Urotherapy in children with dysfunctional voiding and the responsiveness of two condition-specific questionnaires
Abstract
Aims
We sought to establish the responsiveness of the Dutch Vancouver Symptom Score for Dysfunctional Elimination Syndrome (VSSDES) and Pediatric urinary incontinence Quality of life (PinQ) questionnaires. Secondary, we evaluated the outcome of urotherapy extended for children with dysfunctional voiding (DV).
Methods
This cross-sectional multicenter study was done in one tertiary and two community hospitals. Children with DV were included, also when refractory to previous urotherapeutic treatment. The questionnaires were completed before and after urotherapy. The primary outcome measure was the responsiveness of the Dutch VSDESS and PinQ. Secondary outcome was the initial success (defined by the International Children's Continence Society) of extended urotherapy.
Results
Between June 2014 and May 2016, 64 children (median age 7 years, IQR 6-10) received urotherapy (median 18 weeks, IQR 11-28). In contrast to the VSSDES, the PinQ showed good responsiveness. For children and parents, respectively, the area under the ROC-curve was 0.79 (P = 0.01) and 0.72 (P = 0.03) for the PinQ and 0.50 (P = 0.98) and 0.55 (P = 0.62) for the VSSDES. Fifty children received extended urotherapy, 27 had complete, and 14 had partial response. Sixteen children had been refractory to previous treatment; four showed complete, and six showed partial response.
Conclusion
The PinQ is able to detect clinically important changes in continence-specific quality of life after treatment. We support the use of the VSSDES questionnaire in addition to the current diagnostics for the diagnosis of DV. Extended urotherapy showed to be a successful treatment for children with DV, also for those who had received previous unsuccessful treatment.
ABBREVIATIONS
-
- AUC
-
- area under the receiver operating characteristic curve
-
- DV
-
- dysfunctional voiding
-
- EBC
-
- expected bladder capacity
-
- ICCS
-
- International Children's Continence Society
-
- IQR
-
- interquartile range
-
- LUTS
-
- lower urinary tract symptoms
-
- PinQ
-
- pediatric urinary incontinence quality of life score
-
- VSSDES
-
- vancouver symptom score for dysfunctional elimination syndrome
1 INTRODUCTION
Lower urinary tract symptoms (LUTS) are a common reason for children to visit the pediatrician or pediatric urologist.1-3 Symptoms related to the voiding and storage phase of bladder can contribute to numerous functional elimination disorders.3, 4 Dysfunctional voiding (DV) is a common cause of LUTS in neurologically intact children.4 Voiding symptoms such as straining, hesitancy, dysuria, and storage symptoms such as frequency, urgency, or incontinence are suggestive for DV.3-5 Furthermore, DV is often associated with recurrent urinary infections, and bowel dysfunction such as constipation or fecal incontinence.5, 6 The symptoms of DV can have a negative impact on a child's quality of life and self-esteem.1, 2, 6 The International Children's Continence Society (ICCS) has defined DV as habitual contractions of the urethral sphincter or pelvic floor during voiding. The uroflowmetry curve demonstrates a staccato pattern with or without an interrupted flow concomitant with activity on EMG.4, 5 The exact epidemiology of DV is unknown.5 The prevalence of DV in the general population has a wide range: 4.2-46.4%.5, 6
Urotherapy and pelvic muscle floor retraining can be a successful treatment in the majority of these children.3, 6 Urotherapy is a non-standardized conservative based treatment option for children with voiding dysfunctions.4, 5 According to the ICCS, urotherapy includes education about lower urinary tract anatomy and function, as well as life-style advices (balanced fluid intake, diet, proper voiding posture without holding maneuvers, regular bladder, and bowel emptying patterns).4, 5 During follow up the child will be encouraged to comply with therapy and the LUTS will be monitored by bladder, bowel, and intake diary.4, 5 Urotherapy can be extended with visual biofeedback by two approaches: feedback of the uroflow curve and teaching perineal muscle identification by EMG electrodes.1, 3, 5, 6 The physical therapist can extend the urotherapy with pelvic floor muscle retraining, which implies learning how to relax the pelvic floor during voiding.1, 3-6
Administering a condition-specific questionnaire can be useful to evaluate LUTS and effect of the treatment. Subjective complaints can be translated objectively into a total score.4 Two English-language questionnaires were translated into Dutch and proved to have good validity and reliability. One is the Pediatric urinary incontinence Quality of life score (PinQ), which measures the continence-specific quality of life in children with bladder dysfunction.7,8 The other is the Vancouver Symptom Score for Dysfunctional Elimination Syndrome (VSSDES), which evaluates the symptoms of patients with DV.9
In the study presented here we followed children with DV during their treatment. Our primary aim of this study was to evaluate the responsiveness of the Dutch-language versions of the VSSDES and PinQ. Secondary, we evaluated the results of extended urotherapy.
