Fish and Prey Resources in Reservoir Coves with and without Offshore Artificial Habitat Structures
Abstract
Artificial habitat structures are used to mitigate habitat loss within aquatic ecosystems and to increase angler catch rates; however, the potential trophic outcomes of concentrating fish at structure additions are rarely evaluated. We compared fish and fish prey assemblages between reservoir coves with and without newly added offshore habitat structures (PVC cubes enclosing 2.25 m3). Further, we tested whether offshore habitat additions changed fish assemblage and abundance in littoral habitats of treated coves. Coves with offshore structures had higher concentrations of fish and an assemblage structure dominated by Pomoxis and Lepomis species at offshore sites compared to coves without offshore structures. There was no evidence for an effect of offshore structures on fish relative abundance and assemblage structure in nearby littoral habitats. Zooplankton and benthic macroinvertebrate abundances did not change because of the addition of habitat structures. Lack of a response of lower trophic organisms may be due to low predation mortality or else losses to predation are replaced by increased prey production or immigration at the offshore structures. Our results indicated that artificial structures effectively concentrated fish, but increases in fish density may be partitioned among newly available structure in offshore habitats and existing structure within littoral habitats. Future studies are needed to test for a relationship between foraging space and food resources provided by artificial habitats, which may be useful for optimizing the number, size, and material composition of structures used in habitat enhancement programs.
Habitat abundance and location can play an important role in structuring aquatic ecosystems by altering encounter rates among predators and prey. Artificial habitat structures are commonly used to supplement existing habitat or replace structured habitat that has been degraded or lost in aging reservoirs (Tugend et al. 2002; Miranda et al. 2010) and are expected to concentrate fish and increase angler catch rates through a variety of mechanisms (Tugend et al. 2002; Wills et al. 2004). A considerable amount of research has shown that artificial structures of varying material composition, shape, and complexity attract and concentrate fish (Johnson et al. 1988; Walters et al. 1991; Rold et al. 1996; Feger and Spier 2010; Baumann et al. 2016; Driscoll et al. 2020). How fish attraction to artificial habitats affects fish density in natural habitats can be complex, involving the extent to which artificial habitat redirects fish away from natural habitats and the potential for subsequent compensatory increases in fish within the natural habitat (Osenberg et al. 2002). Responses beyond fish aggregation, however, are rarely addressed in studies assessing the outcomes of structure additions, leading to an incomplete understanding of the mechanisms underlying fish attraction to added habitats and their influence on lake and reservoir fisheries (Sass et al. 2006, 2012, 2019). Testing for other factors such as the added structure's ability to attract and concentrate food resources for fish is likely also important for understanding whether artificial habitat structures function as fish attractors and their potential to increase fish production (Bohnsack 1989; Santos et al. 2011; Sass et al. 2019).
Despite evidence that fish associate with added structure (Bolding et al. 2004; Newbrey et al. 2005; Ahrenstorff et al. 2009), few studies have tested for the potential trophic outcomes of concentrating fish abundance at locations with structure additions in lakes and reservoirs (Sass et al. 2006). As a substrate for the colonization of algae and other basal food resources (Vadeboncoeur and Lodge 2000), added structure presumably changes the amount of habitat available for macroinvertebrates and other fish prey resources (Smokorowski et al. 2006; Helmus and Sass 2008; Ferreiro et al. 2011; McArtor et al. 2021). Added habitats are typically structurally complex (Bolding et al. 2004), and benthic macroinvertebrates tend to associate with areas of higher habitat complexity (Diehl 1992; Schneider and Winemiller 2008). Similar to artificial reefs in marine ecosystems and macrophytes in freshwaters systems, added vertical structure may also attract zooplankton (Burks et al. 2001; Oh et al. 2011; Detmer and Wahl 2021). Depending on the fish assemblage of the reservoir or which fish species are attracted to the added structure, habitat additions could change prey communities at the scale of the added structure or at larger spatial scales. For example, invertebrate prey densities could be reduced near habitat structures through direct predation by fish as invertivorous fish are known to be able to suppress local prey (Knapp 2005; Detmer et al. 2017). Alternatively, macroinvertebrate density may be enhanced by aggregations of noninsectivorous fishes that increase nutrient availability through excretion, leading to increased rates of nutrient regeneration and primary producer abundance (Collins and Wahl 2017). In marine systems, artificial structures have been found to support increased abundance and diversity of invertebrates over areas much larger than the structures themselves (e.g., Reeds et al. 2018). Whether or not similar effects of added habitat structures occur in reservoirs remains to be tested.
