with overweight or obesity than in the brain donors with normal weight. Our findings are in agreement with several neuroimaging studies. Clinical implications of a lower neuronal density in individuals with obesity can be observed by the presence of cognitive decline, especially in executive functions, because frontal lobe measures were obtained. Nonetheless, there is a need to further explore the underlying biology and histology of those brain changes in populations with overweight or obesity.
Acknowledgments
The authors thank Eilis C. Welsh for her contribution in the proofreading of the manuscript.