2 MATERIALS AND METHODS
2.1 Study design and population
The local ethics committee approved this multicenter prospective cross-sectional study (MEC-2014-290). Children with the age between 4 and 17 years presenting with DV at the pediatric, pediatric urology, or pelvic floor physical therapy outpatient clinics at two community hospitals and one tertiary hospital in the Netherlands between June 2014 and May 2016 were eligible for inclusion in the study. Children who had a previous unsuccessful treatment in a different setting were included as well. Patients with a neurogenic disease, anatomic abnormalities of the urinary tract, and previous urological surgery were excluded. The diagnosis DV was based on clinical symptoms, and a staccato and/or intermittent uroflowmetry with increased activity on pelvic floor EMG. After signing informed consent, patients, and parents were asked to fill out the questionnaires twice: after inclusion and after finishing urotherapy.
2.2 Questionnaires
Prior to urotherapy, patients, and parents filled out the VSSDES and the PinQ. The VSSDES is a 14-item condition-specific measure to evaluate the symptoms of bladder and bowel dysfunction. The last question addresses the ease with which the questionnaire can be completed, and the response to this question is excluded from the total score. Responses are given on a 5-point Likert scale, ranging from zero (no complaints) to four (severe symptoms) except for question 3 about voiding frequency (5-6 times: score of 0; 3-4 times or 7-8 times: score of 2; 1-2 times or >8: score of 4). All items are weighted equally. A total score is obtained by summating the item scores; the higher the total score the more severe the symptoms.10 A cutoff score of 11 is established for the Dutch version.9
The PinQ is a 20-item questionnaire to evaluate the quality of life of children with urinary incontinence. All items are scored on a 5-point Likert scale. A higher total score corresponds with a lower quality of life.8 Incompletely filled out questionnaires were accepted if no more than two answers were missing. Then the total score is calculated by multiplying the number of items in the questionnaire by the mean value of responses to the answered questions. No cutoff score is published for the PinQ. Thibodeau et al2 made an assumption to grade the severity of impact on quality of life: mild <20, moderate 21-50, and severe >51.
After urotherapy had finished, patients, and parents again filled out the VSSDES and the PinQ and answered an additional question derived from the RAND-36-Item Health Survey (RAND 36-HTI): “How is your voiding problem compared to one year ago?” (response categories: much better, somewhat better, about the same, somewhat worse, much worse).11
2.3 Outcome measure questionnaires
The primary outcome measure of this study was the responsiveness of the two questionnaire's. A questionnaire's responsiveness is its ability to detect clinically important changes over time in patients when treatment is given. A measure of the responsiveness is the area under the receiver operating characteristic (ROC) curve (AUC), according to an external criterion. The answer to the question derived from the RAND 36-HTI and the initial outcome served as external criteria. The AUC shows the ability of a questionnaire to discriminate between improvement and no improvement. An AUC of at least 0.7 was considered to reflect adequate responsiveness.12
2.4 Urotherapy
Our secondary objective was to evaluate the outcome of “extended” urotherapy. All included children received “standard” urotherapy consisting of initial evaluation, education, and management as described by the ICCS.4 Standard urotherapy with visual biofeedback by uroflowmetry and EMG electrodes and/or retraining of the pelvic floor was defined as “extended” urotherapy.