In this study we evaluated fish and fish prey densities and assemblages associated with artificial habitat structures in a large reservoir. Our goals were to test for differences in the relative abundance and assemblage structure of fish, benthic macroinvertebrates, and zooplankton between reservoir coves with and without newly added offshore habitat structures. We also tested for potential effects offshore structures had on the relative abundance and assemblage structure of fish species in littoral habitats within coves (i.e., beyond the immediate scale of the habitat structures). We sampled all reservoir coves during the spring and fall because we anticipated fish use of the added structures and existing littoral habitats to be highest during these seasons (Johnson et al. 1988; Pope and Willis 1996; Fischer and Quist 2014) and we wanted to evaluate fish use at times when anglers also target the added structures, which are predominantly spring and fall.
METHODS
Study Site
Lake Shelbyville is a 4,500-ha reservoir in central Illinois (Moultrie County and Shelby County) created in 1970 by the damming of the Kaskaskia River. The water level in Lake Shelbyville fluctuates seasonally (about 1.5 m between the summer and winter pools) and is regulated by the US Army Corps of Engineers for flood control, water supply, and recreation purposes. The reservoir has a mean depth of 5.8 m (maximum 20.4 m) and branches into irregular arms and coves such that its surface width rarely exceeds 1.5 km. Lake Shelbyville is eutrophic (chlorophyll-a range: 1.2–50.9 mg/L; total phosphorus range: 0.15–0.68 mg/L during this study) and its 193-km shoreline is characterized by steep, eroded banks with little to no littoral vegetation or natural structural habitat (Figure 1). Partially submerged dead trees and stumps are present in some coves, but the quantity and complexity of coarse woody habitat (CWH) has degraded with reservoir age. In October 2017, artificial habitat structures were added to a subset of coves in Lake Shelbyville for the purpose of improving the recreational value of the fishery.

Study Design
We conducted our study within six coves in Lake Shelbyville. All coves were similar in shoreline morphometry and quantity of submerged natural habitat (about 1–2 logs per kilometer of shoreline) as estimated with sidescan sonar. Cube-shaped artificial habitat structures, aggregated in pairs, were installed in three coves at water depths of 4–5 m and at a density of about two structures per 0.4 ha, resulting in 16–22 structures per cove. Structures were constructed from PVC pipe frames (3.81-cm pipe) fitted with about 28 m of corrugated tubing (10.16-cm tubing) and were similar to the cube-shaped PVC structures used by the Georgia Department of Natural Resources (Figure 1). Each 1.22- × 1.22- × 1.52-m structure contained six alternating (parallel and diagonal) layers of corrugated tubing within the interior of the frame for structural complexity. Each structure had a total surface area of about 10.41 m2 and a volume of about 2.25 m3. All structures were built and deployed by the U.S. Army Corps of Engineers, St. Louis District; the Illinois Department of Natural Resources; and volunteers. The remaining three study coves received no artificial habitat structures. Coves with added offshore structures had a mean ± SE surface area of 3.72 ± 0.32 ha and a mean depth of 4.47 ± 0.07 m. Coves without offshore structures had a mean surface area of 4.23 ± 0.83 ha and mean depth of 4.17 ± 0.04 m.
