Clinicopathological Features of Mixed Connective Tissue Disease-Related Myositis: A Case Series
Funding: This work was supported by Intramural Research Grant (5-6) to M.A. for Neurological and Psychiatric Disorders provided by the National Center of Neurology and Psychiatry of Japan; Grants-in-Aid for Research on Rare and Intractable Diseases (23FC1014) provided to M.A. from the Ministry of Health, Labour and Welfare of Japan; and KAKENHI (23K18260), Grant-in-Aid for Early Career Scientists from the Japan Society for the Promotion of Science (JSPS) provided to R.I.
ABSTRACT
Introduction
Mixed connective tissue disease (MCTD) patients often have myositis, however, myopathological and clinical data for MCTD are limited. Recent reports have shown that the pathology of MCTD myositis resembles that of immune-mediated necrotizing myopathy (IMNM), whereas earlier reports described perifascicular atrophy or inflammatory infiltrates predominantly in the perivascular region in MCTD myositis. We aim to describe the clinical and myopathological features of MCTD myositis.
Methods
We analyzed the clinical and myopathological findings of nine myositis patients with U1-RNP antibodies who fulfilled the diagnostic criteria for MCTD.
Results
Eight patients had muscle weakness in the proximal extremities, and overall, six patients had atypical weakness in the face, neck, wrist, or fingers. Four of those patients required additional intensive treatment (intravenous immunoglobulin or methylprednisolone). Therapeutic responses were consistently favorable overall, and there were no deaths during the observation period. In biopsied muscle specimens, common findings were mild myogenic change, increased necrotic and regenerating fibers, and inflammatory infiltrates predominating in the perivascular region. Two specimens were classified into the spectrum of dermatomyositis (DM); the remaining seven specimens, which had a smaller number of necrotic fibers and nonspecific infiltration, were unclassifiable.
Discussion
Patients with MCTD myositis often exhibit an axial or atypical distribution of muscle weakness, which may require intensive therapy. Histological study demonstrates the heterogeneity of myopathology of MCTD myositis and suggests that DM and underlying vasculopathy might be present in these patients.
1 Introduction
Mixed connective tissue disease (MCTD) is an autoimmune disorder characterized by features of systemic lupus erythaematous (SLE), systemic sclerosis (SSc), and myositis. A positive marker is a high blood level of anti-U1-ribonucleoprotein (RNP). In previous cohorts, 9.8%–28% of MCTD patients had myositis at the time of diagnosis, and 51% had myositis over their lifetimes [1, 2]. However, the myopathological features of MCTD and its clinical manifestations have not been fully defined. An immunohistochemical study revealed mixed mechanisms of both dermatomyositis (DM) and polymyositis (PM) [3]. Recent reports have highlighted the similarities between MCTD myositis and immune-mediated necrotizing myopathy (IMNM) [4, 5]. Thus, further detailed clinicopathological reports are needed to characterize the muscular phenotype in MCTD.
In the present study, we performed clinicopathological analyses of patients with MCTD who fulfilled international criteria and exhibited myopathic symptoms.
2 Methods
2.1 Patients and Study Design
We performed a retrospective study using the medical records of patients with MCTD who underwent muscle biopsies that were analyzed in our department between 1997 and 2023. Inclusion criteria required meeting international MCTD criteria as previously published [6-8] and having undergone a muscle biopsy. This study was approved by the Ethics Committee of the Tohoku University School of Medicine (2017-1-862).
2.2 Clinical Assessment and Investigations
The following clinical information at the time of muscle biopsy was reviewed: age; sex; titer of U1 RNP antibody; clinical symptoms associated with SLE, SSc, and myositis; past medical history; and other positive autoantibodies. Muscle strength was assessed by multiple neurologists with manual muscle testing (MMT) at the time of muscle biopsy. Information on detailed myopathic symptoms, that is, titers of serum myogenic enzymes, general scores of MMT, grip strength, and biopsy site, was collected. The presence of either muscle weakness or elevated serum creatine kinase (CK) was defined as indicative of muscle involvement in MCTD. Tests such as needle electromyography (EMG) and muscle CT/MRI were performed as needed, based on the clinician's judgment. The presence of paraspinal muscle atrophy was assessed by CT. The treatment and clinical course of the patients after muscle biopsy were also reviewed.
2.3 Muscle Biopsy
In our department, when patients with MCTD present with muscle weakness or elevated serum CK levels, we aim to perform a muscle biopsy to exclude other conditions, whenever possible, before starting steroid therapy. The decision of which muscle to biopsy was made by the clinicians based on a comprehensive assessment of muscle strength, EMG, CT, and MRI findings. The skin was anesthetized with 1% lidocaine. A 3 cm incision was made in the skin and fascia, and muscle tissue was obtained with a small pean and scissors.