In one of the community hospitals only standard urotherapy was given. Children visited the pediatrician combined with a trained nurse once or twice. When standard urotherapy failed children returned to the outpatient clinic of the pediatrician for additional treatment with medication or were referred to a physical therapist.
Children included in the tertiary hospital and in the other community hospital received extended urotherapy given by a trained nurse or physical therapist. Those children had approximately 5-7 sessions in 4 months. During this period the children were discussed 2-3 times with the pediatrician or pediatric urologist. When additional treatment with anticholinergics was needed because of persistent urgency with acceptable post void residual urine, the pediatrician or pediatric urologist started tolterodine (slow release) 2 mg 1 daily, or solifenacine 5 mg 1 daily, or oxybutynine 0.4 mg/kg 3 daily for 4-12 weeks. The use of anticholinergics was re-evaluated with the pediatrician or pediatric urologist during urotherapy. After approximately 4 months urotherapy was finished. When extended urotherapy failed an invasive treatment as botulinum toxin A injections into the bladder wall or into the urethral sphincter could be considered. Injections in the urethral sphincter were given when an increased activity of pelvic floor muscles or external urethral sphincter was seen during voiding on urodynamic study. A total of 100 IU of botulinum toxin A was injected in equal dose into the external sphincter at the 3, 9, and 12 o'clock positions under general anesthesia and antibiotic prophylaxis. For boys a cystoscope was used. The transurethral approach to the sphincter is more difficult for girls, therefore, injections were placed paraurethral.13 In cases of persisted symptoms of an overactive bladder without post void residual urine botulinum toxin A injections (total dose of 70 UI) into the bladder wall were given under general anesthesia and antibiotic prophylaxis by cystoscopy.
2.5 Outcome measures extended urotherapy
The outcome of extended urotherapy was defined by the definition of initial success proposed by the ICCS: no response (<50% reduction of LUTS), partial response (50% to 99% reduction of LUTS), and complete response (100% reduction of LUTS).4 The children who received only standard urotherapy were excluded in this evaluation. The initial success and symptoms were evaluated after the last visit of urotherapy. The initial success and effect on symptoms of an invasive treatment (botulinum toxin A injections) were not evaluated. Data such as symptoms, post void residual, urinary frequency, maximum voided volume, and fluid intake before urotherapy and at the last visit of urotherapy were retrospectively collected. The maximum voided volume was retrieved from the voiding chart and refers to the largest volume voided, excluding the first morning void. The maximum voided volume was considered small or large if <65% or >150% of expected bladder capacity (EBC), respectively.4 The EBC was defined by the formula (30× [age in years + 1]mL).4 The maximum level was 390 mL at 12 years.4
2.6 Statistical analysis
Statistical analyses were performed using SPSS version 21.0 (IBM Corp., Armonk, NY). A two-sided P-value <0.05 was considered significant. Descriptive statistics were calculated and are presented as median (interquartile range [IQR]), frequency and percentiles. To evaluate differences in symptoms and results of the two questionnaires between before and after treatment, the paired-samples t-test and the McNemar test were used for continuous and categorical variables, respectively. One-way analysis of variance (ANOVA) was used for the evaluation of more than two independent groups. The answer on the RAND 36-HTI question was dichotomized to “not improved” (including “about the same,” “somewhat worse,” and “much worse”) and “improved” (including “much better” and “somewhat better”). The AUC was calculated to determine the responsiveness.12
3 RESULTS
The study population consisted of 64 children with dysfunctional voiding and their parents. Fifty children received extended urotherapy with or without pelvic floor retraining. The fourteen children who received standard urotherapy where only included to evaluate the responsiveness of the questionnaires. Patient characteristics are displayed in Table 1.