Beginning in October 2017, 2 weeks after the addition of artificial structures, fish surveys (n = 6) were conducted each fall (mid-October) and spring (mid-May) in the study coves during 2017–2020. Surveys were not conducted during spring 2020 due to the COVID-19 pandemic. Surveys of zooplankton (n = 5) and benthic macroinvertebrates (n = 5) in the study coves were conducted on the same fall–spring schedule and concluded after fall 2019. We used deepwater AC boat electrofishing to sample the fish assemblages occupying the offshore structures because standard boat electrofishing gear was ineffective for collecting fish at the depths the artificial structures were placed. The deepwater electrofishing setup consisted of a 230-V, three-phase AC Multiquip GDP-5HA generator and three poles with one electrode on each pole outfitted for deepwater sampling via an insulated cable that only exposed the electrodes at a desired depth (e.g., 0–0.5 m above the structures). The length of the exposed electrodes was adjusted to account for differences in ambient conductivity and a target of 7–8 A was used for all sampling events. Stationary deepwater electrofishing surveys were conducted for 10 min each at three fixed sites per cove for a total of 30 min of offshore electrofishing per cove per survey event; all coves were sampled on the same day. A single dipnetter worked from the stationary electrofishing boat, and two dipnetters worked from a separate chase boat that circled the area. In the coves containing offshore structures, each fixed site was directly above a pair of submerged artificial structures. In coves without added structures, each fixed site contained no vertical structure but had similar depths and bottom contours to sites that contained artificial structures. All offshore sites within a cove were separated by at least 80 m. We sampled fish communities within the littoral zone of each cove using pulsed-DC boat electrofishing (60 Hz, 25% duty cycle). Each cove's littoral habitat was electrofished within a fixed transect for 15 min using an ETS MBS-1D electrofisher with a 3,000 W power goal based on water temperature and conductivity; all cove shorelines were sampled on the same day. All fish collected were identified to species, measured to total length (mm), and released.
The zooplankton and macroinvertebrate assemblages in each study cove were sampled at the same fixed sites used for deepwater electrofishing in coves with and without added structures. Zooplankton samples were collected using a 4-L Kemmerer water bottle at the depth of the artificial structures (about 1 m above the substrate) and filtered through 63-μm mesh. Zooplankton were preserved in 4% Lugol's solution and processed later in the laboratory, where samples were viewed under a stereomicroscope (40×−100×) and individual organisms were identified and enumerated. Identifications were pooled into four general taxonomic categories: Cladocera, Copepoda, Rotifera, and “other” (Chaoborus, Hydrachnidia, and Ostracoda). Benthic macroinvertebrate samples were collected using a petite Ponar dredge deployed about 1 m from the artificial structures. Side- and down-scan sonar was used to ensure that zooplankton and dredge samples were collected in close proximity to structures. Dredge samples were rinsed through a 500-μm sieve and preserved in the field in 95% ethanol with rose Bengal stain. In the laboratory, benthic macroinvertebrates were separated from debris and were identified and enumerated under a stereomicroscope (7 × to 45× magnification).
Statistical Analysis
Catch per unit effort (CPUE) was calculated as fish per 10-min sample for deepwater electrofishing at offshore sites and fish per 15-min sample for DC electrofishing of littoral fish assemblages. Total fish CPUE and total invertebrate density (zooplankton = organisms/L; macroinvertebrates = organisms/m2) data from coves with and without offshore structures were compared using Wilcoxon signed rank tests. Length–frequency distributions of fish collected at sites with offshore structures were compared between fall and spring using a Kolmogorov–Smirnov (K–S) cumulative distribution test.
Fish, zooplankton, and macroinvertebrate assemblage structures were compared between coves with and without added structure using permutational multivariate analysis of variance (PERMANOVA) tests in PRIMER (PRIMER-E, Albany, New Zealand). Our tests used repeated measures of taxa abundance or density through time and included site as a random variable nested within cove. Fall and spring assemblages were analyzed separately. Prior to PERMANOVA analyses, we log10(x + 1) transformed the data and constructed matrices based on the Bray–Curtis similarity index (Clarke and Green 1988; Clarke and Warwick 2001). We used nonmetric multidimensional scaling (NMDS, package Vegan; Oksanen et al. 2015) to visualize how similar assemblages were in coves with and without artificial structures. We identified the taxa driving differences between assemblages using a similarity percentages breakdown analysis (SIMPER). Bootstrapped t-tests were used to test whether or not a significant difference in mean CPUE existed between coves with and without structure for the top contributing taxa identified by SIMPER analysis. For all analyses, we considered treatment effects to be significant at α = 0.05. All statistical analyses were performed with PRIMER (v. 7.0.21) and R software (v. 4.0.2; R Foundation for Statistical Computing, Vienna).
