Modifying influence of dietary sugar in the relationship between cortisol and visceral adipose tissue in minority youth
Funding agencies: This work was supported by the following: Grants: U54CA116848 from the National Cancer Institute, R01HD0033064 from the National Institute of Child Health and Human Development, 1P60MD002254-03 from the Office of Minority Health, 1K01DK078858 from the National Institute of Diabetes and Digestive and Kidney Disease, and USC Graduate Provost Fellowship.
Disclosure: The authors declared no conflict of interest.
Abstract
Objective
Cortisol has been associated with preferential visceral adipose tissue (VAT) deposition; however, findings in humans are mixed, which may be clarified when diet is considered.
Design and Methods
Participants included 165 African-American and Latino, overweight adolescents (BMI% 97.2±3.2%, ages 13-18, 67% Latino, 66% female). Body composition was determined by dual energy X-ray absorptiometry, abdominal fat depots [VAT, subcutaneous (SAT)] by multiple-slice MRI, time-controlled serum sample to measure cortisol, and 2-day multi-pass 24-hour dietary recall. Linear regression analysis examined the cross-sectional relationship between cortisol, and the interaction of diet and cortisol on adiposity measures. Sex, race, age, and total body fat were a priori covariates.
Results
There was a significant interaction between cortisol and sugar (total and added) in the prediction of VAT (Pinteraction ≤ 0.05). Amongst participants with high total or added-sugar intake, cortisol was significantly associated with VAT (ß = 0.031 P < 0.001; ß = 0.026 P < 0.001), with no relationship in low consumers of total or added-sugar.
Conclusion
Dietary sugar may play an important role in modifying the relationship between cortisol and VAT, such that cortisol is significantly associated with elevated VAT under conditions of high sugar intake.
Introduction
Adolescence is a critical time period when large changes in body composition naturally occur, and negative changes in fat distribution could prime youth for increased immediate and long-term disease risk. Hispanic and African-Americans youth and adults experience some of the highest rates of obesity, insulin resistance, metabolic syndrome, type 2 Diabetes, cardiovascular disease, and other obesity-related diseases (1-3). While overall body weight translates into increased obesity-related comorbidities, it has been argued that abdominal fat deposition, particularly visceral adipose tissue (VAT), is one of the strongest factors associated with disease risk (4, 5).
Understanding modifiable factors that lead to abdominal deposition, particularly VAT, is key in preventing this harmful fat deposition and reducing future disease risk. One promising pathway involves the hormone cortisol, an adrenal steroid involved in stress reactivity and macronutrient metabolism. Cortisol receptors (glucocorticoid receptors) have the highest concentration in VAT compared to other fat depots, potentially allowing for an accentuated relationship between cortisol exposure and VAT development (6).
Disruptions in stress reactivity, specifically increased exposure to cortisol, have been implicated in total and abdominal obesity, metabolic syndrome, and insulin resistance (7-10). Furthermore, models of chronic exposure to elevated cortisol, such as in Cushing's Syndrome and animal models of exogenous cortisol treatment or increased stress, have directly shown that cortisol exposure leads to abdominal obesity and cardiometabolic disease (11-14). Whereas these models of chronic exposure provide evidence for the causal role of cortisol in obesity and its related co-morbidities, the level of cortisol exposure in these models are substantially elevated beyond that experienced by humans in normal daily living. Variations in stress reactivity because of daily living may affect humans in more subtle ways, which can be seen in the mixed human literature of reported stress and cortisol levels (15, 16). This suggests that factors beyond simple serum cortisol levels need to be explored to elucidate this relationship in humans.
