Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner
Funding agencies: This work was supported by NIH DK084310, U01 DK08505 (RK), and Ethicon Endo-Surgery (RJS, RK), and NIH P30 DK078392.
Conflicts of Interest: RJS receives research support from Ablaris, Johnson and Johnson, Novo Nordisk, and Pfiezer, is a paid speaker for Johnson and Johnson, Merck, Novo Nordisk, and Pfizer, serves as a consultant for Angiogen, Eli Lilly, Johnson and Johnson, Novartis, Novo Nordisk, Takeda and Zafgen, and has equity in Zafgen. RK receives research support from Johnson and Johnson. The other coauthors have no disclosures.
Author contributions: AM, MK, and RK conceived and carried out experiments; KKR, KDRS, BA, and RJS conceived experiments and analyzed data. WJ, PJ, and PJD carried out experiments. All authors were involved in writing the paper and had final approval of the submitted and published versions.
Abstract
Objective
Our objective was to investigate the role of bile acids in hepatic steatosis reduction after vertical sleeve gastrectomy (VSG).
Design and Methods
High fat diet (HFD)-induced obese C57Bl/6 mice were randomized to VSG, Sham operation (Sham), Sham operation with pair feeding to VSG (Sham-PF), or nonsurgical controls (Naïve). All mice were on HFD until sacrifice. Mice were observed postsurgery and data for body weight, body composition, metabolic parameters, serum bile acid level and composition were collected. Further hepatic gene expression by mRNA-seq and RT-PCR analysis was assessed.
Results
VSG and Sham-PF mice lost equal weight postsurgery while VSG mice had the lowest hepatic triglyceride content at sacrifice. The VSG mice had elevated serum bile acid levels that positively correlated with maximal weight loss. Serum bile composition in the VSG group had increased cholic and tauroursodeoxycholic acid. These bile acid composition changes in VSG mice explained observed downregulation of hepatic lipogenic and bile acid synthetic genes.
Conclusion
VSG in obese mice results in greater hepatic steatosis reduction than seen with caloric restriction alone. VSG surgery increases serum bile acids that correlate with weight lost postsurgery and changes serum bile composition that could explain suppression of hepatic genes responsible for lipogenesis.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in the developed world today. Hepatic steatosis or liver lipid accumulation defines this disease and is a precursor to its more severe manifestation; nonalcoholic steatohepatitis (NASH) (1). Obesity is the most common cause for hepatic steatosis and the rise in obesity has resulted in sharply increasing rates of NAFLD (2). These alarming trends in obesity and NAFLD rates have led to the prediction that NASH will soon be the foremost indication for adult liver transplantation in the United States. Bariatric surgery is an important option to stem this tide as it achieves greater and more durable weight loss as compared to life style change or drug therapy (3).
The most widely used bariatric surgeries are Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy (VSG) (4, 5). While, the weight loss achieved by bariatric surgery has a profound metabolic impact, we recognize that the benefits of bariatric procedures go beyond their ability to produce weight loss (6). These bariatric procedures are now recognized to alter critical signaling and metabolic pathways to improve type 2 diabetes and dyslipidemia, even before significant weight loss occurs (7, 8). Specifically for NAFLD, VSG results in a significant improvement of liver enzymes and liver triglyceride levels (9). Given the potent effect of weight loss to improve liver function, the critical question is whether the effect of VSG on NAFLD improvement is beyond what results from weight loss and if so, what is the mechanism by which VSG could influence liver steatosis and metabolism.
Recently, patients undergoing bariatric procedures were reported to have increased serum bile acids (BA) postsurgery (10). In addition to BA's key role in lipid absorption, there is a growing body of evidence that points to BA as signaling molecules that act on both the nuclear farnesoid X receptor (FXR), and the cell-surface G protein coupled receptor TGR5 (11). Further, cholic acid administration lowers hepatic triglyceride content via the FXR, small heterodimer partner (SHP) and sterol regulatory element-binding transcription factor 1 (Srebp-1c) pathway (12). We hypothesized that mice after VSG surgery would have improvement in hepatic steatosis that would be signaled in part by increased serum BA. To test this hypothesis we performed VSG on diet-induced obese mice and measured BA levels and composition along with assessing their impact on hepatic gene targets. We present novel data wherein VSG was associated with changes in BA composition and metabolism that point to a critical role for altered BA metabolism in the NAFLD improvement seen after VSG surgery that is beyond what can be explained by the observed body weight loss alone.
Methods
Animals and Diet
Animal studies were approved by the Institutional Animal Care and Use Committee at Cincinnati Children's Hospital Medical Center. Eight-week-old male C57Bl/6 mice (Jackson laboratory, Bar Harbor, ME) were housed at 22 ± 2°C on a 12-hour light cycle. All animals received 60 kcal% HFD (Research Diets, New Brunswick, NJ) for 6 weeks prior to surgery.
