Clinical Utility of Far-Field Potentials in Amyotrophic Lateral Sclerosis
ABSTRACT
Introduction/Aims
Far field potentials (FFP) have been proposed as a reliable neurophysiological prognostic biomarker in amyotrophic lateral sclerosis (ALS). This study evaluates the diagnostic utility of ulnar nerve FFP in ALS.
Methods
Comprehensive peripheral neurophysiological assessments were conducted in 62 ALS and 43 ALS-mimicking disorder participants. The ulnar nerve was stimulated at the wrist, recording motor responses over the abductor digit minimi (ADM) muscle. Conventional compound muscle action potentials (CMAP), FFP, and near field potential amplitudes were recorded, alongside the split-hand index, neurophysiological index, motor unit number estimation (MScanFit-MUNE), and motor unit index (MUNIX). Diagnostic utility was evaluated using receiver operating characteristic (ROC) analysis.
Results
In ALS, FFP amplitude was significantly lower (5.07 ± 0.36 mV) compared to ALS mimics (8.25 ± 0.40 mV, p < 0.001). FFP amplitude exhibited a moderate-to-strong correlation with neurophysiological biomarkers, including CMAP amplitude (ρ = 0.77, p < 0.001), split-hand index (ρ = 0.53, p < 0.001), neurophysiological index (ρ = 0.52, p < 0.001), MUNIX (ρ = 0.69, p < 0.001), and MScanFit-MUNE (ρ = 0.66, p < 0.001). Weak-to-moderate correlations were also observed with clinical measures of disease progression, including upper limb muscle strength, ALS functional rating score-revised (ALSFRS-R) and the rate of decline in the ALSFRS-R fine motor subscore. ROC analysis demonstrated that FFP amplitude reliably distinguished ALS from mimicking disorders (AUC = 0.80, 95% CI: 0.71–0.89), with consistent diagnostic accuracy across ALS phenotypes.
Discussion
The diagnostic capability of FFP amplitude was comparable to established neurophysiological biomarkers utilized in ALS. It is a promising prognostic and diagnostic biomarker for ALS. Its simplicity and reproducibility complement traditional neurophysiological measures, offering potential for clinical application in ALS diagnosis and monitoring.
1 Introduction
Timely diagnosis of ALS remains challenging, particularly in early stages of the disease or in atypical phenotypes [1, 2]. Consequently, reliable and sensitive biomarkers are essential for early detection and for monitoring disease progression in both clinical and research settings [3].
Among currently available tools, measurement of the compound muscle action potential (CMAP) amplitude is one of the simplest and most accessible methods for quantifying lower motor neuron dysfunction. CMAP amplitude reflects the combined effects of denervation and collateral reinnervation, but it may not reliably indicate axonal loss. First, electrode positioning can affect reproducibility. Second, in chronic denervation, collateral sprouting may preserve CMAP amplitudes despite significant motor unit loss, as healthier axons reinnervate muscles previously served by degenerating motor neurons [4].
To address these limitations, more refined neurophysiological biomarkers have emerged, such as the split hand index (SHI), neurophysiological index (NI), and MScanFit motor unit number estimation (MScan). These have demonstrated improved sensitivity in detecting motor unit loss in ALS [5-9].
Far-field potentials (FFPs) are triphasic electrical signals, recorded between proximal (ulnar styloid) and distal (proximal distal phalanx) reference electrodes [10-12]. They have emerged as a novel neurophysiological measure of lower motor neuron integrity. Higashihara et al. [13] have demonstrated that FFPs are reproducible and correlate with clinical progression in ALS, supporting their potential as a promising biomarker. Notably, because FFPs originate remotely from both the active and reference electrodes, they are less affected by electrode placement, enhancing consistency [10].
Given their reproducibility and correlation with disease progression, FFP-CMAPs may offer a reliable and robust lower motor neuron biomarker in ALS [13]. Their diagnostic utility, however, relative to established neurophysiological markers, remains unclear. In this study, we evaluated the diagnostic performance of the ulnar FFP-CMAP in comparison to established neurophysiological biomarkers, with the aim of determining its clinical utility as a reproducible and accessible measure of lower motor neuron dysfunction in ALS.