This nuanced relationship between stress pathways, and fat deposition or metabolic health may be dependent upon environmental factors, such as dietary intake. Animal models have shown that the full range of deleterious effects of stress or cortisol upon visceral adipose deposition and metabolic health only appeared when the mice were fed a high sugar/fat diet (17). The independent effects of diets high in fat and sugar upon excessive adiposity and metabolic disease, insulin resistance have been well characterized in adolescents and adults; however, the specific interaction of cortisol and diet upon fat deposition has not be tested in humans, especially in high-risk youth. Based on the pattern seen in animal models, the detrimental effects of stress or cortisol may only be seen in those who consume diets high in fat and/or sugar. Thus, the purpose of this study was to assess the modifying role of diet, particularly high fat and sugar, on the association between stress and visceral fat in overweight minority youth. We hypothesized that serum cortisol levels will positively associate with visceral fat in those who consume high levels of dietary sugar and/or fat. Despite the negative effects of total fat intake, a type of fat, a long-chain polyunsaturated fatty acid (FA), omega-3, has shown to be protective against VAT accumulation (18) and to mitigate the effects of a cortisol correlate (dexamethasone) (19). We therefore also explored the potential mitigating effect of a subset of dietary fat, omega-3 FA.
Methods
Participants
A cross-sectional study of 165 overweight (≥85th BMI percentile) African-American and Hispanic adolescents was conducted, using data from participants who met shared inclusion/exclusion criteria across observational (n = 64 (20)) and intervention (n = 101 (21, 22)) studies conducted in the University of Southern California (USC) Childhood Obesity Research Center, using standardized methods for adiposity, metabolic and dietary data collection. Participants with complete and valid adiposity, cortisol, and dietary data were included in this analysis and all had to meet the following selection criteria: Latino or African-American descent via self-report, Tanner stage ≥4 and 10-18 years old, no personal history of diabetes or other major illness; no use of inhaled or dermal corticosteroids. Two hundred and seventy participants met the inclusion/exclusion criteria, however, 69 were excluded for missing or invalid (<2 days recall) dietary data, 25 were excluded for missing cortisol data, and eight for missing adiposity measures, resulting in the 165 used for this analysis. Data were missing for participants because of incomplete study visits or invalid measurement. This study was approved by the institutional review board of the USC, and informed written parental consent and child assent were obtained before participation.
Adiposity and abdominal fat measures
Weight and height were measured to the nearest 0.1 kg and 1 cm, respectively, using a beam medical scale and wall-mounted stadiometer; body mass index (BMI, kg/m2) and BMI percentiles for CDC age- and sex-specific values were determined using EpiInfo 2005 (CDC, Atlanta, GA). Total fat mass and total lean mass were measured by dual energy X-ray absorptiometry using Hologic QDR 4500W-XP vs. 12.6 (Hologic, Bedford, MA).
Abdominal fat distribution (VAT and SAT) was assessed by magnetic resonance imaging (MRI) using GE 1.5 (n = 101) or 3.0 (n = 64) Tesla magnet at the USC imaging center, depending on the specific MRI machine available at the time of study. The two methods are highly correlated (r = 0.95 for the VAT, r = 0.90 for SAT). Multi-slice MRI was done using dual double-gradient-echo, and VAT was determined using the commercially available SliceOmatic software (Tomovision). For the subset of study participants (n = 64) measured using a 3.0 T magnet, a conversion factor of VAT 1.5 T = 1.260(VAT 3.0T) − 0.256 and SAT 1.5 T = 3.244 + 1.751 (SAT 3T) was applied to create comparable 1.5 T VAT and SAT values, generated from 18 young adults in a comparable study (23) who were measured on the same day by both the 1.5 and 3.0 T magnets.
Cortisol
Participants slept overnight in the clinical trials unit (CTU) at USC and had a standardized waking time of approximately 5:30 am. Intravenous catheters were placed in the anticubital vein with the use of local anesthesia (4 % topical LMX cream) and heating pads to limit pain and stress associated with catheter insertion. Fasting blood samples were obtained at 7:30 am. Blood samples were centrifuged immediately for 10 min at 2500 RPM and 8-10°C to obtain plasma, and plasma aliquots were frozen at −70°C until assayed. Morning plasma cortisol concentrations were determined using RIA kits (Siemens, Deerfield, IL) with a minimal detection limit of 0.47 µg/dL.