Surgery and Postoperative Care
Surgeries were carried out under isoflurane anesthesia and buprenorphine analgesia. VSG: Briefly, after the incision of the abdominal wall, stomach was exposed and transected with Endopath™ Linear Cutter (Ethicon Endo-Surgery, Guaynabo, PR) with complete removal of fundus and greater curvature (Figure 1A). In our first experiment, only Sham and VSG groups were used.
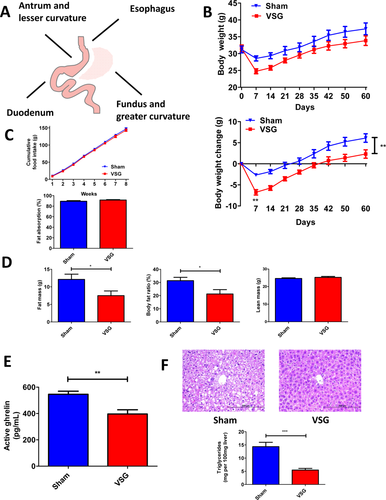
A. Illustration of completed vertical sleeve gastrectomy. B. Absolute body weight and body weight change over the 60 days postsurgery period. The difference in body weight loss between VSG and Sham mice was observed as early as 4 days postsurgery with more profound loss in the VSG group. By the end of the experiment Sham mice gained much more weight compared to VSG mice. N: Sham = 9; VSG = 9. **P < 0.01; two-way ANOVA. C. Cumulative food intake and intestinal fat absorption. Except first few days postsurgery no significant difference was observed in cumulative food intake between VSG and Sham mice. N: Sham = 9; VSG = 9; two-way ANOVA. Intestinal fat absorption was not different between two groups. N: Sham = 9; VSG = 9. t-test. D. Body fat mass, fat ratio, and lean mass 49 days postsurgery. Sham mice contained much more body fat compared to VSG mice. However, no difference was observed in lean mass between VSG and Sham groups. N: Sham = 9; VSG = 9. *P < 0.05; t-test. E. Plasma active ghrelin levels 14 days postsurgery. Sham mice had much higher plasma active ghrelin levels than mice after VSG. N: Sham = 8; VSG = 8. **P < 0.01; t-test. F. Hematoxylin and eosin staining of the liver and hepatic triglyceride content at day 60 postsurgery. Representative 20× magnification liver sections show no pathological changes in the liver of mouse which underwent VSG, while multiple cytoplasmic lipid droplet accumulation is visible in case of Sham mouse liver. VSG mice showed significantly lower hepatic triglyceride levels compared to Sham mice. N: Sham = 9; VSG = 9. ***P < 0.0001; t-test. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Our second experiment had four groups: Nonsurgery controls (Naïve); Sham operation (Sham) involving ligament dissection and pressure application on stomach; Sham pair-fed (Sham-PF) where mice received a diet equal to the average day prior food intake of VSG group. All mice were on a HFD except for a 4-day perioperative period wherein mice were provided a liquid diet Osmolite (Abbott Laboratories, Columbus, OH). Fasting serum was collected at 7 days presurgery and 14, 28, 42, and 56 days postsurgery. To account for known natural diurnal variations in serum BA levels, samples were collected at 1:00 p.m. after 4 hours of fasting period each time.
Similarly, all mice were sacrificed and samples collected in a postprandial state between 10:00 a.m. and 2:00 p.m. at the end of each experiment. Groups were staggered while samples were accrued in both fasting and postprandial collections. Mice were fasted overnight and then 1 hour before sacrifice 0.5 ml of Osmolite was administered by oral gavage. Liver samples were snap-frozen in liquid nitrogen, immersed in Tissue-Tek® OCT compound (Electron Microscopy Sciences, Fort Washington, PA), and fixed in formalin. Other data from this second cohort of animals (not included in this publication) is included in another manuscript under consideration.
Body Composition Analysis
Lean and fat mass of Sham and VSG mice was assessed at 49 days postsurgery by Echo MRI Whole Body Composition Analyzer (Echo Medical Systems, Houston, TX).
Fat Absorption Quantification
To assess intestinal fat absorption mice from our first experiment were fed for 4 days with a diet containing behenic acid at week 3 postsurgery. At day 3 after the introduction of this diet all mice were transferred to clean cages. Five stool samples from each mouse of Sham and VSG groups were collected in the morning of the following day into the eppendorf tubes. Samples were stored at −20°C until behenic acid quantification was performed as previously described (13).
Active Ghrelin Quantification
Blood was collected 21 days postsurgery in ad libitum fed mice 2 hours after the beginning of the dark cycle. Approximately 500 µl was collected into the tubes, which contained 200 mM AEBSF and 0.5 mg EDTA. 1N chloric acid was added immediately. Active ghrelin was quantified by the Rat/Mouse Ghrelin (Active) ELISA Kit (Millipore, Billerica, MA).