2 Methods
2.1 Subjects
Participants with suspected ALS were prospectively recruited from two multidisciplinary ALS clinics affiliated with Sydney Health Partners, University of Sydney, according to STARD criteria [14]. All participants exhibited either a lower motor neuron or a mixed upper and lower motor neuron syndrome with a progressive neuromuscular course lasting at least 6 months, as noted by the referring physician. Recruitment occurred at the Brain and Nerve Research Centre (Sydney, Australia). ALS diagnoses were made according to the Gold Coast Criteria [15]. Exclusion criteria were marked wasting of the abductor digiti minimi (precluding CMAP amplitude recording) and the presence of hereditary motor neuronopathy or demyelinating sensorimotor neuropathy, which could confound neurophysiological interpretation.
Prior to participation, all patients provided written informed consent in accordance with the Declaration of Helsinki. The study was approved by the Sydney Local Health District Human Research Ethics Committee.
2.2 Clinical Assessment
2.3 Neurophysiology Assessments
Far-field potentials were recorded by positioning the E1 electrode over the ulnar styloid and E2 over the distal fifth phalange. Baseline-to-positive peak FFP amplitude was recorded from the abductor digiti minimi (ADM, Figure S1A) [10]. Near-field potentials (NFP) were recorded by positioning the E1 electrode over the muscle belly and the E2 electrode over the ulnar styloid process (Figure S1B). Baseline-to-negative peak NFP amplitudes were recorded. The conventional baseline-to-peak CMAP amplitudes were recorded by positioning the E1 electrode over the ADM muscle belly while the E2 electrode was positioned over the fifth distal phalanx (Figure S1C).
The FFP, NFP, and CMAP amplitudes were recorded from the right hand using a Synergy EMG machine (Version 22.1.0.151, Natus Neurology Inc., Middleton, WI, USA). In the same sitting, the split hand index (SHI) and neurophysiological index (NI) were calculated using the previously reported formula [9, 19].
Motor unit number index studies were undertaken on the right ulnar nerve, with recordings made from the ADM muscle in accordance with established methodology [20-22]. Briefly, surface EMG interference pattern (SIP) was recorded during stable muscle contraction and the force of muscle contraction was varied from minimal to maximal level in five steps. A minimum of 20 SIPs were recorded, each SIP being of 500 ms duration with 5 s of rest provided after collecting 10 SIPs to avoid fatigue. SIP epochs with unstable contractions or duration less than 300 ms were excluded from further analysis [23]. Signals with SIP area > 20 mV.ms, SIP area/CMAP area > 1, and ideal case motor unit count (ICMUC) < 100 were used in the calculation of MUNIX [21]. Motor unit size index (MUSIX) values were calculated by dividing the MUNIX value by the baseline-peak CMAP amplitude. All studies were undertaken on the Synergy EMG machine (Version 22.1.0.151, Natus Neurology Inc., Middleton, WI, USA).
MScanFit-MUNE was performed on the right ulnar nerve utilizing a previously described technique [8]. The responses were recorded from the ADM muscle with the E1 (active) electrode positioned over the belly of the ADM and E2 (reference) over the distal fifth phalanx. The number of motor units and single motor unit amplitudes were recorded. All motor responses were recorded using AgCl disc electrodes (10-mm diameter; 3 M, Maplewood, Minnesota).
2.3.1 Statistical Analysis
The data was assessed for normality by the Shapiro–Wilk test. For normally distributed data, student's t-test was used, while for non-parametric data, the Mann–Whitney U test was used. Pearson's correlation coefficient or Spearman's rho was used for analyzing associations between parameters. A standard reference was used to grade the strength of correlations [24]. Multivariate linear regression analysis was performed to identify independent predictors of FFP in ALS patients. Analysis of covariance (ANCOVA) was undertaken to assess the effects of covariates. Receiver operating characteristic (ROC) analysis was performed to assess the diagnostic accuracy of FFP amplitude in comparison to other neurophysiological parameters. Normally distributed data were expressed as mean ± standard error of the mean (SEM), and non-normally distributed data as median (interquartile range, IQR). p-value < 0.05 were considered significant. All statistical analyses were performed using SPSS version 28 (IBM, Armonk, NY).
