Long-term treatment of older patients with overactive bladder using a combination of mirabegron and solifenacin: a prespecified analysis from the randomized, phase III SYNERGY II study
Abstract
Aims
SYNERGY II was a 12-month phase III trial in patients with overactive bladder (OAB) symptoms that investigated the safety and efficacy of the combination of mirabegron and solifenacin in comparison with each monotherapy. This analysis evaluated the trial findings using four age subgroups (<65, ≥65, <75, and ≥75 years).
Methods
Eligible patients were ≥18 years with symptoms of “wet” OAB (urinary frequency and urgency with incontinence) for ≥3 months. Patients were randomized to receive once-daily solifenacin succinate and mirabegron (5 mg/50 mg; combination), solifenacin succinate, or mirabegron (4:1:1). Safety evaluations: treatment-emergent adverse events (TEAEs), vital signs, and electrocardiogram, post-void residual volume, and laboratory assessments. Primary efficacy variables: change from baseline to end of treatment in number of incontinence episodes/24 h and micturitions/24 h.
Results
Of 1794 patients (full analysis set), 614 (34.2%) and 168 (9.4%) were ≥65 and ≥75 years old, respectively. Overall, 856 (47.2%) patients experienced ≥1 TEAE. Higher TEAE incidences were typically observed for the combination versus both monotherapies (eg, constipation) and in the older versus younger age groups (eg, urinary tract infection). Increases in mean pulse rate from baseline of >1 bpm were noted in the combination and mirabegron younger age groups only. No clinically significant findings were observed in the other safety parameters. The efficacy variables improved with all treatments and the greatest improvements were typically observed with combination therapy.
Conclusions
Mirabegron and solifenacin combination therapy was a well-tolerated and effective treatment for patients with OAB symptoms irrespective of their age.
1 INTRODUCTION
Overactive bladder (OAB) syndrome is diagnosed if patients experience urinary urgency, with or without urgency urinary incontinence, usually with nocturia and frequency, that is not due to an obvious pathogenic cause.1 Several studies have demonstrated that OAB symptoms increase with age. In a large population-based study, 3.4% of men and 8.7% women aged 40-44 years reported symptoms of OAB, which increased to 41.9% of men and 31.3% of women aged ≥75 years.2 Older individuals also frequently report symptoms of nocturia, with >80% of individuals aged 80-90 years having at least one void per night.3 As the proportion of older individuals in the global population is expected to rise,4 OAB is likely to place an increasing strain on healthcare resources.
Physiological changes in older individuals partially explain the association between increasing age and OAB. Changes in the urinary tract that occur with increasing age include decreased bladder capacity5 and sensation,6 impaired detrusor contractile strength,6 and increased residual volume.5 Alterations in brain activity may also be associated with incontinence severity and degree of bother.7
Treating older individuals with OAB can be a complicated process. The urinary symptoms experienced by patients impact quality of life8 and are associated with an increased risk of various conditions including falls and fractures.9, 10 The management of OAB is potentially complicated by the presence of baseline frailty and multiple comorbidities.11, 12 Older patients commonly receive treatment with several anticholinergic therapeutics13 and such polypharmacy is associated with an increased risk of cognitive decline.14 Additionally, older patients may be more likely than younger individuals to experience a poor treatment response15 and receive subsequent re-treatment.16 Therefore there is an unmet clinical need to identify therapeutics that are well tolerated and effective in older individuals.
Antimuscarinics, for example solifenacin, and mirabegron, a β3-adrenoreceptor agonist, constitute the principal pharmacotherapy approaches for treating OAB symptoms. Solifenacin and mirabegron combination therapy has been investigated in previous short-term studies. Several 12- to 16-week trials found that solifenacin and mirabegron combination therapy was associated with clinically relevant improvements in incontinence and micturition frequency.17-20 Combination therapy was well tolerated, with dry mouth and constipation among the most commonly reported treatment-emergent adverse events (TEAEs).17-19 The efficacy and safety of solifenacin and mirabegron has been previously investigated according to age (<65, ≥65, <75, and ≥75 years) in the 12-week phase III BESIDE study.18, 21 Combination therapy was associated with greater improvements in incontinence episodes and micturitions versus solifenacin monotherapy for all age groups.21 There were also no notable differences in vital signs or TEAE frequency between treatment groups, irrespective of age group.
To date, SYNERGY II is the only clinical trial to evaluate the long-term tolerability and effectiveness of mirabegron and solifenacin in combination. This study was a 12-month phase III trial involving patients with OAB symptoms that investigated the safety and efficacy of the combination of solifenacin and mirabegron (5 mg/day and 50 mg/day, respectively) versus each monotherapy.22 With combination therapy, statistically significant improvements in changes from baseline in mean number of incontinence episodes and micturitions were observed versus each monotherapy. Although all three regimens were well tolerated, a slightly higher frequency of TEAEs was reported with the combination. A prespecified analysis from SYNERGY II was to evaluate the safety and efficacy findings from the trial using four patient age subgroups (<65, ≥65, <75, and ≥75 years). The results of this analysis are presented herein.
2 MATERIALS AND METHODS
2.1 Study design
The methodology for this study has previously been reported.22 Briefly, patients aged ≥18 years with symptoms of “wet” OAB (urinary frequency and urgency with urgency urinary incontinence or mixed incontinence with urgency as the predominant factor) for ≥3 months were eligible for inclusion. Exclusions included clinically significant cardiovascular or cerebrovascular disease within 6 months prior to screening, severe hypertension, moderate/severe hepatic impairment, and severe renal impairment. Patients were predominately recruited from the short-term SYNERGY19 or BESIDE18 studies.
The study was comprised of a 2-week, single-blind, placebo run-in period (for washout of prior OAB medication); a 12-month, double-blind, randomized, active-controlled, treatment period; and a 2-week follow-up period. At the start of the treatment period, patients were randomized to receive a combination of once-daily solifenacin succinate 5 mg and mirabegron 50 mg, solifenacin succinate, or mirabegron (4:1:1 ratio). For this subgroup analysis, the safety and efficacy findings from the SYNERGY II study were stratified by age into four patient subgroups (<65, ≥65, <75, and ≥75 years).