Glucose and Insulin Quantification
Fasting samples collected at day 56 postsurgery were utilized. One Touch Glucometer (LifeScan, Milpitas, CA) for glucose and ELISA Kit (Crystal Chem Inc., Downers Grove, IL) for insulin levels were used.
Hepatic Triglyceride, Alanine Aminotransferase, Cholesterol, Bilirubin, BA Quantification, and BA Composition Analyses
TG quantification: 100 mg of liver was homogenized in 1 ml of 20 mM Tris buffer and analyzed by Triglycerides Reagent Set (Pointe Scientific, Canton, MI). Plasma ALT: Kinetic absorbance was measured at 340 nm using Discret Pak™ ALT Reagent Kit (Catachem, Bridgeport, CT). Plasma cholesterol: Quantification Kit (Millipore, Calbiochem, Billerica, MA). Serum bilirubin: Total Bilirubin Reagent Set (Pointe Scientific, Canton, MI). BA were measured in serum by Total Bile Acids Assay Kit (Bio-Quant, San Diego, CA). Serum BA composition was assayed using electro-spray ionization liquid chromatography mass spectrometry ESI-LC-MS as previously described (14).
Histology
H&E stained and frozen sections were prepared by the Pathology Core Services. Frozen sections were stained for neutral fat using Oil Red O kit (American MasterTech, Lodi, CA). Images taken using Olympus BX51TF microscope (Olympus Corporation, Tokyo, Japan).
Next Gen mRNA-seq Assay
RNA was prepared out of snap-frozen liver tissues according to the TRIzol reagent protocol (Molecular Research Center, Cincinnati, OH). RNA extracted from the VSG and Sham-PF samples (n = 5 each group) was utilized to examine hepatic transcriptome using Next Gen mRNA-seq assay in the Gene Sequencing and Expression Core. RNA expression results were normalized and analyzed to identify genes that were differentially expressed in VSG group (t-test, >2 fold change at P < 0.05; FDR 0.05).
qPCR Gene Expression Evaluation
TaqMan™ Reverse Transcription kit and protocol were used for cDNA preparation (Applied Byosystems, Carlsbad, CA). Reaction was processed in an Eppendorf Mastercycler (Eppendorf, Hamburg, Germany). mRNA expression was evaluated by the FAM real-time kinetic PCR on a Stratagene Mx-3005 Multiplex Quantitative PCR Machine (Stratagene, Agilent Technologies, La Jolla, CA). All results were normalized to a standard ribosomal 18S gene. TaqMan™ primers of all genes were utilized (Applied Biosystems, Carlsbad, CA). Standard curve method was used to calculate relative expression.
Statistical Analysis
All values are expressed as mean ± SEM. Statistical significance was evaluated by one- or two-way ANOVA and, were indicated, by Student's t-test in two groups. Pearson's analysis was utilized for the correlation evaluation. P values less than 0.05 were considered to be significant. BA composition data was not normally distributed and was therefore expressed as log transformed.
Results
Sham and VSG surgery were performed on HFD-induced obese mice. VSG lost more weight during the first week postsurgery (−6.77 ± 0.64 g) compared to the Sham (−2.662 ± 0.34 g) and despite having ad libitum access to a HFD, VSG gained less weight overall postsurgery (2.318 ± 0.99 g vs. 6.11 ± 1.01 g) (Figure 1B). There was no difference in cumulative food intake and intestinal fat absorption between the groups (Figure 1C). Total body fat mass was lower in VSG (7.5 ± 1.34 g) compared to the Sham (12.12 ± 1.49 g). There was no difference in lean mass between the groups (Figure 1D). Plasma active ghrelin was lower in the VSG compared to Sham (396.3 ± 32.17 pg/ml vs. 545.9 ± 23.49 pg/ml) (Figure 1E). Liver histology and quantitative hepatic triglyceride analysis showed reduced lipid accumulation in VSG (5.47 ± 0.63 mg per 100 mg wet liver) compared to Sham mice (14.34 ± 1.66 mg) (Figure 1F).