2.4 Pathological Analysis of Muscle Biopsy Specimens
Biopsied skeletal muscles were rapidly frozen with isopentane cooled with liquid nitrogen and serially sectioned at 10 μm. A portion of the tissue was fixed in 10% buffered formalin and embedded in paraffin. The frozen sections were processed for hematoxylin and eosin (HE), modified Gomori trichrome (mGT), nicotinamide adenine dinucleotide tetrazolium (NADH-tetrazolium), cytochrome C oxidase (COX), alkaline phosphatase (ALP), ATPase, periodic acid Schiff, and oil red O staining according to standard procedures.
We performed immunohistochemistry on the frozen sections as previously described [9]. To clarify the features of inflammatory infiltrates, the frozen sections were immunostained for CD3, CD4, CD8, CD20, CD68, C5b-9, HLA class I, intercellular adhesion molecule-1 (ICAM-1) (Atlas, HPA002126, dilution 1:400), vascular cell adhesion molecule-1 (VCAM-1) (Proteintech, 11,444-1-AP, dilution 1:400), and myxovirus resistance protein 1 (MxA) (Santa Cruz, sc-166,412, dilution 1:100). For seven patients for whom no frozen sections were available, the paraffin-embedded sections were stained.
3 Results
3.1 Clinical and Laboratory Data
We identified 9 patients who met inclusion criteria. Clinical features, laboratory results, treatments, and treatment responses of all patients are summarized in Table 1. Patients were all Japanese and predominantly female (66.7%), with a median age of 40 years at the time of muscle biopsy.
| N (%) or median (IQR) | |
|---|---|
| Female | 6 (66.7%) |
| Age at the time of biopsy (years) | 40 (22–55) |
| Positivity of U1 RNP antibody | 9 (100%) |
| Extramuscular symptoms | |
| Raynaud's phenomenon | 9 (100%) |
| Swollen fingers or hands | 7 (77.8%) |
| Polyarthritis | 7 (77.8%) |
| Lympadenopathy | 3 (33.3%) |
| Facial erythaema | 1 (11.1%) |
| Pericarditis or pleuritis | 1 (11.1%) |
| Leukopaenia or thrombocytopaenia | 1 (11.1%) |
| Sclerodactyly | 2 (22.2%) |
| Pulmonary fibrosis, restrictive pattern on pulmonary tests, or reduced DLCO | 7 (77.8%) |
| Pulmonary arterial hypertension | 0 (0%) |
| Muscular symptoms | |
| Proximal weakness | 8 (88.9%) |
| Distal weakness | 3 (33.3%) |
| Facial weakness | 1 (11.1%) |
| Neck weakness | 4 (44.4%) |
| Muscle-related laboratory data | |
| CK level (IU/L) | 2059 (1171–5290) |
| Other positive antibodies | |
| Anti-SSA | 3 (33.3%) |
| Anti-thyroglobulin | 3 (33.3%) |
| Anti-Sm | 3 (33.3%) |
| Anti-centromere | 1 (11.1%) |
| Anti-Scl70 | 1 (11.1%) |
| Anti-ssDNA | 1 (11.1%) |
| Anti-TPO | 1 (11.1%) |
| Initial treatment | |
| Oral prednisolone | 9 (100%) |
| Additional treatment | |
| Intravenous methylprednisolone | 6 (66.7%) |
| Intravenous immunoglobulin (IVIG) | 4 (44.4%) |
| Favorable therapeutic response | 9 (100%) |
- Abbreviations: CK, creatine kinase; DLCO, diffusing capacity of the lungs for carbon monoxide; IQR, interquartile range.
Common extramuscular symptoms at the time of muscle biopsy were Raynaud's phenomenon (RP), polyarthritis, swollen fingers or hands, and lung involvement, including interstitial lung disease and restrictive changes in pulmonary function tests (Table 1). All the patients manifested one to two diagnostic features of SLE, and eight patients, except for patient 7, had one to three features of SSc (Table 2). No patients had pulmonary arterial hypertension (PAH) at the time of muscle biopsy or during the observation period. Common co-morbidities were reflux oesophagitis (33.3%) and hypothyroidism (22.2%). Sjögren's syndrome and rheumatoid arthritis were each present in one patient (Table 3). CK levels were elevated in all patients. All patients were positive for U1-RNP antibodies, and the median titer among eight patients was 163.1 U/mL (normal range; 0.0–9.9 U/mL); one patient's titer was above 550.0 U/mL, and the exact titer was unavailable. With regard to autoantibodies, anti-SS-A and anti-thyroglobulin (Tg) antibodies were frequently observed (Table 1).