2.2 Study assessments
Safety evaluations (primary objective) involved assessing TEAE frequency throughout the treatment period, which included TEAEs that are relevant to older patients (eg, urinary tract infections [UTIs], hypertension, tachycardia). Vital sign, electrocardiogram (ECG), post-void residual (PVR) volume, and laboratory evaluations were performed at months 1, 3, 6, 9, and 12 and at follow-up. PVR volume was sorted into three categories (≥0-<150, ≥150-<300, and ≥300 mL). An increase was identified if PVR volume escalated by ≥1 category from baseline.
To acquire efficacy data (secondary objective), a validated electronic device was used by the patients to complete a micturition (bladder) eDiary for 7 days (3 days for volume voided) prior to each visit. The primary efficacy variables were change from baseline to end of treatment (EoT) in mean number of incontinence episodes/24 h and micturitions/24 h. Secondary efficacy variables were change from baseline to EoT in mean volume voided (MVV) per micturition, overactive bladder questionnaire (OAB-q) Symptom Bother score, and treatment satisfaction-visual analog scale (TS-VAS) score. Apart from MVV per micturition (months 3, 6, and 12 only), efficacy assessments were completed at months 1, 3, 6, 9, and 12.
2.3 Statistical analyses
SAS® version 9.3 or higher (SAS, Cary, NC) was used to perform the statistical analyses. Sample size calculations were based on the probability of observing at least one case of a TEAE in all of the treatment groups. Randomization was stratified by sex, age group (<65, ≥65 years), and geographic region. Further details about the sample size calculations and the randomization process have been published previously.22
For the efficacy analyses, the least-squares mean estimates and two-sided 95% confidence intervals (CIs) for changes from baseline within each treatment group and subgroup were estimated using an analysis of covariance (ANCOVA) model with treatment group, age group (<65, ≥65 years or <75, ≥75 years), sex, previous study history, geographic region, and interaction between age group and treatment group as fixed factors and baseline value as a covariate. A similar ANCOVA model was also used to analyze the vital sign results. No P values for differences between treatment groups were calculated for this subgroup analysis.
3 RESULTS
3.1 Study population
Of the 1794 patients included in the full analysis set, 614 (34.2%) patients were ≥65 years old and 168 (9.4%) were ≥75 years old (Supplementary Table S1). Demographics and baseline characteristics were generally similar across age groups, except that patients from the older age groups typically had a longer disease duration and a greater number of urgency urinary incontinence episodes/24 h (Table 1). In addition, the older age groups were generally comprised of higher proportions of males and patients who had received previous OAB medications. In total, 1441 (79.4%) of patients received at least one concomitant non-OAB medication during the treatment period.
| <65 years | ≥65 years | |||||
|---|---|---|---|---|---|---|
| Parameter | Mirabegron 50 mg (n = 200) | Combination 5 + 50 mg (n = 784) | Solifenacin 5 mg (n = 196) | Mirabegron 50 mg (n = 102) | Combination 5 + 50 mg (n = 409) | Solifenacin 5 mg (n = 103) |
| Sex, n (%) | ||||||
| Male | 38 (19.0) | 142 (18.1) | 35 (17.9) | 25 (24.5) | 97 (23.7) | 23 (22.3) |
| Female | 162 (81.0) | 642 (81.9) | 161 (82.1) | 77 (75.5) | 312 (76.3) | 80 (77.7) |
| Age in years, median (range) | 55.0 (19–64) | 54.0 (20–64) | 56.0 (19–64) | 70.5 (65–83) | 70.0 (65–86) | 71.0 (65–86) |
| Race, n (%) | ||||||
| White | 172 (86.0) | 665 (84.8) | 171 (87.2) | 90 (88.2) | 377 (92.2) | 88 (85.4) |
| Black or African American | 5 (2.5) | 23 (2.9) | 4 (2.0) | 0 | 4 (1.0) | 0 |
| Asian | 20 (10.0) | 92 (11.7) | 20 (10.2) | 11 (10.8) | 26 (6.4) | 13 (12.6) |
| Other | 3 (1.5) | 4 (0.5) | 1 (0.5) | 1 (1.0) | 2 (0.5) | 2 (1.9) |
| BMI in kg/m2, mean (SD) | 28.23 (5.30) | 28.39 (6.08) | 28.24 (5.42) | 29.94 (6.33) | 29.03 (5.46) | 28.97 (4.90) |
| Geographic region, n (%) | ||||||
| North America | 39 (19.5) | 156 (19.9) | 38 (19.4) | 23 (22.5) | 94 (23.0) | 22 (21.4) |
| Latin America | 1 (0.5) | 2 (0.3) | 1 (0.5) | 0 | 2 (0.5) | 2 (1.9) |
| Western Europe | 22 (11.0) | 85 (10.8) | 23 (11.7) | 15 (14.7) | 62 (15.2) | 15 (14.6) |
| Eastern Europe | 114 (57.0) | 449 (57.3) | 112 (57.1) | 53 (52.0) | 208 (50.9) | 52 (50.5) |
| Asia | 16 (8.0) | 66 (8.4) | 16 (8.2) | 6 (5.9) | 25 (6.1) | 7 (6.8) |
| Southern Hemisphere | 8 (4.0) | 26 (3.3) | 6 (3.1) | 5 (4.9) | 18 (4.4) | 5 (4.9) |