Dietary assessment
Dietary intake was collected on at least three separate days (1 weekend, 2 weekday) using the 24-hour recall multiple pass method, and only participants with two or more recalls were included in the analysis (84% of sample collected at least 1 weekend recall). Dietary recalls are a validated and widely used instrument in the assessment of dietary intake in youth (24, 25). The computer program Nutrition Data System for Research (NDS-R) developed by the Nutrition Coordinating Center at the University of Minnesota was used to analyze all dietary records. This program is updated regularly to reflect the most recent nutrient data available, and was used to calculate key dietary variables for this analysis, such as total fat, sugar, added sugar, omega-3 FA intake. Plausibility of dietary intake was assessed by examining the linear regression of energy intake with body weight, and any recalls over three standard deviations above or below the regression line were excluded from final analyses. This led to the exclusion of one participant, prior to analysis. Any recall in which participants reported illness or eating more or less than usual was excluded from analysis. Furthermore, to account for the phenomenon of under-reporting in overweight youth, we assessed the effects of a more stringent dietary exclusion criteria for under-reporting based upon the Schofield age and sex specific equations for BMR (26), and used the low energy reporters (LER) cut-point based off 0.9 for n = 1 and 1 day of recall (energy intake (EI):BMR, the Goldberg cutoff technique) (27, 28). Participants were characterized as LER or not (above or below 0.9).
Statistics
The normality of outcome variable and regression residuals were assessed and VAT was square root transformed to meet the assumptions of linear regression. Independent sample t-tests and chi-square analyses was performed to compare the age, total body fat, and lean mass, BMI, BMI%, SAT, VAT, cortisol, gender, and race of the participants (n = 105) excluded for missing data, to those included in these analyses (n = 165). Bivariate analyses with all study parameters were performed to identify significant correlations between study variables (dietary variables, VAT, SAT, waist circumference, body fat, weight, BMI, cortisol). Hierarchical linear regressions were performed to test the independent and interaction effects of dietary intake, expressed as both grams and percent of caloric intake (%kcals), and cortisol on VAT and SAT. The hypothesized dietary variables were dietary sugar, fat, and omega-3 FA; however, the data were analyzed in a linear progression from total macronutrient categories to their subsets to thoroughly investigate the effects of dietary quality. First the total macronutrient categories, total carbohydrate, and total fat, were assessed for main and interaction effect with cortisol on VAT and SAT. Then the subcategories, total and added sugar intake, fiber (total, insoluble and soluble), saturated FAs, monounsaturated FAs, polyunsaturated FAs, and omega-3 FAs, and were analyzed for main and interaction effect with cortisol on VAT and SAT. All interaction terms were created from the continuous variables, cortisol*diet; therefore to prevent collinearity and model instability, dietary and cortisol measures and their cross-product interaction term were centered in all regression models. Stratified scatter plots with regression lines were used to display significant interactions. Dietary variables were stratified by mean intake or World Health Organization (WHO) intake recommendations, as applicable (i.e. if a statistically significant modifier) for graphical representation. All significant predictors and interactions were included in one final regression model with covariates to quantify variance of adipose depots explained by combined significant predictors. The following a priori covariates were included: age, sex, race, total fat mass, energy intake (when dietary variable expressed in grams was used). Interactions between race, sex, and diet were also tested in all models, as was the potential confound of LER (factor modeled as yes/no for LER). All analyses were performed using SPSS version 16.0, (SPSS, Chicago, IL), with P < 0.05 considered statistically significant.