In the next experiment, HFD-induced obese mice (32-35 g) were randomized into four groups; VSG, Sham, Sham-PF, and Naïve. VSG lost the most weight during the first week postsurgery (9.95 ± 2.6 g), compared to Naïve (0.14 ± 0.9 g), Sham (5.22 ± 1.8 g), and Sham-PF (5.06 ± 1.7 g) (Figure 2A). Beyond the first week, VSG consumed the same amount of food as Naïve and Sham (Figure 2B). Postoperatively all groups gained weight on a HFD but VSG never reached their baseline. At sacrifice body weights of Sham were not different from Naïve and similarly Sham-PF were not different from the VSG (Figure 2A). Fasting glucose was lower in VSG and Sham-PF (162.9 ± 4.5 mg/dl and 159.3 ± 5.61 mg/dl) compared to Naïve and Sham (191.7 ± 5.3 mg/dl and 185.3 ± 7.05 mg/dl, respectively). Fasting insulin was lowest in VSG (0.56 ± 0.05 ng/ml) compared to Naïve (2.46 ± 0.49 ng/ml), Sham (1.57 ± 0.29 ng/ml), and Sham-PF (0.95 ± 0.13 ng/ml) prior to sacrifice. HOMA-IR was the lowest in the VSG (0.23 ± 0.02) (Figure 2C). At day 14 postsurgery serum cholesterol was lower in VSG (6.77 ± 3.65 mg/dl) compared to Naïve and Sham-PF (46.67 ± 3.33 mg/dl and 17.34 ± 6.52 mg/dl, respectively) (Figure 2D). Also at 14 days ALT was lowest in VSG mice (36.58 ± 2.71 IU/l) compared to Naïve (73.15 ± 17.17 IU/l), Sham (66.26 ± 1.99 IU/l), and Sham-PF (47.83 ± 2.945 IU/l) (Figure 2E). Oil Red O staining was least in VSG compared to Naïve, Sham, and Sham-PF (Figure 2F). Liver triglycerides were lower in VSG (4.39 ± 0.55 mg per 100 mg wet liver) compared to Naïve (12.28 ± 1.72 mg), Sham (10.44 ± 1.1 mg), and Sham-PF (9.06 ± 0.79 mg) (Figure 2F). Thus, VSG mice were metabolically improved after surgery compared to all three control groups.
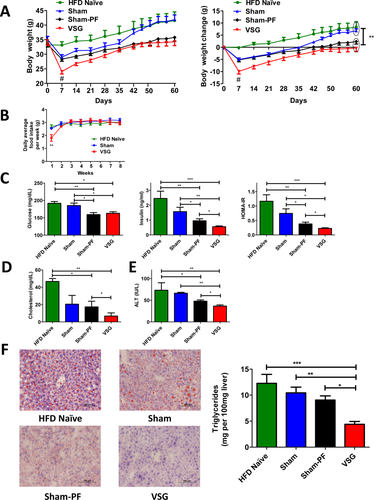
A. Absolute body weight and body weight change over the 60 days postsurgery period in four groups. VSG mice lost the most body weight compared to HFD Naïve, Sham, and Sham-PF mice at day 7 postsurgery. However, no significant difference in body weight gain was observed between VSG and Sham-PF groups at day 60 postsurgery. Both these groups gained much less weight compared to HFD Naïve and Sham groups by the end of the study and were significantly lighter. N: HFD Naïve = 6; Sham = 7; Sham-PF = 6; VSG = 6. #P < 0.01; VSG vs. HFD Naïve, Sham, and Sham-PF; two-way ANOVA; **P < 0.01 VSG and Sham-PF vs. HFD Naïve and Sham; two-way ANOVA. B. Average daily food intake per week in HFD Naïve, Sham, and VSG groups. Except for the first week postsurgery no significant difference was observed in food intake between HFD Naïve, Sham, and VSG mice. N: HFD Naïve = 6; Sham = 7; VSG = 6. **P < 0.01; two-way ANOVA. C. Fasting serum glucose and insulin levels, and HOMA-IR 56 days postsurgery. Serum glucose levels were lower in the VSG group compared to HFD Naïve and Sham groups, and serum insulin levels and HOMA-IR were the lowest in the VSG group compared to other three groups. N: HFD Naïve = 6; Sham = 7; Sham-PF = 6; VSG = 6. *P < 0.05; **P < 0.01; ***P < 0.0001; one-way ANOVA; and t-test. D. Serum cholesterol levels at day 14 postsurgery. Fasting serum cholesterol levels were significantly lower in VSG compared to HFD Naïve and Sham-PF groups. N: HFD Naïve = 3; Sham = 4; Sham-PF = 6; VSG = 6. *P < 0.05; **P < 0.01; one-way ANOVA; and t-test. E. Serum ALT levels at day 14 postsurgery. ALT levels were the lowest in the VSG group compared to HFD Naïve, Sham, and Sham-PF groups. N: HFD Naïve = 3; Sham = 4; Sham-PF = 6; VSG = 6. *P < 0.05; **P < 0.01; one-way ANOVA; t-test. F. Liver histology and hepatic triglyceride content 60 days postsurgery in four groups. Representative 20× magnification Oil Red O stained frozen sections show very slight neutral lipid accumulation in the livers of VSG mice compared to the mice of other groups, pointing that liver steatosis was markedly reduced in the VSG group. Liver triglyceride levels were the lowest in the VSG group compared to HFD Naïve, Sham, and Sham-PF groups. N: HFD Naïve = 6; Sham = 7; Sham-PF = 6; VSG = 6. *P < 0.05; **P < 0.01; ***P < 0.001; one-way ANOVA. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Next, we evaluated the role of BA, and fasting serum BA levels were the highest at 14 and 28 days postsurgery in the VSG compared to Naïve, Sham, and Sham-PF groups (Figure 3A and C). These increased BA were not associated with biliary tract obstruction given that total bilirubin levels were not different between the groups (Figure 3B). Fasting serum BA levels were no longer higher in the VSG group at day 42 postsurgery, however, postprandial BA levels remained higher in VSG mice even at sacrifice 60 days postsurgery (Figure 3D). Further, a correlation was observed between fasting serum BA on day 14, postprandial serum BA on day 60, and maximal weight loss postsurgery in the VSG group (R2 = 0.83; P = 0.021 and R2 = 0.86; P = 0.008, respectively) (Figure 3E). This correlation was absent in other groups (data not shown).