| Type of OAB at screening, n (%) | ||||||
| Urgency urinary incontinence only | 138 (69.0) | 556 (70.9) | 144 (73.5) | 71 (69.6) | 293 (71.6) | 81 (78.6) |
| Mixed incontinence | 62 (31.0) | 228 (29.1) | 52 (26.5) | 31 (30.4) | 116 (28.4) | 22 (21.4) |
| Duration of “wet” OAB symptoms in months, mean (SD) | 68.8 (69.8) | 64.5 (72.7) | 72.4 (88.5) | 77.2 (84.7) | 89.2 (101.2) | 86.3 (109.9) |
| Received previous OAB medications, n (%)a | 103 (51.5) | 415 (52.9) | 107 (54.6) | 56 (54.9) | 240 (58.7) | 58 (56.3) |
| Received previous treatment with solifenacin, n (%)a,b | 56 (54.4) | 244 (58.8) | 45 (42.1) | 28 (50.0) | 133 (55.4) | 27 (46.6) |
| Received previous treatment with mirabegron, n (%)a,b | 10 (9.7) | 43 (10.4) | 6 (5.6) | 5 (8.9) | 28 (11.7) | 4 (6.9) |
| Seven-day micturition eDiary baseline characteristics | ||||||
| Incontinence episodes/24 h, mean (SD) | 2.93 (3.64) | 2.94 (3.35) | 3.14 (3.56) | 3.60 (3.38) | 3.22 (2.78) | 3.04 (3.63) |
| Micturitions/24 h, mean (SD) | 10.59 (2.62) | 10.65 (3.03) | 10.73 (2.77) | 10.36 (1.94) | 10.37 (2.03) | 10.80 (2.96) |
| MVV per micturition in mL, mean (SD) | 162.31 (60.05) | 159.00 (60.09) [n = 781] | 161.98 (58.67) | 158.44 (60.58) | 159.85 (55.10) | 158.26 (58.36) |
| Urgency urinary incontinence episodes/24 h, mean (SD)c | 2.67 (3.41) | 2.62 (2.84) [n = 779] | 2.90 (3.46) [n = 194] | 3.23 (3.03) | 2.98 (2.63) [n = 408] | 2.92 (3.57) |
| <75 years | ≥75 years | |||||
|---|---|---|---|---|---|---|
| Parameter | Mirabegron 50 mg (n = 273) | Combination 5+50 mg (n = 1089) | Solifenacin 5 mg (n = 264) | Mirabegron 50 mg (n = 29) | Combination 5+50 mg (n = 104) | Solifenacin 5 mg (n = 35) |
| Sex, n (%) | ||||||
| Male | 60 (22.0) | 213 (19.6) | 49 (18.6) | 3 (10.3) | 26 (25.0) | 9 (25.7) |
| Female | 213 (78.0) | 876 (80.4) | 215 (81.4) | 26 (89.7) | 78 (75.0) | 26 (74.3) |
| Age in years, median (range) | 60.0 (19–74) | 59.0 (20–74) | 59.0 (19–74) | 77.0 (75–83) | 78.0 (75–86) | 77.0 (75–86) |
| Race, n (%) | ||||||
| White | 235 (86.1) | 942 (86.5) | 227 (86.0) | 27 (93.1) | 100 (96.2) | 32 (91.4) |
| Black or African American | 5 (1.8) | 24 (2.2) | 4 (1.5) | 0 | 3 (2.9) | 0 |
| Asian | 29 (10.6) | 118 (10.8) | 31 (11.7) | 2 (6.9) | 0 | 2 (5.7) |
| Other | 4 (1.5) | 5 (0.5) | 2 (0.8) | 0 | 1 (1.0) | 1 (2.9) |
| BMI in kg/m2, mean (SD) | 28.74 (5.53) | 28.68 (6.00) | 28.55 (5.34) | 29.39 (7.34) | 27.82 (4.42) | 28.04 (4.55) |
| Geographic region, n (%) | ||||||
| North America | 57 (20.9) | 223 (20.5) | 51 (19.3) | 5 (17.2) | 27 (26.0) | 9 (25.7) |
| Latin America | 1 (0.4) | 3 (0.3) | 2 (0.8) | 0 | 1 (1.0) | 1 (2.9) |
| Western Europe | 32 (11.7) | 127 (11.7) | 33 (12.5) | 5 (17.2) | 20 (19.2) | 5 (14.3) |
| Eastern Europe | 152 (55.7) | 603 (55.4) | 146 (55.3) | 15 (51.7) | 54 (51.9) | 18 (51.4) |
| Asia | 21 (7.7) | 91 (8.4) | 21 (8.0) | 1 (3.4) | 0 | 2 (5.7) |
| Southern Hemisphere | 10 (3.7) | 42 (3.9) | 11 (4.2) | 3 (10.3) | 2 (1.9) | 0 |
| Type of OAB at screening, n (%) | ||||||
| Urgency urinary incontinence only | 191 (70.0) | 775 (71.2) | 195 (73.9) | 18 (62.1) | 74 (71.2) | 30 (85.7) |
| Mixed incontinence | 82 (30.0) | 314 (28.8) | 69 (26.1) | 11 (37.9) | 30 (28.8) | 5 (14.3) |
| Duration of “wet” OAB symptoms in months, mean (SD) | 70.5 (74.4) | 71.8 (83.9) | 75.6 (88.5) | 81.9 (83.2) | 85.1 (88.9) | 88.7 (144.1) |
| Received previous OAB medications, n (%)a | 142 (52.0) | 602 (55.3) | 145 (54.9) | 17 (58.6) | 53 (51.0) | 20 (57.1) |
| Received previous treatment with solifenacin, n (%)a, b | 78 (54.9) | 344 (57.1) | 60 (41.4) | 6 (35.3) | 33 (62.3) | 12 (60.0) |
| Received previous treatment with mirabegron, n (%)a, b | 14 (9.9) | 60 (10.0) | 8 (5.5) | 1 (5.9) | 11 (20.8) | 2 (10.0) |
| Seven-day micturition eDiary baseline characteristics | ||||||
| Incontinence episodes/24 h, mean (SD) | 3.06 (3.59) | 2.98 (3.20) | 3.06 (3.45) | 4.01 (3.19) | 3.61 (2.79) | 3.39 (4.47) |
| Micturitions/24 h, mean (SD) | 10.56 (2.44) | 10.59 (2.79) | 10.73 (2.65) | 10.11 (2.09) | 10.18 (2.03) | 10.92 (3.99) |
| MVV per micturition in mL, mean (SD) | 161.68 (61.32) | 159.87 (58.16) [n = 1086] | 162.21 (58.54) | 154.60 (48.26) | 153.31 (60.82) | 149.27 (57.65) |
| Urgency urinary incontinence episodes/24 h, mean (SD)c | 2.79 (3.34) | 2.70 (2.80) [n = 1083] | 2.85 (3.35) [n = 262] | 3.54 (2.77) | 3.16 (2.49) | 3.33 (4.46) |
- Data shown for the FAS. BMI, body mass index; FAS, full analysis set; MVV, mean volume voided; OAB, overactive bladder; SD, standard deviation
- a Previous OAB medication was defined as medication that was received prior to starting, or after the end, of the SYNERGY19 or BESIDE18 studies.