Results
Table 1 shows the baseline characteristics of the participants with complete dietary, MRI and cortisol data (n = 167). There was no significant difference in the age, total body fat and lean mass, BMI, BMI%, SAT, VAT, cortisol, gender, and race of the participants excluded for missing data (n = 107) and those included in these analyses. Participants' ages ranged from 13 to 18 years, with a mean age ± SD of 15.5 ± 1.7 years, a mean BMI percentile of 97.2 ± 3.2%, 76% in Tanner stage 5, 67% Latino, and 66% of the sample were female.
| Combined (n = 165) | African-Americans (n = 55) | Latino (n = 110) | ||||
|---|---|---|---|---|---|---|
| mean (SD) | range | mean (SD) | range | mean (SD) | range | |
| Age | 15.5 (1.7) | 4.9 | 15.6 (1.1) | 4.9 | 15.5 (1.2) | 4.8 |
| Tanner (4/5)* | 40/125 | - | 7/48 | - | 33/77 | - |
| Sex (M/F)* | 56/109 | - | 14/41 | - | 42/68 | - |
| Serum cortisol (µg/dL) | 10.2 (3.9) | 18.3 | 9.8 (3.4) | 13.8 | 10.3 (4.2) | 18.3 |
| Adiposity | ||||||
| BMI (kg/m2) | 34.4 (6.5) | 33.6 | 35.2 (6.5) | 32.2 | 34.0 (6.4) | 27.6 |
| BMI %ile | 97.2 (3.2) | 14.5 | 97.7 (2.4) | 9.9 | 96.9 (3.6) | 14.5 |
| BMI z-score | 2.1 (0.5) | 2.9 | 2.2 (0.4) | 1.6 | 2.1 (0.5) | 2.9 |
| Weight (kg) | 93.7 (20.2) | 97.8 | 97.6 (20.2) | 87.5 | 91.7 (20.1) | 97.8 |
| Height (cm) | 164.9 (8.2) | 44.4 | 166.3 (8.0) | 39.3 | 164.2 (8.3) | 37.5 |
| Waist (cm) | 106.6 (14.9) | 65.5 | 106.0 (14.9) | 62.0 | 107.1 (15.4) | 71.3 |
| Waist/Height (ratio) | 0.65 (0.09) | 0.43 | 0.64 (0.09) | 0.41 | 0.65 (0.09) | 0.41 |
| Total body fat (kg) | 35.4 (12.6) | 67.8 | 35.5 (11.2) | 56.8 | 35.3 (13.0) | 67.8 |
| Total lean mass (kg) | 54.0 (9.6) | 47.9 | 55.8 (9.0) | 31.9 | 53.1 (9.7) | 47.9 |
| SAT (L) | 13.8 (6.7) | 30.4 | 15.9 (6.4) | 29.3 | 12.8 (6.6) | 28.3 |
| VAT (L) | 1.7 (1.1) | 5.7 | 1.3 (0.8) | 3.3 | 1.9 (1.1) | 5.7 |
| Dietary Intake | ||||||
| Energy (kcal) | 1876.4 (613.2) | 2693 | 1875.3 (614.7) | 2252 | 1876.4 (615.3) | 2660.2 |
| Total Sugar (g) | 108.0 (51.9) | 223.87 | 107.5 (58.8) | 223.7 | 108.3 (48.4) | 219.4 |
| Added Sugar (g) | 76.0 (46.9) | 255.87 | 79.1 (46.7) | 225.8 | 74.5 (47.2) | 225.8 |
| Total Fat (g) | 73.2 (29.9) | 157.65 | 77.4 (31.5) | 132.7 | 71.2 (29.0) | 157.65 |
| Total Fiber (g) | 14.1 (5.8) | 40.6 | 12.4 (5.0) | 22.34 | 15.0 (9.1) | 40.6 |
| PUFA (g) | 16.6 (9.2) | 54.3 | 18.0 (9.4) | 44.8 | 15.9 (9.1) | 54.3 |
| Omega-3 FA (g) | 1.5 (0.8) | 4.7 | 1.6 (0.9) | 4.4 | 1.4 (0.7) | 3.6 |
| % Total Sugar | 22.7 (7.3) | 39.2 | 22.4 (8.7) | 39.2 | 22.9 (6.5) | 32.0 |
| % Added Sugar | 15.8 (7.2) | 34.2 | 16.6 (7.4) | 29.8 | 15.3 (7.0) | 34.2 |
| % Total Fat | 33.6 (6.2) | 33.6 | 35.6 (6.1) | 28.2 | 32.7 (6.1) | 31.0 |
- Reported means (SD) for continuous variables, as frequency for categorical variables (*); BMI, Body Mass Index; F, Female; FA, Fatty Acids; M, Male; PUFA, polyunsaturated fatty acid; SAT, subcutaneous abdominal adipose tissue; VAT, visceral adipose tissue.