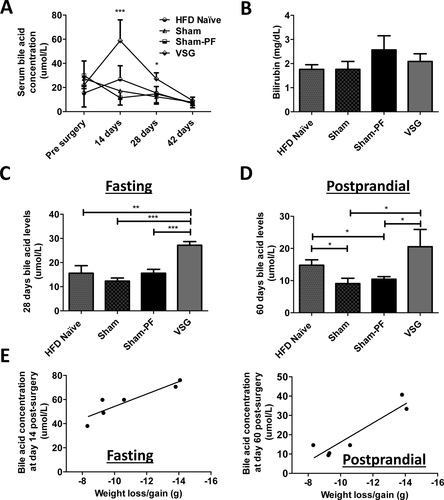
A. Fasting serum total bile acid levels presurgery, at day 14, 28, and 42 postsurgery. Fasting serum bile acid levels were the highest in VSG group at day 14 and 28 postsurgery compared to all other groups, while there was no difference in bile acid levels between the groups before surgery and at day 42 postsurgery. N: HFD Naïve = 6; Sham = 7; Sham-PF = 6; VSG = 6. *P < 0.05; ***P < 0.001; one-way ANOVA. B. Serum total bilirubin levels. There was no difference in serum total bilirubin levels between four groups at day 60 postsurgery. N: HFD Naïve = 6; Sham = 7; Sham-PF = 6; VSG = 6. C. Fasting serum total bile acid levels at day 28 postsurgery. Fasting serum bile acid levels were the highest in the VSG group compared to all other groups at 28 days postsurgery. N: HFD Naïve = 6; Sham = 7; Sham-PF = 6; VSG = 6. **P < 0.01; ***P < 0.0001; one-way ANOVA. D. Postprandial serum total bile acid levels at day 60 postsurgery. Postprandial serum bile acid levels were higher in VSG mice compared to Sham and Sham-PF mice at time of sacrifice. N: HFD Naïve = 6; Sham = 7; Sham-PF = 6; VSG = 6. *P < 0.05; one-way ANOVA; t-test. E. Serum bile acid levels correlation with maximal body weight loss after VSG. A strong correlation was seen between fasting total serum bile acid levels at day 14, postprandial total serum bile acid levels at day 60 after VSG with maximal weight loss postsurgery. N = 6. R2 = 0.83; P = 0.021 and R2 = 0.86; P = 0.008, respectively. Pearson correlation analysis.
To further understand changes in BA physiology in VSG mice we assessed serum BA composition (Figure 4A). VSG mice had significantly higher serum cholic acid levels compared to Sham-PF (2.33 ± 0.18 vs. 1.57 ± 0.22). Among the taurine-conjugated BA, specifically, levels of tauroursodeoxycholicacid (TUDCA) were significantly elevated in the VSG mice (2.12 ± 0.11) compared to Naïve, Sham, and Sham-PF groups (1.62 ± 0.03, 1.53 ± 0.07, and 1.7 ± 0.05, respectively) (Figure 4B).