3 Results
3.1 Patient Demographics
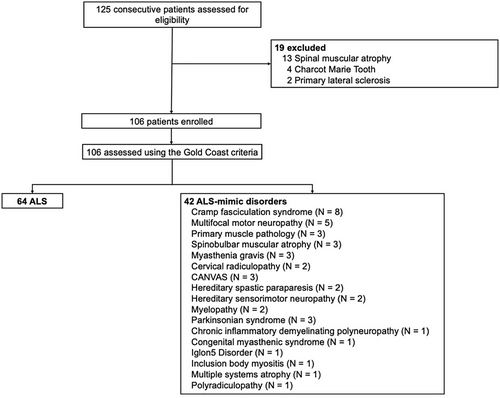
Between February 2024 and February 2025, a total of 125 participants were screened. From this cohort, 106 patients met the inclusion criteria for this study. A total of 64 ALS and 42 ALS-mimic participants were recruited (Figure 1). Most ALS participants presented with limb-onset disease and demonstrated moderate muscle weakness and functional impairment (Table 1). Disease progression in the ALS cohort was moderate, while the degree of upper motor neuron dysfunction was significantly more pronounced in ALS participants compared to ALS-mimics.
| ALS (n = 64) | ALS mimic disorders (n = 42) | p | |
|---|---|---|---|
| Demographic data | |||
| Median age at assessment (years) | 67 (60–72.8) | 58 (38.8–69) | 0.003 |
| Men (%) | 38 (59.4) | 32 (76.2) | |
| Women (%) | 26 (40.6) | 10 (23.8) | |
| Disease duration (months) | 12.79 (7.48–27.52) | 39.62 (12.48–157.39) | 0.001 |
| Onset of disease | |||
| Limb onset (%) | 46 (71.9) | ||
| Bulbar onset (%) | 18 (28.1) | ||
| ALSFRS-R | 39 (34–43) | ||
| ALSFRS-R (fine motor) | 10 (8–11) | ||
| Rate of Decline | |||
| ALSFRS-R | 0.54 (0.33–1.03) | ||
| ALSFRS-R (fine motor) | 0.12 (0.03–0.34) | ||
| Upper limb MRC score | 55 (48–59) | 60 (57–60) | < 0.001 |
| ADM MRC score | 4 (4–5) | 5 (4–5) | < 0.001 |
| UMN score | 8.5 (6–10) | 0 (0–1) | < 0.001 |
| Peripheral neurophysiology | |||
| Ulnar FFP amplitude (mV) | 5.07 ± 0.36 | 8.25 ± 0.40 | < 0.001 |
| Ulnar NFP amplitude (mV) | 4.95 ± 0.32 | 8.65 ± 0.44 | < 0.001 |
| Ulnar CMAP amplitude (mV) | 7.02 ± 0.38 | 10.81 ± 0.43 | < 0.001 |
| Split hand index | 3.33 (1.29–6.34) | 7.47 (5.57–10.20) | < 0.001 |
| Neurophysiological index | 0.51 (0.26–1.14) | 1.91 (1.29–2.69) | < 0.001 |
| MUNIX | 100 (69–149) | 159 (131–203) | < 0.001 |
| MScan number of motor units | 51 (33–88.5) | 92 (67.5–110.5) | 0.008 |
- Note: Data are expressed as median (interquartile range) mean ± standard error of the mean or median (interquartile range).
- Abbreviations: ALSFRS-R = amyotrophic lateral sclerosis functional rating score-revised. MRC = Medical Research Council. UMN = upper motor neuron. CMAP = compound motor action potential. FFP = far-field potential. NFP = near field potential. MUNE = motor unit number estimate.
3.2 Neurophysiological Parameters
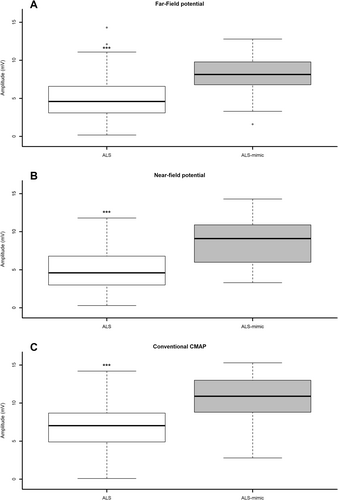
The FFP amplitude was significantly reduced in ALS participants compared to ALS-mimics (Table 1; Figure 2A). Similarly, NFP amplitude was markedly lower in ALS patients (Table 1; Figure 2B). Conventional CMAP amplitude was significantly reduced in ALS participants (Table 1; Figure 2C). In addition, the SHI, NI, MUNIX, and the number of motor units derived from MScan were all significantly lower in ALS patients (Table 1).