- b Percentages shown use the number of patients who had received previous OAB medications as the denominator.
- c Descriptive statistics were only calculated for patients with at least one urgency urinary incontinence episode at baseline.
3.2 Safety
Overall, 856 (47.2%) patients enrolled in this study experienced at least one TEAE.22 Regardless of age group, higher incidences of overall TEAEs were observed in the combination group versus both monotherapies (Table 2). Higher overall incidences of TEAEs were also typically observed in the older versus the younger age groups. When the TEAE results were stratified by sex, a slightly higher incidence was observed for females (701/1449 patients; 48.4%) versus males (155/365 patients; 42.5%).
| Patients, n/n (%) | ||||
|---|---|---|---|---|
| TEAE | Age group in years | Mirabegron 50 mg (n = 305) | Combination 5+50 mg (n = 1206) | Solifenacin 5 mg (n = 303) |
| Overall | <65 | 83/201 (41.3) | 359/792 (45.3) | 80/199 (40.2) |
| ≥65 | 43/104 (41.3) | 237/414 (57.2) | 54/104 (51.9) | |
| <75 | 111/276 (40.2) | 539/1100 (49.0) | 121/268 (45.1) | |
| ≥75 | 15/29 (51.7) | 57/106 (53.8) | 13/35 (37.1) | |
| TEAEs by PT (≥4.0% for any treatment and age group) | ||||
| Dry mouth | <65 | 7/201 (3.5) | 41/792 (5.2) | 7/199 (3.5) |
| ≥65 | 5/104 (4.8) | 33/414 (8.0) | 11/104 (10.6) | |
| <75 | 9/276 (3.3) | 65/1100 (5.9) | 16/268 (6.0) | |
| ≥75 | 3/29 (10.3) | 9/106 (8.5) | 2/35 (5.7) | |
| Nasopharyngitis | <65 | 9/201 (4.5) | 27/792 (3.4) | 11/199 (5.5) |
| ≥65 | 7/104 (6.7) | 16/414 (3.9) | 4/104 (3.8) | |
| <75 | 15/276 (5.4) | 42/1100 (3.8) | 13/268 (4.9) | |
| ≥75 | 1/29 (3.4) | 1/106 (0.9) | 2/35 (5.7) | |
| Urinary tract infection | <65 | 6/201 (3.0) | 26/792 (3.3) | 6/199 (3.0) |
| ≥65 | 5/104 (4.8) | 15/414 (3.6) | 6/104 (5.8) | |
| <75 | 8/276 (2.9) | 35/1100 (3.2) | 10/268 (3.7) | |
| ≥75 | 3/29 (10.3) | 6/106 (5.7) | 2/35 (5.7) | |
| Constipation | <65 | 3/201 (1.5) | 17/792 (2.1) | 3/199 (1.5) |
| ≥65 | 0 | 23/414 (5.6) | 4/104 (3.8) | |
| <75 | 3/276 (1.1) | 35/1100 (3.2) | 7/268 (2.6) | |
| ≥75 | 0 | 5/106 (4.7) | 0 | |
| Escherichia urinary tract infection | <65 | 1/201 (0.5) | 14/792 (1.8) | 2/199 (1.0) |
| ≥65 | 5/104 (4.8) | 21/414 (5.1) | 1/104 (1.0) | |
| <75 | 4/276 (1.4) | 25/1100 (2.3) | 3/268 (1.1) | |
| ≥75 | 2/29 (6.9) | 10/106 (9.4) | 0 | |
| Bronchitis | <65 | 9/201 (4.5) | 14/792 (1.8) | 1/199 (0.5) |
| ≥65 | 3/104 (2.9) | 10/414 (2.4) | 4/104 (3.8) | |
| <75 | 11/276 (4.0) | 23/1100 (2.1) | 4/268 (1.5) | |
| ≥75 | 1/29 (3.4) | 1/106 (0.9) | 1/35 (2.9) | |
| Urinary tract infection, bacterial | <65 | 0 | 10/792 (1.3) | 0 |
| ≥65 | 1/104 (1.0) | 16/414 (3.9) | 1/104 (1.0) | |
| <75 | 0 | 20/1100 (1.8) | 1/268 (0.4) | |
| ≥75 | 1/29 (3.4) | 6/106 (5.7) | 0 | |
| Osteoarthritis | <65 | 0 | 4/792 (0.5) | 2/199 (1.0) |
| ≥65 | 1/104 (1.0) | 9/414 (2.2) | 0 | |
| <75 | 1/276 (0.4) | 8/1100 (0.7) | 2/268 (0.7) | |
| ≥75 | 0 | 5/106 (4.7) | 0 | |
| Edema peripheral | <65 | 0 | 6/792 (0.8) | 0 |
| ≥65 | 2/104 (1.9) | 4/414 (1.0) | 0 | |
| <75 | 0 | 10/1100 (0.9) | 0 | |
| ≥75 | 2/29 (6.9) | 0 | 0 | |
| TEAEs relevant to the older population | ||||
| Hypertension | <65 | 4/201 (2.0) | 8/792 (1.0) | 3/199 (1.5) |
| ≥65 | 0 | 15/414 (3.6) | 1/104 (1.0) | |
| <75 | 4/276 (1.4) | 19/1100 (1.7) | 4/268 (1.5) | |
| ≥75 | 0 | 4/106 (3.8) | 0 | |
| Tachycardia | <65 | 3/201 (1.5) | 16/792 (2.0) | 1/199 (0.5) |
| ≥65 | 2/104 (1.9) | 7/414 (1.7) | 0 | |
| <75 | 5/276 (1.8) | 20/1100 (1.8) | 1/268 (0.4) | |
| ≥75 | 0 | 3/106 (2.8) | 0 | |
| Dizziness | <65 | 2/201 (1.0) | 5/792 (0.6) | 0 |
| ≥65 | 2/104 (1.9) | 8/414 (1.9) | 0 | |
| <75 | 3/276 (1.1) | 12/1100 (1.1) | 0 | |
| ≥75 | 1/29 (3.4) | 1/106 (0.9) | 0 | |
| Urinary retentiona, b | <65 | 0 | 1/792 (0.1) | 1/199 (0.5) |
| ≥65 | 1/104 (1.0) | 5/414 (1.2) | 0 | |
| <75 | 1/276 (0.4) | 6/1100 (0.5) | 1/268 (0.4) | |
| ≥75 | 0 | 0 | 0 | |