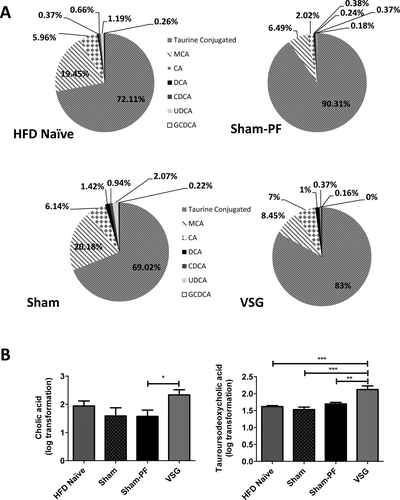
A. Serum bile acid composition analysis. Bile acid composition analysis showed that the majority of bile acids in all groups were taurine-conjugated. Proportion of taurine-conjugated acids was higher in Sham-PF and VSG groups, while Naïve and Sham groups had higher proportion of muricholic acid. Percentage of cholic acid was the highest in VSG mice. The following bile acids are also indicated: muricholic acid (MCA), cholic acid (CA), deoxycholate (DCA), chenodeoxycholate (CDCA), ursodeoxycholate (UDCA), and glycine-conjugated deoxycholate (GCDCA). N: HFD Naïve = 6; Sham = 7; Sham-PF = 6; VSG = 6. B. Cholic and tauroursodeoxycholic acid levels at day 60 postsurgery. Postprandial serum cholic acid levels were higher in VSG compared to Sham-PF mice, while levels of tauroursodeoxycholic acid were significantly elevated in the VSG compared to all other groups. N: HFD Naïve = 6; Sham = 7; Sham-PF = 6; VSG = 6. *P < 0.05; **P < 0.01; ***P < 0.001; t-test; one-way ANOVA.
Next, using mRNA-seq we identified 590 genes up or downregulated in VSG compared to Sham-PF mice at time of sacrifice (Figure 5A). BA metabolism was among the biological processes identified by this approach (Table 1). Specifically, several FXR target genes were identified, including cholic acid sensitive BA synthesis gene Cyp8b1. By RT-PCR analysis we confirmed that BA synthesis genes were downregulated in VSG mice at time of sacrifice compared to all three control groups; viz; Cyp7a1 (0.51 ± 0.19), Cyp8b1 (0.32 ± 0.09) (Figure 5B). Genes responsible for the transport of BA into hepatocytes were also suppressed in VSG group, including Na+-taurocholate cotransporting polypeptide (Ntcp) and organic anion transporting polypeptide 4 (Oatp4), (0.66 ± 0.09 and 0.75 ± 0.04, respectively) (Figure 5C). Fatty acid synthase (Fasn) mRNA relative expression was significantly lower in VSG (0.7915 ± 0.06) versus Sham-PF group (1.042 ± 0.14). The expression of gene responsible for the transport of long-chain fatty acids carnitine palmitoyltransferase 1 (Cpt1) was also the lowest in VSG mice (0.8 ± 0.05) (Figure 5D). Thus, both BA synthesis and lipogenesis genes were downregulated in the VSG group.
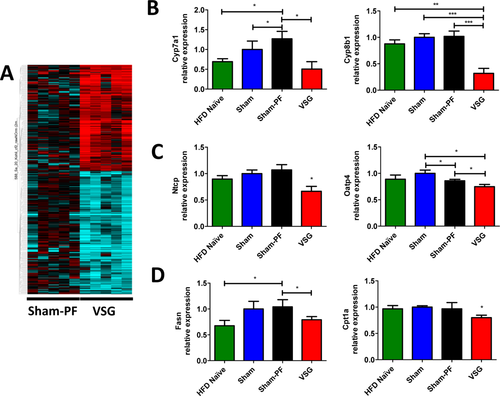
A. Next Gen mRNA-sequencing gene expression heat map of Sham-PF and VSG mice at day 60 postsurgery. Multiple genes responsible for bile acid synthesis were downregulated in the livers of VSG mice (blue rows) compared to Sham-PF mice at 60 days postsurgery. N: Sham-PF = 5; VSG = 5. B. Hepatic bile acid synthesis gene expression at day 60 postsurgery. mRNA levels of the genes coding for the bile acid production (Cyp7a1 and Cyp8b1) were measured by RT-PCR and expressed in relative expression units. These bile acid production rate limiting genes were downregulated in the VSG group compared to HFD Naïve, Sham, and Sham-PF groups. HFD Naïve = 4; Sham = 5; Sham-PF = 5; VSG = 5. *P < 0.05; **P < 0.01; ***P < 0.0001; one-way ANOVA; t-test. C. Hepatocyte bile acid uptake gene expression at day 60 postsurgery. mRNA levels of the genes coding for the bile acid import into the hepatocyte (Ntcp and Oatp4) were measured by RT-PCR and expressed in relative expression units. These hepatocyte bile acid uptake genes were downregulated in the VSG group compared to HFD Naïve, Sham, and Sham-PF groups. HFD Naïve = 4; Sham = 5; Sham-PF = 5; VSG = 5. *P < 0.05; one-way ANOVA; t-test. D. Hepatic lipid metabolism gene expression at day 60 postsurgery. mRNA levels of the genes coding for the lipid metabolism (Fasn and Cpt1a) were measured by RT-PCR and expressed in relative expression units. Fasn mRNA was decreased in VSG mice compared to Sham-PF mice. Cpt1a mRNA was the lowest in the VSG group compared to other groups. N: HFD Naïve = 4; Sham = 5; Sham-PF = 5; VSG = 5. *P < 0.05; t-test. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
| Gene abbreviation | Function | Fold expression |
|---|---|---|
| Atp8b1 | Protection from bile salt overexpression | 1.646 |
| Cyp51a1 | Cholesterol synthesis | 0.61 |
| Ugt3a2 | Bile acid glycosilation | 0.609 |
| Slco1a4 | Bile acid transmembrane import | 0.351 |
| Slco1a2 | Bile acid transmembrane import | 0.351 |
| Cyp3a11 | Cholesterol hydroxylation | 0.348 |
| Cyp8b1 | Cholic acid synthesis | 0.331 |
- In total, 590 genes were significantly overexpressed or downregulated in the VSG group compared to the Sham-PF group at 60 days postsurgery. Among these, there were several genes, which were taking part in bile acid metabolism.