Bivariate correlations showed no significant relationship between serum cortisol and any of the dietary intake variables, and only serum cortisol and total body fat were significantly associated with VAT (r = 0.22, P = 0.005; r = 0.58, P < 0.001, respectively). Cortisol was not significantly associated with SAT, total body fat, BMI, weight, or waist circumference (P > 0.1).
In regression analyses there were no significant relationships between any dietary variables and VAT after adjusting for sex, race, and total body fat. Furthermore, there were no significant interactive effects between cortisol and total macronutrient categories, carbohydrate, and fat. However, when looking at subcategories of macronutrients, there were significant interactions between cortisol and the dietary categories upon VAT, specifically total and added sugar (Pinteraction: P = 0.008; P = 0.029, respectively). While sugar was significant expressed as grams or as percent of kcals, percent total and added sugar were displayed in all subsequent tables and figures for ease of interpretation.
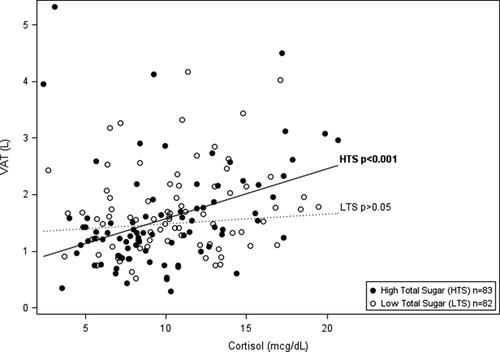
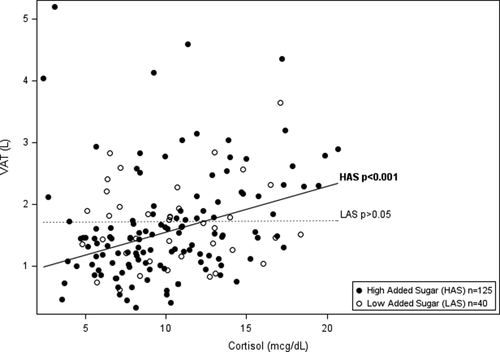
Tables 2 and 3 show the hierarchical regression of cortisol, percent total or added sugar, and the interaction between cortisol and total or added sugar with VAT using three progressive models. Regression analysis indicated a significant interaction of cortisol and total sugar, and cortisol and added sugar on VAT (ß = 0.002 P = 0.008; ß = 0.002 P = 0.029, respectively). To display the significant interaction between cortisol and total sugar intake, the sample was divided by the mean sample intake of total sugar (mean = 22.7%) to represent high and low consumers of total sugar intake. Higher cortisol significantly associated with greater VAT in those with high intake of % total sugar and had no association with VAT in those with low % total sugar (shown in Figure 1 and Table 2). Specifically, amongst participants with high total sugar intake, cortisol was significantly associated with VAT (ß = 0.031 P < 0.001) and explained an additional 8.4% percent of the variance in VAT beyond age, race, sex, total fat mass, and total energy intake. Figure 1 displays the linear relationships between cortisol and VAT by high and low consumers of total sugar (above and below mean % total sugar intake), adjusted for age, race, sex, total fat mass, and total energy intake. The relationship with between added sugar, cortisol, and VAT paralleled that of total sugar, however, there is a dietary recommendation for % added sugar intake set by the World Health Organization (WHO), wheread there is not one set for total sugar. Therefore, to display the significant interaction between cortisol and % added sugar intake, the WHO recommendation for added sugar intake at 10% of calories was used to divide high and low consumers of added sugar. Higher cortisol significantly associated with greater VAT in those with high intake of added sugar (≥10%) and had no association with VAT in those with low % added sugar (<10%) (shown in Figure 2 and Table 3). Specifically, amongst participants with high added sugar intake, cortisol was significantly associated with VAT (ß = 0.026 P < 0.001) and explained an additional 5.7% of the variance in VAT beyond sex, race, and total body fat. Figure 2 displays the linear relationships between cortisol and VAT by high and low consumers of added sugar (above and below 10%), adjusted for age, race, sex, total fat mass, and total energy intake.