RESULTS
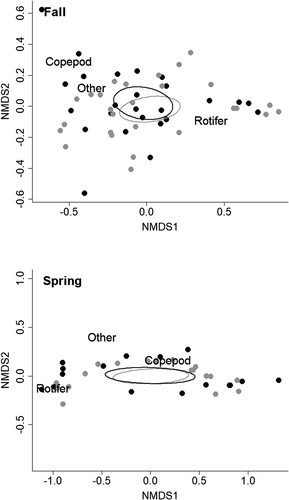
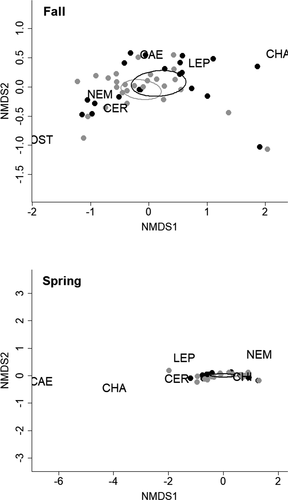
The addition of artificial habitat structures did not result in differences in invertebrate prey. Zooplankton and macroinvertebrate densities did not differ between coves with or without added offshore-habitat structure during fall or spring (Table 1) and showed similar trends through time (Figure S1 available in the Supplement in the online version of this article). Zooplankton assemblages did not differ between coves during the fall (PERMANOVA, pseudo-F1, 4.01 = 0.27, P = 0.801) or spring (pseudo-F1, 4.07 = 0.31, P = 0.824; Figure 2). Similarly, no difference in macroinvertebrate assemblages was detected between coves with and without added offshore-habitat structure during fall (pseudo-F1, 5.34 = 0.73, P = 0.715) or spring (pseudo-F1, 4.07 = 2.62, P = 0.158; Figure 3; Table S1 available in the Supplement in the online version of this article).
| Without structures | With structures | W | P | |
|---|---|---|---|---|
| Fall | ||||
| Zooplankton | 217.8 (16.8) | 218.8 (15.3) | 167 | 0.829 |
| Macroinvertebrates | 3,290.8 (505.0) | 2,502.5 (493.1) | 133 | 0.296 |
| Littoral fish | 199.1 (31.9) | 163.1 (25.2) | 68 | 0.023 |
| Littoral fish (no GZS) | 83.3 (18.6) | 81.5 (7.5) | 28 | 0.424 |
| Offshore fish | 2.6 (0.6) | 8.0 (1.1) | 74 | <0.001 |
| Spring | ||||
| Zooplankton | 439.1 (88.9) | 524.4 (112.0) | 47 | 0.163 |
| Macroinvertebrates | 3,484.4 (730.0) | 3,216.5 (725.1) | 89 | 0.579 |
| Littoral fish | 88.8 (5.3) | 137.8 (19.8) | 7 | 0.063 |
| Offshore fish | 1.2 (0.2) | 3.5 (0.7) | 15 | 0.005 |
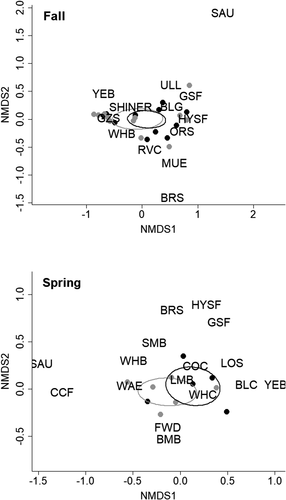
Mean CPUE of fish in littoral habitats was higher in coves without added structures in the fall surveys but not in the spring surveys; however, removing Gizzard Shad from the analysis resulted in no difference in overall littoral fish abundance between cove types (Table 1). No temporal trend in CPUE of fish in littoral habitats was observed (Figure S1). Littoral fish assemblage structures did not differ between coves with or without added structure in either season (fall: pseudo-F1, 4 = 0.55, P = 0.721; spring: pseudo-F1, 4 = 1.51, P = 0.207; Figure 4). Bluegill Lepomis macrochirus, Black Crappie Pomoxis nigromaculatus, White Crappie P. annularis, Gizzard Shad, and Largemouth Bass Micropterus salmoides comprised the majority of littoral fish collected from coves with and without structure (Tables S2 and S3).