| Vision blurred | <65 | 0 | 3/792 (0.4) | 1/199 (0.5) |
| ≥65 | 1/104 (1.0) | 2/414 (0.5) | 0 | |
| <75 | 0 | 4/1100 (0.4) | 1/268 (0.4) | |
| ≥75 | 1/29 (3.4) | 1/106 (0.9) | 0 | |
| Fall | <65 | 1/201 (0.5) | 2/792 (0.3) | 0 |
| ≥65 | 0 | 2/414 (0.5) | 0 | |
| <75 | 1/276 (0.4) | 4/1100 (0.4) | 0 | |
| ≥75 | 0 | 0 | 0 | |
| Palpitations | <65 | 0 | 4/792 (0.5) | 0 |
| ≥65 | 0 | 1/414 (0.2) | 0 | |
| <75 | 0 | 5/1100 (0.5) | 0 | |
| ≥75 | 0 | 0 | 0 | |
| Nocturia | <65 | 0 | 0 | 0 |
| ≥65 | 2/104 (1.9) | 1/414 (0.2) | 0 | |
| <75 | 1/276 (0.4) | 1/1100 (0.1) | 0 | |
| ≥75 | 1/29 (3.4) | 0 | 0 | |
| Residual urine volume increased | <65 | 0 | 2/792 (0.3) | 0 |
| ≥65 | 0 | 1/414 (0.2) | 0 | |
| <75 | 0 | 2/1100 (0.2) | 0 | |
| ≥75 | 0 | 1/106 (0.9) | 0 | |
| Electrocardiogram QT prolonged | <65 | 1/201 (0.5) | 1/792 (0.1) | 0 |
| ≥65 | 0 | 0 | 0 | |
| <75 | 1/276 (0.4) | 1/1100 (0.1) | 0 | |
| ≥75 | 0 | 0 | 0 | |
- Data shown for the SAF. Evaluating the safety of the combination regimen and both monotherapies was the primary objective of this study. PT, preferred term; SAF, safety analysis set; TEAE, treatment-emergent adverse event
- a In total, the lower level term of urinary retention was reported by one (0.3%) patient, three (0.2%) patients, and one (0.3%) patient from the mirabegron, combination, and solifenacin groups, respectively, and the lower level term of feeling of residual urine was reported by three (0.2%) patients from the combination group.
- b One patient (a 66-year-old White male) from the combination group was catheterized after 279 days of treatment. The event of urinary retention resolved after 8 days and the catheter was removed. Study treatment was discontinued after 284 days of treatment.
The most commonly reported TEAEs in this study were dry mouth and nasopharyngitis. With the exception of the ≥75-year-old age group, dry mouth was typically reported more frequently in the combination and solifenacin groups versus the mirabegron group. For all age groups, the frequency of constipation was lower for both monotherapies versus the combination. In addition, UTI was reported more frequently in the older patients versus the younger patients regardless of treatment group. Low incidences of the TEAEs relevant to the older population were typically observed in this subgroup analysis. However, UTI, UTI specifically caused by Escherichia spp., and UTI with an unspecified bacterial etiology were all reported by ≥4.0% of patients from at least one of the treatment and age groups. All three of these UTI-related TEAEs were reported more frequently by females versus males, regardless of treatment group. Importantly, there were no reports of confusion or cognitive changes during this study.
In terms of vital signs, increases in mean pulse rate from baseline of >1 bpm were noted for younger patients following mirabegron and combination treatment, whereas negligible changes were observed with solifenacin (0.78 and 0.38 bpm; Table 3). For older patients, no mean pulse rate increases of >1 bpm were reported. Apart from patients who were <65 years old, all three treatments were associated with numerical increases in systolic blood pressure, with the smallest increases from baseline typically observed following combination treatment. In addition, with the exception of the patients who were <75 years old and received combination therapy, the changes in diastolic blood pressure were minimal for all of the groups investigated. In terms of comparisons between treatment groups, combination therapy was typically associated with lower or similar changes from baseline in systolic and diastolic blood pressure versus both monotherapies for all age groups. Where a numerically higher mean change from baseline was observed for combination therapy, the difference from monotherapy was small and was not considered to be clinically relevant.