- N: Sham-PF = 5; VSG = 5.
Discussion
The main endpoint of our study was the improvement in the hepatic steatosis after VSG surgery that could not be explained by weight loss alone. While VSG is often categorized as a “restrictive” procedure, a wide range of evidence points to the fact that the weight loss is not a direct product of physical restriction (15-17). This extends importantly to metabolic beneficial effects on the liver. VSG is able to reduce hepatic glucose production in a manner that is beyond what occurs with weight loss alone (8). Using appropriate calorie-restricted and weight-matched controls we observed that VSG surgery in obese mice resulted in sustained weight loss, and improvement in parameters of the metabolic syndrome, including a calorie-intake and weight-independent reduction of hepatic steatosis (see Figure 2).
The effects of VSG surgery on weight in our murine model are confounded by the fact that the mice continued to be on a 60 kcal% HFD postsurgery. This is unlike most clinical scenarios wherein patients are intensively counseled to alter both lifestyle and adopt healthy eating habits postsurgery. However, despite being on a 60 kcal% HFD these mice gained less weight than their Sham ad libitum controls (Figure 2A). Further, the VSG mice had improved metabolic outcomes when compared to the Sham-PF controls that had similar body weights. Thus, we speculate that the effect of murine VSG surgery on metabolic outcomes is caused by more than just the early weight lost immediately postsurgery.
Our hypothesis was that alterations in BA may provide an explanation for this phenomenon. We therefore studied changes in BA physiology post-VSG to understand the role of BA in this weight-independent reduction of hepatic steatosis. BA are produced in the liver and absorbed in the ileum where they can have important impacts on signaling hepatic lipogenesis and BA production (12, 18, 19). We and others have shown in recent clinical studies that serum BA are elevated after certain bariatric procedures (RYGB) but not in weight-matched controls or weight-loss matched patients from procedures such as adjustable gastric banding (10, 20-22). On the other hand, levels of several intestinal hormones and growth factors positively correlated with BA levels after RYGB, that is, adiponectin, glucagon-like peptide-1, and fibroblast growth factor 19 (21, 22). It may be speculated that these changes observed in BA enterohepatic circulation in RYGB patients are the result of significant surgical modifications in the upper gastrointestinal tract which result in a dramatic change in the location where BA and chyme mix. However, the same argument cannot be made for the VSG surgery.
Nevertheless, we present novel data in this report that demonstrate VSG resulting in increased serum levels of total BA with a particular increase in cholic acid and TUDCA (see Figure 4B). Recently patients undergoing bariatric procedures were reported to have increased serum BA postsurgery. One of these reports included a small subset of patients that underwent VSG surgery as well (10). It has further been shown that taurine-conjugated BA levels are decreased in obese patients; however, their postprandial levels are significantly increased after RYGB indicating that taurine-conjugated subset of BA may contribute to the improvement in meal-related physiology seen after this bariatric procedure (23). Haeusler et al. reported elevated liver and plasma triglyceride levels in FoxO1-deficient mice that were associated with a deficiency of 12α-hydroxylated cholic acid and its synthetic enzyme Cyp8b1. This triglyceride imbalance was ameliorated when mice received FXR agonist cholic acid in their diet (24). However, it had been shown that oral administration of TUDCA markedly improved hepatic steatosis in ob/ob mice by reducing expression of de novo lipogenesis genes (25). In our study, both cholic acid and TUDCA serum levels were higher in the VSG group. These data are consistent with the findings of Haeusler et al. and offer up a further explanation for the BA-driven improvement of hepatic steatosis seen in our VSG mice (Figure 6).
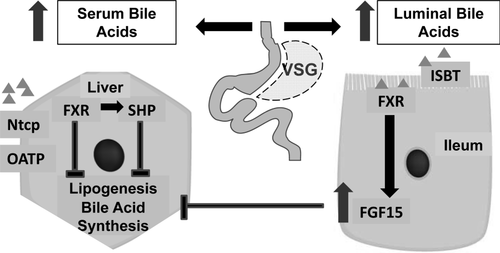
Scheme of bile acid driven signaling pathway in liver and ileum.