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Variable n = 165 | beta (95% CI) | beta (95% CI) | beta (95% CI) |
| Constant | 0.176 (−0.527, 0.879) | 0.202 (−0.487, 0.891) | 0.283 (−0.395, 0.961) |
| Age | 0.019 (−0.020, 0.058) | 0.015 (−0.023, 0.053) | 0.013 (−0.024, 0.051) |
| Sexa | −0.191 (−0.297, −0.084)e | −0.174 (−0.278, −0.071)e | −0.186 (−0.288, −0.085)e |
| Raceb | 0.254 (0.157, 0.351)e | 0.246 (0.152, 0.340)e | 0.233 (0.141, 0.326)e |
| Total Fat (kg) | 0.019 (0.016, 0.023)e | 0.020 (0.016, 0.023)e | 0.020 (0.017, 0.024)e |
| Caloric intake (kcal) | 0.000 (0.000, 0.000) | 0.000 (0.000, 0.000) | 0.000 (0.000, 0.000) |
| Cortisolc | 0.020 (0.009, 0.031)e | 0.019 (0.008, 0.030)e | |
| % Total Sugarc | −0.002 (−0.009, 0.004) | −0.002 (−0.008, 0.004) | |
| Cortisolc x % Total Sugarc | 0.002 (0.001, 0.003)d | ||
| R2 Total | 0.498 | 0.520 | 0.538 |
| Δ R2 | 0.498e | 0.042e | 0.021d |
- Model 1 includes age, sex, race, total body fat and energy intake; model 2 includes age, sex, race, total body fat, energy intake, serum cortisol and % total sugar intake; model 3 includes age, sex, race, total body fat, energy intake serum cortisol, % total sugar, and the interaction of serum cortisol and % total sugar intake.
- a Male is the comparison group.
- b African American is the comparison group
- c Standardized (centered on mean).
- d P < 0.05.
- e P ≤ 0.01.
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Variable n = 165 | beta (95% CI) | beta (95% CI) | beta (95% CI) |
| Constant | 0.176 (−0.527, 0.879) | 0.253 (−0.436, 0.941) | 0.262 (−0.418, 0.942) |
| Age | 0.019 (−0.020, 0.058) | 0.013 (−0.025, 0.051) | 0.013 (−0.023, 0.053) |
| Sexa | −0.191 (−0.297, −0.084)e | −0.179 (−0.283, −0.076)e | −0.189 (−0.291, −0.087)e |
| Raceb | 0.254 (0.157, 0.351)e | 0.244 (0.150, 0.338)e | 0.240 (0.147, 0.333)e |
| Total Fat (kg) | 0.019 (0.016, 0.023)e | 0.020 (0.016, 0.023)e | 0.020 (0.016, 0.023)e |
| Caloric intake (kcal) | 0.000 (0.000, 0.000) | 0.000 (0.000, 0.000) | 0.000 (0.000, 0.000) |
| Cortisolb | 0.021 (0.010, 0.032)e | 0.019 (0.008, 0.030)e | |
| % Added Sugarb | 0.000 (−0.006, 0.007) | 0.000 (−0.006, 0.006) | |
| Cortisolc x % Added Sugarc | 0.002 (0.000, 0.003)d | ||
| R2 Total | 0.498 | 0.539 | 0.553 |
| Δ R2 | 0.498e | 0.040d | 0.014d |
- Model 1 includes age, sex, race, total body fat and energy intake; model 2 includes age, sex, race, total body fat, energy intake, serum cortisol and % added sugar; model 3 includes age, sex, race, total body fat, energy intake, serum cortisol, % added sugar, and the interaction of serum cortisol and % added sugar.