| <65 years | ≥65 years | |||||
|---|---|---|---|---|---|---|
| Parameter | Mirabegron 50 mg (n = 198) | Combination 5+50 mg (n = 783) | Solifenacin 5 mg (n = 199) | Mirabegron 50 mg (n = 102) | Combination 5+50 mg (n = 413) | Solifenacin 5 mg (n = 103) |
| Systolic blood pressure in mmHga | ||||||
| Baseline, mean (SE) | 122.99 (0.87) | 122.43 (0.45) | 123.50 (0.93) | 129.63 (1.30) | 130.46 (0.71) | 130.47 (1.25) |
| Adjusted change from baseline, mean (SE) | 0.12 (0.76) | −0.31 (0.39) | 0.93 (0.76) | 4.05 (1.07) | 2.97 (0.54) | 3.90 (1.06) |
| 95%CI | −1.38, 1.62 | −1.07, 0.45 | −0.56, 2.42 | 1.96, 6.14 | 1.91, 4.02 | 1.82, 5.98 |
| Difference: combination vs monotherapy, mean (SE) | −0.43 (0.85) | – | −1.24 (0.85) | −1.09 (1.19) | – | −0.93 (1.18) |
| 95%CI | −2.11, 1.24 | −2.91, 0.43 | −3.41, 1.24 | −3.25, 1.38 | ||
| Diastolic blood pressure in mmHgb | ||||||
| Baseline, mean (SE) | 75.74 (0.55) | 75.62 (0.31) | 76.34 (0.59) | 74.69 (0.80) | 74.37 (0.43) | 74.67 (0.86) |
| Adjusted change from baseline, mean (SE) | 0.42 (0.50) | 0.29 (0.25) | 0.37 (0.50) | 0.09 (0.70) | 0.23 (0.35) | 0.26 (0.69) |
| 95%CI | −0.57, 1.40 | −0.20, 0.79 | −0.60, 1.35 | −1.27, 1.46 | −0.46, 0.91 | −1.10, 1.62 |
| Difference: combination vs monotherapy, mean (SE) | −0.12 (0.56) | – | −0.08 (0.56) | 0.14 (0.78) | – | −0.03 (0.77) |
| 95%CI | −1.22, 0.97 | −1.17, 1.01 | −1.39, 1.66 | −1.55, 1.48 | ||
| Pulse rate in bpmc | ||||||
| Baseline, mean (SE) | 71.84 (0.66) | 72.26 (0.33) | 72.08 (0.68) | 71.04 (0.97) | 70.55 (0.48) | 69.94 (0.92) |
| Adjusted change from baseline, mean (SE) | 1.77 (0.54) | 1.27 (0.27) | 0.78 (0.54) | 0.10 (0.75) | 0.58 (0.38) | −0.56 (0.75) |
| 95%CI | 0.71, 2.83 | 0.73, 1.80 | −0.27, 1.83 | −1.36, 1.57 | −0.16, 1.32 | −2.02, 0.91 |
| Difference: combination vs monotherapy, mean (SE) | −0.50 (0.60) | – | 0.49 (0.60) | 0.48 (0.84) | – | 1.13 (0.83) |
| 95%CI | −1.68, 0.68 | −0.69, 1.66 | −1.16, 2.11 | −0.50, 2.77 | ||
| <75 years | ≥75 years | |||||
|---|---|---|---|---|---|---|
| Mirabegron 50 mg (n = 271) | Combination 5+50 mg (n = 1090) | Solifenacin 5 mg (n = 267) | Mirabegron 50 mg (n = 29) | Combination 5+50 mg (n = 106) | Solifenacin 5 mg (n = 35) | |
| Systolic blood pressure in mmHgd | ||||||
| Baseline, mean (SE) | 124.70 (0.78) | 124.44 (0.41) | 125.32 (0.83) | 130.31 (2.33) | 133.03 (1.39) | 130.11 (2.05) |
| Adjusted change from baseline, mean (SE) | 0.77 (0.66) | 0.85 (0.33) | 1.68 (0.66) | 7.95 (2.01) | 0.51 (1.13) | 3.87 (1.85) |
| 95%CI | −0.52, 2.06 | 0.21, 1.50 | 0.38, 2.97 | 4.01, 11.90 | –1.70, 2.72 | 0.24, 7.50 |
| Difference: combination vs monotherapy, mean (SE) | 0.08 (0.73) | – | –0.82 (0.74) | –7.45 (2.27) | – | –3.36 (2.11) |
| 95%CI | –1.36, 1.52 | –2.27, 0.62 | –11.91, –2.99 | –7.49, 0.77 | ||
| Diastolic blood pressure in mmHge | ||||||
| Baseline, mean (SE) | 75.60 (0.48) | 75.37 (0.26) | 76.13 (0.52) | 73.41 (1.50) | 73.37 (0.84) | 73.04 (1.39) |
| Adjusted change from baseline, mean (SE) | 0.13 (0.43) | 0.44 (0.21) | 0.27 (0.43) | 1.95 (1.31) | −1.49 (0.73) | 0.86 (1.20) |
| 95%CI | −0.70, 0.97 | 0.02, 0.86 | −0.57, 1.11 | −0.61, 4.52 | −2.92, −0.06 | −1.50, 3.22 |
| Difference: combination vs monotherapy, mean (SE) | 0.31 (0.48) | – | 0.17 (0.48) | −3.44 (1.48) | – | −2.35 (1.37) |
| 95%CI | −0.63, 1.24 | −0.77, 1.11 | −6.34, −0.55 | −5.03, 0.33 | ||
| Pulse rate in bpmf | ||||||
| Baseline, mean (SE) | 71.71 (0.56) | 71.83 (0.28) | 71.45 (0.59) | 70.22 (2.13) | 70.01 (0.99) | 70.54 (1.50) |
| Adjusted change from baseline, mean (SE) | 1.25 (0.46) | 1.12 (0.23) | 0.38 (0.46) | 0.78 (1.41) | 0.06 (0.79) | −0.08 (1.30) |
| 95%CI | 0.35, 2.15 | 0.67, 1.57 | −0.53, 1.29 | −1.98, 3.55 | −1.48, 1.60 | −2.63, 2.46 |
| Difference: combination vs monotherapy, mean (SE) | −0.13 (0.51) | – | 0.75 (0.52) | −0.72 (1.60) | – | 0.15 (1.48) |
| 95%CI | −1.14, 0.88 | −0.27, 1.76 | −3.85, 2.41 | −2.75, 3.04 | ||
- Data shown for the SAF. Evaluating the safety of the combination regimen and both monotherapies was the primary objective of this study. Adjusted change from baseline values as well as the 95% CIs were generated from an ANCOVA model with treatment group, age group (< 65, ≥65 years or <75, ≥75 years), sex, previous study history, and geographic region as fixed factors and an age group-by-treatment interaction and baseline value as covariates. Home-based vital sign assessments were also conducted as part of this study. The results from these assessments are not shown herein. Interaction between age group and treatment group (statistically significant if P ≤ 0.10): aP = 0.864, bP = 0.963, cP = 0.574, dP = 0.007, eP = 0.026, fP = 0.895. ANCOVA, analysis of covariance; CI, confidence interval; EoT, end of treatment; SAF, safety analysis set; SE, standard error.