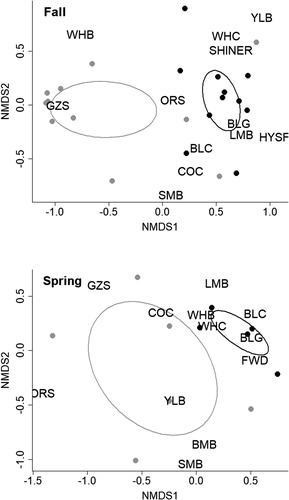
We identified significant differences in the mean CPUE of fish collected at offshore sites in fall and spring, with higher CPUE at sites in coves with added structures compared to coves without added structures (Table 1). Differences in mean CPUE of all fish collected between these sites were greatest 2 years postdeployment of offshore structures (Figure S1). Offshore fish assemblages also differed between coves with and without added structures in fall (pseudo-F1, 6.66 = 9.84, P = 0.001) and spring surveys (pseudo-F1, 5.28 = 2.88, P = 0.044; Figure 5). Differences in the assemblages during both seasons were driven by Gizzard Shad at offshore sites in coves without structures and higher CPUE of Bluegill and White Crappie at offshore sites in coves with submerged structures (Table 2). Length frequency distributions of fish collected at sites with offshore structures were different between seasons (K–S test; D = 0.17, P = 0.04), with a higher proportion of small fish (<200 mm) collected during the fall (Figure S2). Freshwater Drum were more abundant at offshore sites with added structures during spring but appeared to be equally distributed between offshore sites with and without structures during fall (Table 2). In contrast to surveys of littoral habitats, Largemouth Bass were rarely collected at offshore sites in coves with or without added structures. Buffalo species Ictiobus spp., Common Carp, White Bass, and Yellow Bass Morone mississippiensis were all collected at offshore sites, but CPUE was variable among coves with and without structure and between seasons (Tables S4 and S5).
| CPUE (fish per 10-min sample) | P-value | Contribution (%) | ||
|---|---|---|---|---|
| Without structures | With structures | |||
| Fall | ||||
| Bluegill | 0.52 (0.33) | 3.22 (0.62) | <0.01 | 23.05 |
| Gizzard Shad | 0.72 (0.17) | 0.52 (0.15) | 0.34 | 21.88 |
| Freshwater Drum | 0.64 (0.17) | 0.33 (0.16) | 0.08 | 14.74 |
| White Crappie | 0.08 (0.05) | 1.81 (0.37) | <0.01 | 12.22 |
| Black Crappie | 0.06 (0.04) | 0.64 (0.17) | <0.01 | 6.55 |
| Spring | ||||
| Bluegill | 0.11 (0.08) | 1.06 (0.42) | <0.01 | 17.76 |
| White Crappie | 0.22 (0.13) | 1.11 (0.30) | <0.01 | 16.21 |
| Gizzard Shad | 0.28 (0.18) | 0.17 (0.12) | 0.51 | 15.72 |
| Freshwater Drum | 0.11 (0.08) | 0.39 (0.12) | 0.04 | 10.69 |
| Smallmouth Buffalo | 0.17 (0.09) | 0.00 (0.00) | 0.04 | 9.71 |
DISCUSSION
Coves where artificial habitat structures were added showed expected increases in fish abundance, particularly sport fish species, yet we observed no change in zooplankton or macroinvertebrate densities and no effects on littoral fish relative abundances or composition. Overall, electrofishing CPUE was 2.5 times higher during spring sampling and over 3 times higher during fall sampling at offshore sites with artificial structures compared to offshore sites without added structure. Relative abundance increases were predominantly due to Pomoxis and Lepomis species, further supporting that adding structured habitat in reservoirs is effective for attracting and concentrating centrarchids (Daugherty et al. 2014; Driscoll et al. 2020). We also collected several nongame species at sites with artificial structures. Few fish were collected at offshore sites without added structure, and most often, Gizzard Shad made up the largest percentage of catches within these offshore sites. Therefore, the primary effect of the artificial structures was an increase in overall fish in a cove by increasing the relative abundance of centrarchids in the offshore environments.