Subgroup analysis was conducted to evaluate whether FFP amplitude differed across clinical ALS phenotypes. No significant differences in FFP amplitude were observed based on site of disease onset (limb vs. bulbar; p = 0.81), disease duration (early disease < 12 months; later ≥ 12 months, p = 0.49), degree of functional impairment as measured by ALSFRS-R score (mild disease ≥ 39; more severe disease < 39, p = 0.96) and degree of clinical upper motor neuron dysfunction (prominent ≥ 6; less prominent < 6, p = 0.61).
3.3 Correlation With Clinical and Neurophysiological Biomarkers
The FFP amplitude exhibited weak correlations with ADM MRC score, the ALSFRS-R fine motor subscore, rate of disease progression of the ALSFRS-R fine motor score, and the upper limb composite MRC score (Table 2). There were no significant correlations of FFP amplitude with disease duration, total ALSFRS-R score, rate of disease progression, or UMN score. At a neurophysiological level, the FFP amplitude was strongly correlated with the conventional CMAP amplitude, and moderately correlated with the NFP amplitude, the split hand index, neurophysiological index, and motor unit number estimation calculated by MScanFit-MUNE (Table 2).
| Correlation with FFP (ρ) | p | |
|---|---|---|
| Clinical parameters | ||
| ADM MRC score | 0.39 | 0.002* |
| Upper limb composite MRC score | 0.38 | 0.002* |
| ALSFRS-R | −0.03 | 0.83 |
| ALSFRS-R (fine motor) | 0.38 | 0.002* |
| Rate of decline | ||
| ALSFRS-R | 0.06 | 0.67 |
| ALSFRS-R (fine motor) | −0.26 | 0.04* |
| Disease duration | 0.03 | 0.84 |
| UMN score | −0.04 | 0.78 |
| Age | −0.25 | 0.01* |
| Neurophysiological parameters | ||
| Conventional CMAP amplitude | 0.77 | < 0.001* |
| NFP amplitude | 0.60 | < 0.001* |
| Split hand index | 0.53 | < 0.001* |
| Neurophysiological index | 0.52 | < 0.001* |
| MUNIX | 0.69 | < 0.001* |
| MScan number of motor units | 0.66 | < 0.001* |
- Note: Significant correlations are indicated by an asterisk (*).
- Abbreviations: ADM, abductor digit minimi; ALSFRS-R, amyotrophic lateral sclerosis functional rating score-revised; CMAP, compound muscle action potential; FFP, far-field potential; MRC, Medical Research Council; MUNIX, motor unit number index; NFP, near-field potential; UMN, upper motor neuron.
A weak negative correlation was observed between FFP amplitude and age, indicating that FFP amplitude decreases with advancing age. Because ALS patients were significantly older than controls, a univariate ANCOVA was performed with age as a covariate to determine whether it accounted for the group differences in FFP amplitudes. The effect of “group” remained robust, with ALS patients exhibiting a significantly lower FFP amplitude compared to controls (F1,101 = 24.95, p < 0.001, η2p = 0.198). In contrast, age did not significantly predict FFP amplitude in the adjusted model (F1,101 = 1.04, p = 0.311, η2p = 0.010), indicating that the group difference in FFP amplitude was not driven by age.
Multiple linear regression analysis was conducted to identify independent predictors of FFP amplitude in ALS participants. Conventional CMAP amplitude was the strongest predictor of FFP amplitude (B = 0.739, SE = 0.130, p < 0.001; β = 0.710) followed by MUNIX values (B = 0.018, SE = 0.006, p = 0.004, β = 0.35), explaining a substantial proportion of the variance in FFP amplitude (R2 = 0.665, adjusted R2 = 0.638, F4,50 = 24.82, p < 0.001). Collinearity diagnostics were within acceptable limits (Variance Inflation Factor < 3, tolerance > 0.2), and the Durbin–Watson statistic (2.04) indicated no evidence of autocorrelation.