- a Male is the comparison group.
- b African-American is the comparison group.
- c Standardized (centered on mean).
- d P < 0.05.
- e P ≤ 0.01.
No other dietary variables showed any significant interactive or independent associations with VAT. While omega-3 FA did not significantly interact with cortisol (Pinteraction=0.12) as hypothesized, when stratified by mean intake of omega-3 FA, omega-3 FA seemed to mitigate the relationship between cortisol and VAT, such that only in participants with low omega-3 FA intake (below mean value) did cortisol positively associate with VAT ((ß = 0.031 P < 0.01). Furthermore, there were no significant interactions between race or sex with any of the dietary variables or cortisol in any of the models tested. While 55% of our sample (50% male and 57% female) was classified as LER the inclusion of LER as a factor in the regression models did not significantly alter our analyses.
Discussion
As hypothesized, cortisol was significantly associated with VAT in those individuals consuming high levels of dietary total or added sugar, and showed no relationship in those consuming low levels of dietary sugar. No other dietary variable showed any significant interactive or independent associations with cortisol upon VAT. Furthermore, while no dietary variable directly associated with VAT, examining the interactions between dietary variables and cortisol upon VAT elucidated the potential modifying effect of diet.
Independently, serum cortisol levels and reported stress show mixed associations with weight and body composition in humans (15, 16); this could be because of the modifying role that dietary intake can serve, as seen in this study. Furthermore, the highest concentration of cortisol receptors in adipose depots is in VAT (6, 29), and the measurement of whole body weight and composition commonly used in previous studies may be too insensitive to capture this potential depot specific effect. This was seen in our study, where serum cortisol significantly associated with VAT, but not with total body weight, BMI, total body fat, SAT, or waist circumference.
To the best of our knowledge no other human study to date has looked at the modifying effect of diet and serum cortisol upon VAT; however, animal studies have laid the groundwork for this interaction. Rodent models develop striking abdominal obesity and metabolic dysregulation when they are chronically stressed in combination with a high-fat/high-sugar diet; furthermore visceral fat increases by 50% in just two weeks time with the double exposure of chronic stress and high-fat/high-sugar diet (17). In our study there was an interaction between cortisol and sugar, while there was none with fat intake, or combined fat and sugar intake. In the above rodent model, one cannot disentangle the effect of high fat versus high sugar diet as the two were given in combination, however, human studies have shown that sugar intake increases cortisol release, whereas this is not seen in response to fat or protein intake (30, 31). In addition, sugar intake has been shown to alter cortisol metabolism and increase its exposure at the tissue level through the enzyme 11βHSD1, potentially accounting for this depot and diet specific interaction (32). Furthermore, we have previously shown that amongst minority at-risk youth, sugar intake, rather than fat or other macronutrients, negatively predicts insulin action, sensitivity, and beta-cell function (33, 34). While there are data demonstrating the negative effects of added sugars upon metabolic health, beyond those of general caloric consumption, the co-association between sugar intake and total caloric consumption was accounted for in our study; total kilocalories was a covariate in models with grams of sugar intake, and sugar intake as a percentage of kcals was also utilized. Cortisol is hypothesized to cause adipose hypertrophy as long as there is a background of over-nutrition (17), however, our data argues that the effect may be specific to macronutrient quality, particularly sugar intake, rather than just general over-nutrition or elevated caloric intake. Furthermore, increased sugar consumption in the last few decades has been driven by increasing intake of high fructose corn syrup (35), those exposing consumers to more fructose, a monosaccharide with established metabolic risks (36).