Despite higher fish relative abundance at offshore sites with added structures, we found no difference in community composition or catch rates (excluding Gizzard Shad) of fishes occupying the littoral habitats of coves with and without offshore habitat structures. Artificial habitats can redirect fish away from natural habitats (Bohnsack 1989; Wehkamp and Fischer 2013), but fish lost to artificial habitat could be replaced in natural habitat patches through density-dependent habitat selection or production (Osenberg et al. 2002; Lindberg et al. 2006). In all coves examined, CWH was limited and any available structure was observed to concentrate fish. Thus, artificial structures within reservoir coves could be used by managers to effectively concentrate fish and support higher overall densities of fish within coves, but increases in fish density may be partitioned among newly available structure in sublittoral offshore habitats and existing structure within littoral habitats (e.g., Newbrey et al. 2005). Additional research is needed to test whether this pattern of fish distribution and abundance changes through time as littoral CWH would be expected to further degrade, while artificial structures remain intact (Bolding et al. 2004; Driscoll et al. 2020) and possibly support higher densities of fish after several years postdeployment, as indicated by our data and by Baumann et al. (2016). Further, it is unknown whether artificial structures in coves are only redistributing fish within the reservoir or increasing fish production by enhancing recruitment (e.g., through increased refugia) or growth, which may be currently limited by the availability of structured habitat and associated prey resources (Grossman et al. 1997; Powers et al. 2003; Sass et al. 2019).
In contrast to Baumann et al. (2016), who quantified fish use of similar cube-shaped structures, we found CPUE on artificial structures to be consistently higher during fall surveys compared to spring surveys. We also found higher abundances of young and small fish using offshore artificial structures during the fall. This pattern of habitat use may be due to several factors, including reproductive activity and greater use of shallow littoral habitats by crappie species and Bluegill during our spring sampling efforts (May 14–15 each year). Crappie species in Lake Shelbyville typically spawn earlier than when we conducted sampling (Illinois Natural History Survey, unpublished data) but may continue to spawn as late as June and use inshore areas throughout the spring before moving to deeper, offshore habitats (Guy et al. 1992; Sammons et al. 2001). Bluegill spawn at slightly higher water temperatures, have more prolonged spawning seasons than crappies, and often display protracted reproduction due to temporally variable environmental or energetic conditions (Garvey et al. 2002; Warren 2009; Beuchel et al. 2013). Bluegill may have also been more concentrated in shallow littoral habitats during the spring before shifting to structured habitat in deeper water during the summer and fall (Casterlin and Reynolds 1978; Johnson et al. 1988). Similar to our study, Daugherty et al. (2014) found CPUE and structure occupancy by centrarchids to be highest during fall sampling in a Texas reservoir supplemented with clusters of recycled Christmas trees but hypothesized that decreased spring CPUE could have been due to degradation and loss of structure complexity prior to their spring sampling efforts. Given that the structures we evaluated did not degrade between seasons, greater use of artificial structures during the fall compared to the spring may also be due to higher densities of age-0 fish or smaller, cover-seeking fish during the fall, attracting larger piscivores (Wege and Anderson 1979; Bolding et al. 2004), as well as fish seeking structure in areas of thermal refuge because water temperatures remain elevated throughout the fall in Lake Shelbyville (Detmer et al. 2022).
Quantifying abundance and community composition of fishes using artificial structures deployed in deep (>3 m) habitats within turbid reservoirs is challenging but necessary for evaluating artificial habitat enhancements. The deepwater AC electrofishing gear used in our study appeared to be effective for collecting fish proximate to artificial structures; however, further studies should be conducted to evaluate catchability of different species using this gear and the potential for gear bias or sampling inefficiencies. Sonar technologies, such as dual-frequency identification (DIDSON), have been successfully used to quantify fish targets on deep structures within turbid reservoirs (e.g., Baumann et al. 2016) but are limited for collecting all data important to fisheries managers, such as fish length and species identity (Mueller et al. 2010). A combination of electrofishing and sonar gears, however, could simultaneously be used to collect fish for identification and enumeration while assessing behavioral responses or other gear biases that affect electrofishing catchability on offshore structured habitat.