Candidate variables identified through Spearman correlation analysis—including disease duration, ALSFRS-R fine motor subscores, upper limb composite MRC score, MRC score for ADM, rate of decline of the ALSFRS-R fine motor subscore, neurophysiological index, split hand index, and motor unit number estimation from MScanFit-MUNE—were excluded from the final model due to non-significance. Although NFP amplitude showed a significant bivariate correlation with FFP amplitude, its inclusion in the multivariate model with the conventional CMAP amplitude led to a negative coefficient due to shared variance. As the CMAP amplitude provided a stronger predictive value and more consistent interpretation, NFP amplitude was excluded from the final model.
3.4 Diagnostic Utility
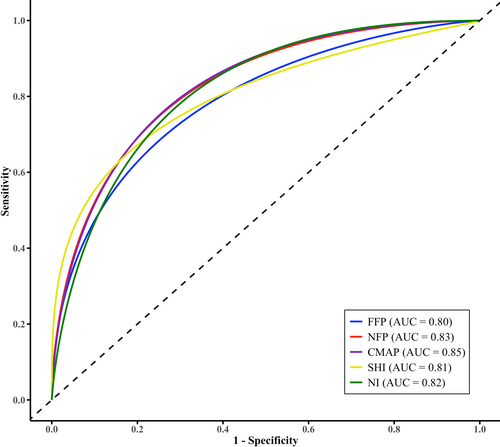
Receiver operating characteristic (ROC) curve analysis demonstrated that FFP amplitude had a very good ability to distinguish ALS from ALS-mimicking disorders, with an area under the curve (AUC) of 0.80 (95% confidence interval [CI]: 0.71–0.89). NFP amplitude exhibited a similar diagnostic performance (AUC = 0.83, 95% CI: 0.75–0.91).
Pairwise AUC comparisons showed no significant difference between FFP and conventional CMAP amplitude (AUC = 0.85, 95% CI: 0.78–0.92, AUCdifference = −0.05 ± 0.28, 95% CI: −0.10–0.01, p = 0.11), the SHI (AUC = 0.81, 95% CI: 0.73–0.89; AUCdifference = −0.009 ± 0.29, 95% CI: −0.11–0.10, p = 0.87), and the NI (AUC = 0.82, 95% CI: 0.73–0.91), (AUCdifference = −0.03 ± 0.30, 95% CI: −0.14–0.08, p = 0.58) (Figure 3). These findings suggest that FFP amplitude offers comparable diagnostic utility to other established neurophysiological indices.
The optimal cutoff value for FFP amplitude, determined using Youden's index, was 6.15 mV. This threshold provided a sensitivity of 71.9% and a specificity of 85%, yielding a Youden's index of 0.57, indicative of good overall diagnostic discrimination. The FFP amplitude showed high positive predictive value (87.96%), supporting its utility as a confirmatory tool for ALS diagnosis in clinically suspected cases. Its moderate negative predictive value (66.49%) suggests a more limited role in confidently excluding ALS, particularly in early stages or atypical disease. The diagnostic odds ratio (DOR) for FFP amplitude at the optimal cutoff (6.15 mV) was 14.5, indicating a moderate-to-good level of overall diagnostic performance, further supporting the utility of FFP amplitude in differentiating ALS from ALS-mimicking disorders. Subgroup analysis across different ALS phenotypes demonstrated that the diagnostic performance of the FFP amplitude was preserved irrespective of the site of disease onset, disease duration, presence of upper motor neuron signs, or level of functional impairment (Table 3). Seven ALS patients did not meet the Gold Coast criteria at baseline. Among them, FFP amplitude was reduced in 6 (86%), CMAP amplitude in 2 (29%), NFP in 6 (86%), SHI in 5 (71%), and NI in 3 (43%).