Bile acids are reabsorbed in the terminal ileum, affect transcription factor FXR in the enterocyte and as a result FGF15 secretion, which through the blood flow is able to suppress bile synthesis and lipid metabolism in the liver. On the other hand, reabsorbed bile acids are transported to the liver by the portal vein blood flow, where their hepatocyte uptake is regulated by the import genes (Ntcp and Oatps). Bile acids' interaction with FXR effects SHP transcription, which in turn inhibits bile and lipid synthesis in the liver. In mice after VSG serum bile acid levels were elevated, expression of bile acid production genes was decreased as a consequence of the bile acid synthesis negative feedback regulation, and lipid metabolism genes were downregulated in the liver which resulted in the reduction of hepatic steatosis.
BA also have the ability to suppress hepatic lipogenesis through activation of the FXR pathway. A clinical study reported that in NAFLD patient levels of Fasn and Srebp-1c were increased, while those of FXR were depleted (26). In a mouse study, it was shown that cholic acid could decrease liver triglycerides via activation of the FXR–SHP pathway (12). Given the BA elevation in our VSG mice we investigated and found that lipogenic genes, viz. Fasn and Cpt1a were downregulated in the VSG group compared to their weight and caloric intake-matched controls (see Figure 5D). We did not measure energy expenditure directly in our experiments but VSG mice had improved metabolic parameters including lower hepatic triglycerides, insulin, and HOMA-IR, compared to the Sham-PF mice. RYGB studies have shown a clear increase in energy expenditure though results from VSG studies to the same effect are lacking (27). The VSG mice in our study also had decreased food intake for the first week postsurgery when compared to other ad libitum fed groups but clearly there is need for additional experiments to explain these metabolic improvements in VSG mice compared to their pair-fed controls. These would include experiments directly assessing energy expenditure and also further dissecting the effects of BA on signaling through the TGR5 and FXR pathways.
The question of course remains as to how these changes in serum BA were brought about despite the lack of biliary obstruction or intestinal manipulation performed during surgery. In a recent study, Kang et al. reported that ghrelin-O-acyltransferase (GOAT) knockout mice exhibited elevated serum BA levels. GOAT is the activating enzyme for the orexigenic hormone ghrelin and GOAT knockout mice also had increased mRNA and protein expression of the ileal sodium-dependent bile acid transporter (ISBT) in the intestinal and biliary tract, therefore establishing a link between ghrelin/GOAT and BA reabsorption (28). Ghrelin is produced primarily in the gastric fundus (28), which is removed as part of the VSG surgery. We observed that active ghrelin levels were lower in VSG compared to Sham mice (Figure 1E). However, mRNA levels of ISBT were not different between these groups at time of sacrifice (data not shown). Such data point away from the hypothesis that the differences in BA after VSG are a result of altered intestinal reuptake.
Another potential explanation for the elevation in serum BA could be increased synthesis in the liver. Two key rate limiting enzymes for total BA are Cyp7a1 and Cyp8b1 (29, 30). Rather than being upregulated however, expressions of both these genes are reduced after VSG. Given that changes of gene expression did not support a role for either increased intestinal absorption or increased hepatic production of BA in the increased serum BA observed after VSG, we sought to see whether there were potential changes in hepatocyte uptake of BA. Higher BA lead to suppression of hepatocyte BA production (Cyp7a1 and Cyp8b1) but more importantly higher BA levels also suppress the liver cell BA uptake mechanisms (31-33). Ntcp is the major BA transporter into hepatocytes from serum (34), while Oatp4 along with other Oatp family members play important roles in this process as well (35). We found that liver expression levels of Ntcp and Oatp4 were downregulated in the VSG mice. These data point therefore to a feedback loop, which we understand to result in a higher steady state level of serum BA in VSG mice though certain elements of this loop will need further investigation including ileal secreted fibroblast growth factor (FGF) 15 (Figure 6).
Thus, in conclusion, bariatric surgery in general and VSG in particular, successfully treat obesity and improve a number of components of the metabolic syndrome including NAFLD. However, due to the invasiveness and potential complications of these procedures, there is an urgent need to identify the mechanisms behind their success. The key observation of the present work was that VSG produces a reduction in hepatic triglyceride levels to a degree that is considerably greater than that is achieved by weight loss via food restriction alone. Furthermore, changes in serum BA levels and composition in our VSG surgery murine model together suggest a putative role for specific BA and their downstream signaling in the hepatic improvements seen after VSG surgery. This work advances our long-term goal to study less invasive “bariatric-mimetic” technologies that would provide similar metabolic benefits to a much wider population of patients at a lower cost and with reduced risk of complications.