| Study population n = 64 | |
|---|---|
| Age, median (IQR) | 7 (6-10) |
| Number of girls, n (%) | 35 (55) |
| Duration of the treatment in weeks, median (IQR) | 18 (11-28) |
| Type of urotherapy, n (%) | |
| •Standard urotherapy | 14 (22) |
| •Extended urotherapy | 50 (78) |
| Extended urotherapy n = 50 | |
| Age, median (IQR) | 8 (7-10) |
| Number of girls, n (%) | 28 (56) |
| Duration of the treatment in weeks, median (IQR) | 18 (11-25) |
| Anticholinergics during urotherapy, n (%) | 14 (28) |
3.1 Questionnaires
The VSSDES questionnaire was completed before and after urotherapy by 50 (78%) children and 49 (77%) parents; the PinQ by 45 (70%) children, and 48 (75%) parents. Table 2 presents the mean difference scores (SD).
| Total score of the questionnaire | Before urotherapy | After urotherapy | Difference | P-value |
|---|---|---|---|---|
| VSSDESb
Child n = 50 Parent n = 49 |
17.9 ± 6.9
17.9 ± 6.5 |
11.6 ± 5.9
11.5 ± 6.2 |
−6.3 ± 6.7
−6.4 ± 6.6 |
<0.001a
<0.001a |
| PinQc
Child n = 45 Parent n = 48 |
23.7 ± 14.8
21.5 ± 11.2 |
17.0 ±15.0
17.1 ± 12.9 |
−6.7 ± 10.9
−4.4 ± 12.0 |
<0.001a
0.015a |
- a Paired t-test.
- b A higher total score indicates more severe symptoms.
- c A higher total score indicates a lower quality-of-life.
The responsiveness of the VSSEDS was measured by the AUC calculated with the RAND-36-HTI as an external criterion; the AUC was 0.50 (P = 0.98) for children, and 0.55 (P = 0.62) for parents. The AUC for the PinQ was 0.79 (P = 0.01) for the children and 0.72 (P = 0.03) for the parents (Table 3).
| VSSDES | Number (%) | Mean ± SDa |
|---|---|---|
| RAND-36-HTI n = 50
•Much better/ a little better •Same •Much worse/a little worse Area under the ROC curve P-value |
41 (82.0) 7 (14.0) 2 (4.0) |
−6.3 ± 7.0 −6.5 ± 5.9 −5.5 ± 2.1 0.50 0.98 |
| Parent RAND-36-HTI n = 49
•Much better/a little better •Same •Much worse/a little worse Area under the ROC curve P-value |
38 (77.5) 9 (18.4) 2 (4.1) |
−6.8 ± 6.3 −6.8 ± 8.0 2.0 ± 1.4 0.55 0.62 |
| PinQ | Number (%) | Mean ± SDb |
| RAND-36-HTI n = 45
•Much better/a little better •Same •Much worse/a little worse Area under the ROC curve P-value |
37 (82.2) 6 (13.3) 2 (4.5) |
−8.3 ± 11.2 −0.9 ± 4.3 5.5 ± 7.8 0.79 0.01 |
| Parent RAND-36-HTI n = 48
•Much better/a little better •Same •Much worse/a little worse Area under the ROC curve P-value |
37 (77.1) 9 (18.8) 2 (4.1) |
−6.7 ± 11.0 1.1 ± 12.4 14.5 ± 5.0 0.72 0.03 |
- The RAND-36-HTI functions as an external criterion.
- a A higher total score indicates more severe symptoms.
- b A higher total score indicates a lower quality-of-life.
3.2 Outcome of extended urotherapy
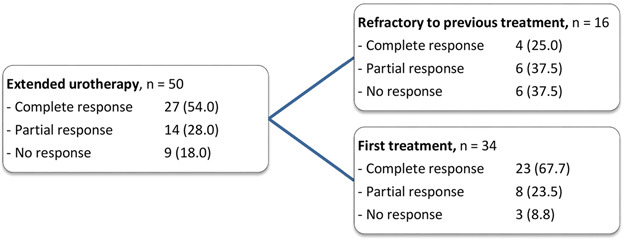
Fifty children received extended urotherapy with or without pelvic floor retraining (Table 1). Sixteen children were refractory to previous urotherapeutic treatment and received urotherapeutic treatment in a different setting for the second time. Fourteen of them had received previous urotherapeutic treatment in combination with pelvic floor physical therapy and two had received group urotherapy. The median duration of urotherapy was 18 weeks (IQR 11-25 weeks).