| Area under curve | Standard error | 95% confidence interval | AUC difference | p | |
|---|---|---|---|---|---|
| Limb onset | 0.80 | 0.05 | 0.71–0.90 | 0.00 | 1.00 |
| Bulbar onset | 0.80 | 0.06 | 0.68–0.92 | ||
| Disease duration < 12 months | 0.80 | 0.06 | 0.68–0.92 | 0.01 | 0.90 |
| Disease duration ≥ 12 months | 0.81 | 0.05 | 0.71–0.90 | ||
| UMN score ≥ 6 | 0.79 | 0.05 | 0.70–0.89 | 0.04 | 0.65 |
| UMN score < 6 | 0.83 | 0.06 | 0.71–0.95 | ||
| ALSFRS-R < 39 | 0.77 | 0.06 | 0.66–0.89 | 0.05 | 0.52 |
| ALSFRS-R ≥ 39 | 0.82 | 0.05 | 0.72–0.92 | ||
| ALSFRS-R (fine motor) < 9 | 0.85 | 0.06 | 0.73–0.97 | 0.07 | 0.37 |
| ALSFRS-R (fine motor) ≥ 9 | 0.78 | 0.05 | 0.68–0.88 |
- Abbreviation: ALSFRS-R = amyotrophic lateral sclerosis functional rating score-revised.
4 Discussion
This study demonstrated a significant reduction in FFP amplitude in ALS, effectively distinguishing ALS from mimicking disorders in an appropriate clinical context. This diagnostic utility remained consistent across disease-onset site, duration, presence of UMN signs, and functional impairment. FFP amplitude correlated significantly with clinical and neurophysiological measures of disease severity, suggesting its potential as a surrogate biomarker of progression. Additionally, conventional CMAP amplitude and MUNIX values were independent predictors of FFP amplitude, reinforcing its role as an outcome biomarker. The diagnostic capability of FFP amplitude was comparable to established neurophysiological biomarkers utilized in ALS [3].
4.1 Clinical Utility of Far-Field Potentials
Major contributors to the FFPs are intrinsic hand muscles, particularly the interossei, which are innervated by the deep motor branch of the ulnar nerve [10, 11, 25, 26]. In contrast, the hypothenar eminence muscles, including the ADM, predominantly contribute to the NFP responses recorded using the ADM belly-proximal reference montage [25]. The marked reduction in FFP amplitude in ALS patients, compared to those with mimicking disorders, highlights a specific vulnerability of C8-T1 motor neurons to degeneration in ALS and aligns with the well-established split-hand pattern of muscle wasting in ALS [6, 9, 22, 27-30], a notion supported by the significant correlation between FFP amplitude and split-hand index observed in this study.
It was recently reported that the decline in FFP amplitude may reflect disease progression in ALS [13]. The present study extends this observation by establishing significant positive correlations between FFP amplitude and clinical measures, suggesting that FFP amplitude may reflect motor function and disease severity in ALS. Additionally, the significant negative correlation with the rate of decline in the ALSFRS-R fine motor subscore suggests that lower FFP amplitudes may be associated with a faster rate of disease progression in fine motor function, further supporting the potential of FFPs as a biomarker of disease dynamics. Despite these significant correlations, it is important to note that FFP amplitude did not correlate significantly with disease duration, total ALSFRS-R score, rate of disease progression, or the UMN score, suggesting that FFPs may be more sensitive to changes in motor function and fine motor decline rather than the broader progression of ALS. Although multiple linear regression analysis did not yield significant results for clinical parameters, the observed significant correlations, particularly with motor function and disease progression, point to FFP amplitude as a potential prognostic and outcome biomarker in ALS. Further studies are required to confirm these findings, particularly with larger sample sizes and longitudinal studies, to solidify the role of FFPs as a biomarker of clinical disease progression in ALS.
In addition to the significant clinical correlations, FFP amplitude was significantly correlated with other peripheral neurophysiological measures. Taken together, these findings provide further support for the utility of FFPs in assessing motor function and disease progression in ALS. Despite these moderate correlations, MLR analysis identified CMAP amplitude and MUNIX as independent predictors of FFP amplitude, highlighting their primary role in reflecting lower motor neuron function. This suggests that CMAP amplitude and MUNIX may be more direct and reliable measures of LMN integrity in ALS, with FFP amplitude serving as a complementary tool in detecting LMN loss. The combination of these measures enhances the diagnostic sensitivity and provides a more comprehensive understanding of motor neuron degeneration.