Neither dietary fat intake nor omega-3 FA a type of fat known for its protective cardiometabolic affects (37), significantly interacted with cortisol to predict accumulation of VAT, as hypothesized. Beyond the established cardiometabolic effects, omega-3 treatment has been shown in rats to decrease visceral fat (18), and in humans omega-3 FA treatment has corrected insulin resistance induced by dexamethasone (a potent pharmacological form of cortisol) (19) and decreased inflammatory pathways (TNF-α and NF-κB) (38), which are known up-regulators of the cortisol activating enzyme, 11-βHSD1. Whereas we did not find a significant interaction between omega-3 FA and cortisol, the stratified results hinted at a protective relationship that may be explored by future investigators.
There are several limitations of this study that should be noted. The cross-sectional nature of this study limits any cause-and-effect assumptions concerning diet, cortisol, and fat deposition. It is always possible that obese youth with high cortisol levels and VAT prefer high sugar intake or that those with higher intakes of sugar and therefore obesity make more cortisol; however, animal model work has demonstrated causal associations in the study's hypothesized directions. The current study is limited by the use of two 24-h diet recalls [84% of participants collected at least 1 weekend recall and this factor did not affect analyses (data not shown)]; diet recalls rely solely on the participants' self-reporting and are often prone to errors; however, many steps were taken to limit these effects including the exclusion of subjects with implausible intake data and checking for the confounding effect of LER. Despite these limitations, diet recalls are still the most widely used and validated measures of dietary intake available. Furthermore, the use of a single serum measurement of cortisol is another limitation because of the known diurnal variation of this hormone; however, the standardized setting, time of awakening, and time of blood draw mitigates this limitation. Although imperfect as a measure of HPA activity, a single morning serum cortisol may still predict metabolic disease risk, as higher morning serum cortisol is associated with the presence of metabolic syndrome cross-sectionally (39) and with deterioration of insulin sensitivity over time (40) in our study population. Total cortisol was measured in this study and future studies should concurrently measure cortisol binding globulin to quantify the levels of free cortisol. While the novel diet interactions with cortisol are significant, this effect is rather modest, accounting for only 8.4% of the variance of VAT beyond standard predictors. This may be because of the homogenous overweight population used for this study. Seventy-nine percent of the participants consumed greater than 10% WHO recommendation of added sugar in their diet. Whereas this homogenous population choice is effective in limiting confounding variables, it also decreases variation, and thus has the potential to limit study power, and it is possible that a stronger relationship may have been seen with a wider range of weight status and dietary intake. In addition, the focus on the development of risk factors in overweight minority adolescents is important, because of their disproportionate risk for obesity-related diseases, however, this may limit the generalizability of the results. Future studies need to be conducted assessing these relationships in broader participant populations with larger sample size and provide independent replication. Despite these limitations, this is the first human study to investigate the how dietary intake may interact with the role cortisol may play upon abdominal obesity development. A recent large scale prospective cohort study showed that VAT accumulation, independent of total obesity was the strongest significant predictor of type 2 diabetes development (4), emphasizing the importance of studies that elucidate potential mechanisms for VAT development, particularly in minority youth.
In conclusion, this study highlights the potential importance of macronutrient quality, such that cortisol is significantly associated with elevated VAT under conditions of high dietary sugar intake. Dietary sugar may play a crucial role in moderating the effects of cortisol, and future intervention programs that target stress reduction in at-risk youth should study the impact of dietary education and modification techniques.
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the sponsoring agencies. We would like to thank all the Childhood Obesity Research Core (CORC) research team, as well as the nursing staff at the clinical trials unit. In addition, we are grateful for our study participants and their families for their involvement. The results of the present study do not constitute endorsement by Obesity Research.