After extended urotherapy symptoms such as daytime and nighttime incontinence, urge, dysuria, and abdominal pain all had improved significantly (Table 4). During urotherapy anticholinergics were prescribed to fourteen children. After urotherapy, 64% of the children had stopped the anticholinergics. Before treatment three girls experienced urinary tract infections (UTIs) with fever (≥2 in 6 months) and nine girls and one boy had UTIs without fever (frequency: 1-3 UTIs in 12 months, five girls unknown). During therapy, none of the children experienced UTIs. The mean fluid intake increased from 985 to 1547 mL (P < 0.001, n = 43). A voiding chart was completed at the last visit of urotherapy in 34 children. Nineteen out of 34 children had a maximum voided volume <65% or >150% of the EBC before therapy, 14 children showed improvement after treatment. At the last visit of urotherapy 37 children had undergone an uroflowmetry, which in 12 (32.4%) of them showed a persistent staccato, and/or intermittent flow pattern. In 7 of those 12 children also with increased activity on pelvic floor EMG. The mean post void residual of these 37 children decreased from 28.1 to 12.8 mL (P = 0.025).
| Symptoms n = 50a | Before urotherapy n (%) | After urotherapy n (%) | P-value |
|---|---|---|---|
| Daytime incontinence
Partial success (50-99%) No success (<50%) |
42 (84.0) | 23 (46.0)
12 (24.0) 11 (22.0) |
<0.001b |
| Dry | 8 (16.0) | 27 (54.0) | |
| Nighttime incontinence
Partial success (50-99%) No success (<50%) |
30 (60.0) | 16 (32.0)
3 (6.0) 13 (26.0) |
<0.001b |
| Dry | 20 (40.0) | 34 (68.0) | |
| Urge (n = 43) | 21 (48.8) | 6 (14.0) | <0.001b |
| Dysuria | 3 (6.0) | 0 | |
| Abdominal pain | 10 (20.0) | 2 (4.0) | 0.008b |
- a Unless stated otherwise.
- b McNemar test.
Based on the definition initial success by the ICCS, the treatment outcome of extended urotherapy could be classified as complete response in 27 (54%), partial response in 14 (28%), and no response in 9 (18%) children (Table 5). There was a significance difference in urotherapy outcome between the children refractory to previous urotherapeutic treatment and those who received urotherapeutic treatment for the first time, in disadvantage of the children refractory to previous urotherapeutic treatment (P = 0.014). Overall, nine out of 50 children had no response to extended urotherapy. Three children and their parents decided to accept the situation. Six children received botulinum toxin A injections in the urethral sphincter (n = 4), or in the bladder wall (n = 1), or in the urethral sphincter and bladder wall (n = 1).
4 DISCUSSION
The evaluation of the responsiveness of the VSSDES and PinQ, and of patient outcomes suggest that the PinQ questionnaire can detect clinically important changes over time (Table 3) and that symptoms had improved after extended urotherapy (Table 4).
The reliability and validity of the original and Dutch versions of both questionnaires were found to be good in earlier studies.7-10 Completing the questionnaires makes the symptoms and feelings transparent and negotiable with the health professional and family-members. This could lead to increased empathy, support, and treatment compliance. Both, Afshar et al and Bower et al, suggest to measure the responsiveness of the questionnaires.7, 10 As far as we know, this is the first study that reports on the responsiveness of the questionnaires. We hypothesized that lower post-treatment scores on the PinQ and the VSSDES compared to the baseline scores would reflect improvements on quality of life and symptoms. According to the external criterion, the RAND-36-HTI question: “How is your voiding problem compared to one year ago?” the AUC was measured. The responsiveness of both the child and parent versions of the PinQ proved to be more than adequate, the AUC was both above >0.7. This was not the case for the VSSDES. Perhaps the RAND-36-HTI question solely addresses the aspect of the voiding dysfunction and does not fully encompass the symptoms. Besides, only two parents and children found the voiding dysfunction to be worse now. We noted that completing the post-treatment questionnaires was not a priority for the parents and children. Resulting in a median interval of 14 weeks (IQR 0-48) between ending urotherapy and completing the last questionnaires.