At a diagnostic level, the present study revealed that reduction of FFP amplitude exhibited very good discriminatory ability in distinguishing ALS from ALS-mimicking disorders. The diagnostic utility was comparable to established neurophysiological biomarkers, including reduction of CMAP amplitude, split-hand index, and neurophysiological index, suggesting that FFP amplitude offers diagnostic utility on par with these well-established biomarkers. The optimal cutoff value for FFP amplitude, determined via Youden's index, demonstrated strong overall diagnostic potential for differentiating ALS from mimicking disorders. The positive predictive value and diagnostic odds ratio further support the utility of reduced FFP amplitude as a surrogate diagnostic tool for ALS. However, the moderate negative predictive value limits its ability to exclude ALS, particularly in early stages or atypical phenotypes. Notably, the diagnostic utility of FFP amplitude was consistent across various ALS phenotypes, regardless of disease onset, duration, or functional impairment, reinforcing its robustness as a potential diagnostic biomarker for ALS.
A key advantage of measuring FFPs over conventional CMAP amplitudes and MUNIX values lies in its exceptional intra- and inter-rater reproducibility [13]. Unlike CMAPs, which are highly sensitive to electrode placement, FFP recordings are largely unaffected by electrode positioning, ensuring more consistent and reliable results across different clinicians and settings [10-12, 31]. Far-field potentials can be easily recorded using standard EMG equipment, unlike more complex measures such as MUNIX and MScan-Fit MUNE, which require specialized devices and expertise. This simplicity and reliability make FFP amplitude a valuable diagnostic tool, particularly for widespread clinical use in the early detection and monitoring of ALS and other neurodegenerative diseases.
It could be argued that the diagnostic utility of reduced FFP amplitude in differentiating ALS from mimicking disorders is limited, particularly in early stages of ALS. The comparable AUCs for reduced FFP amplitude across ALS patients with both slower and faster functional decline, as well as those at earlier and later disease stages (Table 3), suggest that its diagnostic utility is relatively stable across disease progression. Nonetheless, multicentre studies are needed to further validate its diagnostic value. Correlations between FFP amplitude and clinical or most neurophysiological measures were weak to modest, limiting conclusions about prognostic value. Longitudinal studies are warranted to assess the prognostic relevance of reduced FFP amplitude in ALS. Separately, a reduction of FFP amplitude was evident in 86% of patients not meeting the Gold Coast criteria at baseline. Given the small sample size (n = 7), diagnostic interpretation is limited, and larger studies would be required to confirm this finding.
In conclusion, this study demonstrates that FFP amplitude is a valuable diagnostic and prognostic biomarker for ALS. Specifically, FFP amplitude was significantly reduced in ALS patients and strongly correlated with clinical measures of disease progression, particularly those reflecting upper limb weakness and fine motor dysfunction. Notably, an FFP amplitude of < 6.15 mV effectively distinguished ALS from mimicking disorders, underscoring its potential as a complementary diagnostic tool in clinical practice. The simplicity, reliability, and reproducibility of FFP recordings make them an attractive, easily implementable biomarker for routine clinical use. Incorporating FFPs into clinical diagnostics and clinical trials could substantially enhance early diagnosis and disease monitoring, and could serve as a robust surrogate outcome marker for ALS progression, improving both patient care and trial efficiency.
Author Contributions
Aicee Dawn Calma: conceptualization, investigation, methodology, formal analysis, writing – original draft, data curation. Nathan Pavey: conceptualization, methodology, investigation, writing – review and editing, formal analysis, data curation. Claudia Santos Silva: conceptualization, investigation, methodology, writing – review and editing, formal analysis. Yukiko Tsuji: conceptualization, investigation, writing – review and editing, methodology. Mehdi A. J. Van den Bos: writing – review and editing, methodology. Michelle A. Farrar: investigation, writing – review and editing, methodology. Parvathi Menon: conceptualization, investigation, methodology, writing – review and editing, supervision. Steve Vucic: conceptualization, investigation, funding acquisition, writing – review and editing, methodology, validation, formal analysis, supervision.
Acknowledgments
Support from Brain Foundation Australia (Aicee Dawn Calma), MND Research Institute Australia (Nathan Pavey, Mehdi van den Bos, Parvathi Menon and Steve Vucic) and NHMRC (Mehdi van den Bos, Parvathi Menon and Steve Vucic) is gratefully acknowledged. Open access publishing facilitated by The University of Sydney, as part of the Wiley - The University of Sydney agreement via the Council of Australian University Librarians.
Ethics Statement
This study was approved by the Sydney Local Health District Human Research Ethics Committee, Sydney, Australia. Prior to participation, all patients were provided with written informed consent in accordance with the Declaration of Helsinki.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.