Overall, our sampling indicated that the added structures supported a mixed assemblage of fish with different feeding strategies (i.e., planktivores, insectivores, and piscivores), making it difficult to distinguish the relative influence of specific fish predators and different densities of fish between offshore sites with and without structure where invertebrate prey densities appeared equivalent. Similar to assessments of artificial reefs (Grossman et al. 1997; Powers et al. 2003), understanding such patterns is further complicated by the large spatial scale at which planktivorous and insectivorous fish and their prey are distributed in large reservoirs such as Lake Shelbyville (Van Den Avyle et al. 1982; Michaletz and Gale 1999). Thus, high connectivity between coves and the main channel of the reservoir may facilitate immigration and increased evenness of prey and also contribute to a lack of observed differences in densities of prey resources between coves with and without added structures.
We collected data on prey resources to test whether benthic macroinvertebrates and zooplankton were depleted or enhanced in areas proximate to artificial structures, a pattern (i.e., a halo effect) that has been found in some assessments of marine artificial reefs (Sweatman and Robertson 1994; Reeds et al. 2018). We found no evidence that fish using artificial structures were significantly reducing offshore densities of zooplankton or benthic macroinvertebrates. Although smaller in size compared to artificial reefs within marine environments, structures placed within reservoir coves are likely influenced by similar mechanisms as those associated with artificial reefs, such as spatially and temporally complex predator–prey interactions (Overholtzer-McLeod 2004, 2006; Campbell et al. 2011). As a result, similar densities of benthic and planktonic prey between offshore sites with and without structured habitat may be an emergent pattern arising from a balance between increased prey production and predator attraction at habitat patches (Powers et al. 2003). For example, the addition of structurally complex habitats likely attracts and increases benthic macroinvertebrate density (Foster et al. 1994; Sampaolo and Relini 1994; Schneider and Winemiller 2008) but also drives higher predation by insectivorous fishes. Similarly, artificial structures may support high densities of zooplankton (Champion et al. 2015) and, in the presence planktivorous fish, attract zooplankton by providing refuge from predation (Lauridsen et al. 1999; Burks et al. 2002). High zooplankton densities may have been a critical factor driving the abundance of Bluegill at our offshore sites, as the artificial structures likely provided increased cover and access to food. Although unexplored in freshwater systems, this link between zooplanktivory and habitat availability has been proposed as a potential mechanism by which artificial reefs may increase fish production (Champion et al. 2015). Further, the relationship between foraging space and food resources provided by artificial habitats may be useful for optimizing the size of deployed structures, as food availability has been shown to be inversely related to refuge space (i.e., large structures may provide more cover but lower foraging volume; Champion et al. 2015). Size considerations may be particularly important for designing and deploying artificial structures in reservoirs, as costs of materials, construction, and deployment can dictate the number and type of structures used in habitat enhancement programs (Tugend et al. 2002; Allen et al. 2014; Baumann et al. 2016).
Our study demonstrates that responses to habitat additions are likely more nuanced than increases in local abundance of fish. A lack of suppression of zooplankton and benthic macroinvertebrates in spite of increased abundance of planktivorous fishes suggests either a lack of consumptive effects or a potential balance between increased affinity by prey and increased predation occurring at the scale of added habitats. Still unknown, however, is whether habitat additions create local ecological hot spots, alter ecotrophic efficiency (e.g., DeBoom and Wahl 2013), and enhance or diminish the flow of energy. Likewise, a lack of an effect of offshore structures on proximate littoral communities points either to the potential for some separation of influence between these nearshore and offshore areas or to interactions between these habitats introducing compensatory responses in littoral habitat patches. Worth further exploration are the roles of habitat limitation, density dependence, and community structure on the potential for either of these processes. Ultimately, the focus of our study provides a new framework for which to consider potential food web responses to habitat additions in freshwaters.
ACKNOWLEDGMENTS
We thank the staff and students of the Kaskaskia, Sam Parr, and Ridge Lake Biological Stations for help in the field and laboratory and Randy Kramer for help fabricating the deepwater electrofishing rig used in this study. All animals used in this study were ethically collected and handled according to animal care and use guidelines established by the University of Illinois (Institutional Animal Care and Use protocol no. 17068). This study was supported by the Federal Aid in Sportfish Restoration Act (project F-185-R-6) administered through the Illinois Department of Natural Resources. There is no conflict of interest declared in this article.