In this study, the Dutch VSSDES showed to be not useful to detect clinical important changes over time in symptoms after therapy. Still, the questionnaire is a reliable and valid tool to more objectively and systematically evaluate symptoms of patients with DV.9 Our hypothesis for the PinQ could be confirmed. The children and parents who answered the RAND-36-HTI question with “much” or “somewhat better” had mean lower scores on the PinQ after treatment.
The children in our study showed a good initial success rate after extended urotherapy with visual biofeedback by uroflowmetry and EMG electrodes and/or pelvic floor retraining. Judged from three ICCS basic principles of treatment outcomes, extended urotherapy was successful for 82% (complete response 54%) of the children overall. The success of treatment in children who were refractory to previous urotherapy was 63% (complete response 25%). Children who did not respond to previous urotherapy may be more motivated in new and different setting. It is also possible that the moment and the intensity of attention by the healthcare professional or parent is relevant to success. Previous studies have reported success rates of 90-100% of urotherapy with the possibility to extend with biofeedback or pelvic floor retraining or medication (commonly an antimuscarinic) in children with DV.5, 14 Tugtepe et al15 reported on 28 children with DV refractory to three months of standard urotherapy. All children received additional extended urotherapy resulting in 50-100% decrease of LUTS. The outcome of the present study is comparable or slightly less favorable than that of these previous studies, to which the multicenter design and the inclusion of 16 children refractory to a previous treatment may have contributed. Note, however, outcomes of urotherapy are hard to compare between studies with different study populations, treatment approaches, and definitions of success of DV. In our study, the children have received standard urotherapy as defined by the ICCS extended with visual biofeedback by uroflowmetry and EMG electrodes and/or retraining of the pelvic floor. If needed an additional treatment with anticholinergics was started to treat urge-related symptoms. The content of extended urotherapy was similar, despite every child need his or her own stepwise approach. The role of pharmacological therapy can be considered as ancillary in the management of DV.3, 5 A standard protocol for urotherapy following a stepwise approach and uniform reporting of outcomes would be helpful in current clinical practice and facilitate comparison between studies.
One of the strengths of this study is the prospective inclusion of all eligible children and the use of a standard measure to evaluate the responsiveness.12 Children were recruited in different hospital settings and received different types of urotherapy, which makes the results of this study more generalizable for current clinical practice. However, the different approaches of urotherapy could possibly give some bias on the outcome. Limitations include the absence of a control group, the retrospective collection of LUTS data and the long interval between finishing urotherapy and completing the last questionnaires, which may have confounded the results.
5 CONCLUSION
In contrast to the VSDESS, the PinQ is a responsiveness questionnaire. The PinQ is able to detect clinically important changes over time when treatment is given and can be used initially, during follow up and after treatment to evaluate the continence-specific quality of life in children with DV. We support the use of the VSSDES questionnaire in addition to the current diagnostics (voiding diary and uroflowmetry with pelvic floor EMG) for the diagnosis of DV. Urotherapy with visual biofeedback by uroflowmetry and EMG electrodes and/or retraining of the pelvic floor showed to be a successful treatment for children with DV, also for those who had received previous unsuccessful treatment.
ACKNOWLEDGMENTS
We like to-thank Nynke van der Linden for recruiting patients, Astrid Hendrik, and Roelie van Zon for performing follow-up evaluations, and Ko Hagoort for editorial assistance.
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.
ETHICAL APPROVAL
The study has been performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The local ethics committee approved this multicenter prospective cross-sectional study (MEC-2014-290). Study received local ethics committee approval (MEC-2014-290).
INFORMED CONSENT
Parents and patients >12-years old gave their written consent prior to inclusion in the study. Participation in the study was voluntary and anonymous with no explicit incentives provided for participation.