The overall changes in ECG parameters were not considered to be clinically relevant. Mean ± standard deviation changes from baseline to EoT in QT interval corrected for heart rate by Fridericia's formula (QTcF) varied between −1.9 ± 16.9 and 0.6 ± 13.2 ms, 0.2 ± 14.1 and 1.5 ± 12.2 ms, and 0.9 ± 12.8 and 2.1 ± 11.4 ms for the age groups from the mirabegron, combination, and solifenacin groups, respectively.
For both of the younger age groups, a slightly greater proportion of the combination-group patients experienced a categorized increase in PVR volume at EoT versus both monotherapies (< 65 years—mirabegron: 0%, combination: 12/782 [1.5%] patients, solifenacin: 2/199 [1.0%] patients; <75 years—mirabegron: 1/271 [0.4%] patients, combination: 25/1088 [2.3%] patients, solifenacin: 4/267 [1.5%] patients). Conversely, in the older age groups, a slightly greater proportion of the patients who received solifenacin experienced a categorized increase in PVR volume at EoT versus the other treatments (≥65 years—mirabegron: 1/102 [1.0%] patients, combination: 14/412 [3.4%] patients, solifenacin: 4/103 [3.9%] patients; ≥75 years—mirabegron: 0%, combination: 1/106 [0.9%] patients, solifenacin: 2/35 [5.7%] patients). These increases in PVR volume were not considered to be clinically relevant. Furthermore, the laboratory assessments generated no additional safety concerns.
3.3 Efficacy
Regardless of age group, all three treatments were associated with numerical improvements in both of the primary variables (change from baseline to EoT in mean number of incontinence episodes/24 h and micturitions/24 h; Figures 1 and 2). Using combination therapy, greater improvements in mean number of incontinence episodes/24 h were generally achieved versus mirabegron and solifenacin. The only exception was observed for patients who were <65 years old in comparison with solifenacin (combination: −1.93 ± 0.07, solifenacin: −2.00 ± 0.13). Furthermore, the degree of improvement in mean number of micturitions/24 h was generally lower with both monotherapies versus combination treatment. The only exception was for the ≥75 years age group in comparison with solifenacin (combination: −2.55 ± 0.24, solifenacin: −2.62 ± 0.39).
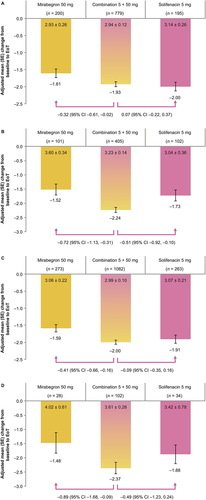
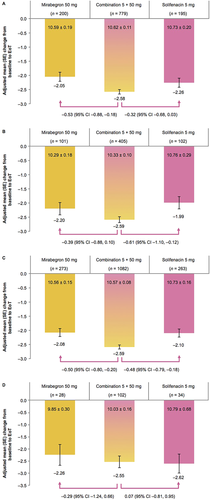
Combination, mirabegron, and solifenacin therapy were all associated with numerical improvements in the secondary efficacy variables (Supplementary Figures S1–S3). For all age groups, greater improvements were consistently achieved with combination therapy for all three variables. The most notable differences between combination therapy and mirabegron were observed for both younger age groups and the ≥65-year-old age group, whereas the most noteworthy contrasts between combination therapy and solifenacin were observed for the ≥65-year-old and <75-year-old age groups.
4 DISCUSSION
This is the first study to provide reassurance about the long-term safety and efficacy of mirabegron and solifenacin combination therapy in both elderly and non-elderly patients with OAB symptoms. Older patients can be a difficult-to-treat population due to the presence of pre-existing frailty and multiple comorbidities,11, 12 a need for multiple anticholinergic medications,13 and the increased likelihood of an inadequate treatment response.15 Therefore the fact that mirabegron and solifenacin combination therapy appears to be an efficacious long-term treatment in this population is particularly promising.
In terms of safety, slightly higher incidences of TEAEs were observed following combination therapy versus mirabegron and solifenacin monotherapy regardless of age group. This observation concurs with the entire study population results22 and previous shorter-term studies involving this combination.19 In agreement with the BESIDE age subgroup analysis,21 slightly higher incidences of overall TEAEs were observed in the older age groups in the present study versus the younger age groups. However, a higher overall incidence of TEAEs was typically observed in the combination and solifenacin groups in this study (37.1–57.2%) versus the combination and solifenacin 5 mg groups from the BESIDE age subgroup analysis (30.3–40.9%).21 This finding was primarily due to the different nature of the studies; the present study had a longer treatment duration and the BESIDE study involved a run-in treatment period with solifenacin,18 so the randomized patients may have displayed a good tolerance to solifenacin treatment. A subgroup analysis from a 1-year trial evaluated the safety of mirabegron monotherapy in patients who were ≥65 years or ≥75 years old.23 In contrast to the BESIDE age subgroup analysis, higher incidences of TEAEs were observed in this mirabegron study (≥65 years: 65.1%, ≥75 years: 68.0%) than for the mirabegron group in the present investigation (≥65 years: 41.3%, ≥75 years: 51.7%).
Dry mouth was the most commonly reported TEAE in this study, which aligns with the results of the BESIDE age subgroup analysis.21 Similar incidences of dry mouth were observed for the combination and solifenacin 5 mg groups in both studies. Overall, low incidences of TEAEs that are relevant to the older population were observed in the present investigation. Only UTI-related events were reported by ≥4.0% of patients from at least one of the treatment and age groups. This result agrees with the BESIDE age subgroup analysis, where the incidences of the TEAEs that are relevant to the older population were typically <2% irrespective of the treatment or age subgroups.21 These findings are also supported by further investigations which have suggested a link between the occurrence of OAB and an increased prevalence of bacteriuria.24 Given the potential for adverse cognitive events in an older group, it is also important to note that incidences of confusion and cognitive changes were not observed in this study.
The changes from baseline in vital signs were not considered to be clinically relevant for any of the age and treatment subgroups in this study. In both the present investigation and the BESIDE age subgroup analysis,21 similar overall changes from baseline in vital signs were observed for the combination and monotherapy groups. In this study, mirabegron and combination treatment were associated with slight increases in pulse rate from baseline for both of the younger age groups only (mirabegron: 1.25-1.77 bpm, combination: 1.12-1.27 bpm). The fact that the same relationship was not observed for the older age groups was not unexpected given that aging is associated with profound decreases in cardiac β-adrenergic responsiveness through various mechanisms including receptor downregulation and decreased agonist binding.25 In contrast to the results presented herein, treatment with solifenacin 5 mg was associated with slight increases from baseline in pulse rate for the younger age groups in the BESIDE age subgroup analysis.21
The finding that all three treatments were associated with numerical improvements in both the primary and secondary efficacy variables agrees with the results from the entire patient population in this study22 and previous investigations that have examined the efficacy of combination, mirabegron, or solifenacin therapy according to patient age subgroups.21, 23 Combination therapy was typically associated with greater improvements in all efficacy variables versus both monotherapies, similar to the findings from the BESIDE age subgroup analysis.21 These data showcase the fact that effective prolonged treatment can be achieved in patients with OAB symptoms using low-dose solifenacin (5 mg) as part of the combination regimen.
The present study has some limitations. Compared with the other subgroups, there was more uncertainty associated with the results for the ≥75 years subgroup due to the low sample size; only 9.4% of the patients enrolled in this study were from this age group. Additionally, this study predominately enrolled patients who had completed the SYNERGY19 or BESIDE18 studies, which may mean that the patient population may have previously experienced good tolerability and a favorable response to combination, mirabegron, or solifenacin therapy. However, as the patients had to display OAB symptoms, the randomization, and double-blinding process would have protected against bias from participation in these earlier trials. Furthermore, this study included the general OAB population, additional useful data could have been acquired if frail or vulnerable elderly patients were specifically enrolled. Last, owing to the length of the treatment period, the inclusion of a placebo arm would have been ethically unjustifiable. Concurrently, the blinded, controlled nature of this study allowed a thorough assessment of the primary objective and the inclusion of a placebo group may not have provided any additional useful data.
5 CONCLUSIONS
The results of this investigation suggest that mirabegron and solifenacin combination therapy is a well-tolerated and effective treatment for patients with OAB symptoms regardless of their age and concomitant drug therapy. This combination regimen allows the use of two agents with different modes of action to be used in conjunction, which maximizes efficacy and enables the use of low-dose antimuscarinic therapy in clinical practice.
ACKNOWLEDGMENTS
The authors would like to thank the SYNERGY II study investigators and all patients who took part in the study. This study was sponsored by Astellas Pharma Europe B.V. All medication used in this study was prepared, packaged, and labeled under the responsibility of qualified staff at Astellas Pharma Europe B.V. Medical writing support was provided by Michael Parsons PhD of Envision Scientific Solutions and funded by Astellas Pharma Global Development.
DISCLOSURES
Elizabeth R. Mueller declares grants, principal investigator, and advisory board member fees from Astellas Pharma and advisory board member fees from Boston Scientific; Rob van Maanen and Matthias Stoelzel were and are employees of Astellas Pharma, respectively; Christopher Chapple declares grants, editorial support, scientific study/trial support, meeting participant/lecturer, and consultancy/advisor fees from Astellas Pharma, grants, scientific study/trial support, meeting participant/lecturer, and consultancy/advisor fees from Allergan, and meeting participant/lecturer fees from Pfizer; Paul Abrams declares grants, lecturer, consultancy, and investigator fees from Astellas Pharma, consultancy and lecturer fees from Pfizer, consultancy fees from Ferring and Ipsen, lecturer on leadership fees from Coloplast and Pierre Fabre, and lecturer fees from Sun Pharma; Sender Herschorn declares grants and personal fees from Astellas Pharma and Ipsen, personal fees from Pfizer, and grants from Ixaltis; Dudley Robinson declares consultancy fees from Astellas Pharma, Pfizer, Ferring, Allergan, and Ixaltis and speaker fees from Astellas Pharma, Pfizer, and Allergan; Salman Al-Shukri declares investigator fees from Astellas Pharma; Tomasz Rechberger declares investigator, travel, lectureship, and advisory board member fees from Astellas Pharma; and Christian Gratzke declares principal investigator fees from Astellas Pharma, lecturer and advisory board member fees from Astellas Pharma, Bayer, Janssen, and GSK, lecturer fees from Pfizer, Amgen, and Ipsen, grants and personal fees from AMS, and outcome review panel fees from Steba. Sang Jin Yoon declares no such interests.