| Patient | Sex | Age (yr) | Common symptoms | Mixed symptoms | Other symptom | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SLE-like symptoms | SSc-like symptoms | Polymyositis-like symptoms | ||||||||||||||
| RP | Swollen fingers or hands | Poly-arthritis | Lymph-adenopathy | Facial erythaema | Peri- carditis or pleuritis | Leuko- paenia or thrombo-cytopaenia | Sclero-dactyly | Pulmonary fibrosis, restrictive pattern on pulmonary function tests, or reduced DLCO | Hypomotility or esophageal-dilation | Muscle weakness | CK (U/L) | Myogenic pattern on EMG | PAH | |||
| 1 | F | 55 | + | + | + | − | − | − | − | − | + | − | + (including neck, wrist, and fingers) | 1171 | + | − |
| 2 | F | 22 | + | + | − | + | − | − | − | − | + | + | + | 5878 | + | − |
| 3 | F | 75 | + | + | + | − | − | ± | − | − | + | − | + (including neck) | 2123 | + | − (Borderline PAH half a year later) |
| 4 | M | 42 | + | + | − | + | − | − | − | + | + | + | + (including fingers) | 5585 | + | − |
| 5 | F | 27 | + | + | + | + | − | − | − | − | + | NA | + (including neck, wrist, and fingers) | 557 | + | − |
| 6 | M | 55 | + | + | + | − | − | − | − | − | + | NA | + (including neck and wrist) | 208 | NA | − |
| 7 | M | 40 | + | − | + | − | − | − | − | − | − | NA | − | 5290 | NA | − |
| 8 | F | 18 | + | + | + | − | + | − | − | − | + | − | + | 2059 | NA | − |
| 9 | F | 20 | + | − | + | − | − | − | + | + | − | − | + (including face) | 1377 | + | − |
- Abbreviations: CK, creatine kinase; EMG, electromyography; F, female; NA, not available; PAH, pulmonary arterial hypertension; RP, Raynaud's phenomenon; yr., years.
| Patient | Duration from muscle weakness to muscle biopsy (months) | Duration from the diagnosis of MCTD to muscle biopsy (months) | Duration from any MCTD related symptoms to muscle biopsy (months) | Titer of U1 RNP antibody (U/mL) | Past medical history | Myositis specific antibodies | Other positive antibodies | Reason for the muscle biopsy | Initial treatment | Additional treatment |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 14 | 30 | 144 | Above 550.0 | Appendicitis, perianal abscess, rheumatoid arthritis, Hashimoto's disease, lumbar disk herniation, reflux oesophagitis | Anti-ARS (−), Anti-Jo-1 (−), Anti-MDA-5 (−), Anti- TIF1-γ (−), Anti Mi-2 (−) | Anti-Tg antibodies | Moderate muscle weakness and high CK level | PSL 0.5 mg/kg | — |
| 2 | 3 | 2 | 72 | 353.0 | Reflux oesophagitis | Anti-ARS (−) | Anti-Tg antibodies, anti-thyroid peroxidase antibodies | High CK level in spite of mild muscle weakness | PSL 1 mg/kg | IVMP, IVIg |
| 3 | 6 | 1 | 6 | 164 | Uterine fibroid, atrial fibrillation | Anti-ARS (−), Anti-Jo-1 (−) | Anti-Tg antibodies | Muscle weakness and muscle pain | PSL 1 mg/kg | IVMP, IVIg |
| 4 | 2 | 3 | 6 | 180 | Ileus, reflux oesophagitis, knee osteoarthritis | Anti-Jo-1 (−) | Anti-SSA antibodies, anti-centromere antibodies | High CK level | PSL 1 mg/kg | IVMP, IVIg |
| 5 | 7 | Not diagnosed with MCTD before muscle biopsy | 180 | 162.1 | Sjögren's syndrome, cholecystitis, tension headache, hypothyroidism | Anti-Jo-1 (−) | Anti-Sm antibodies | Suspicion of myositis | PSL 1 mg/kg | IVMP, IVIg |
| 6 | 8 | 5 | 7 | 161.1 | None | Anti-Jo-1 (−) | Anti-SSA antibodies, anti-Sm antibodies | Suspicion of myositis | PSL 0.5 mg/kg | — |
| 7 | — | 0 | 15 | 183.7 | Natural pneumothorax | Anti-Jo-1 (−) | — | Suspicion of MCTD or polymyositis | PSL 1 mg/kg | IVMP |
| 8 | 1 | 2 | 7 | 135.6 | None | Anti-Jo-1 (−) | Anti-Sm antibodies | High CK level | PSL 1 mg/kg | — |
| 9 | 3 | 1 | 11 | 62.7 | Achilles tendon injury | Anti-Jo-1 (−) | Anti-SSA antibodies, anti-Scl-70 antibodies, anti-ssDNA antibodies | NA | PSL 1 mg/kg | IVMP |
- Abbreviations: CK, creatine kinase; IVIg, intravenous immunoglobulin; IVMP, intravenous methylprednisolone; M, male; NA, not available; PSL, prednisolone.
Concerning myopathic symptoms, as shown in Table 2, all patients except for patient 7 had proximal limb weakness. Four patients (patients 1, 3, 5, and 6) additionally had neck weakness, and 3 of them had mild atrophy in the paraspinal muscles, as observed in CT findings. Facial muscle weakness was observed in one patient (patient 9). Four patients (patients 1, 4, 5, and 6) exhibited proximal plus distal weakness involving the wrist and fingers. Muscle weakness was not severe enough to manifest as gait disturbances.
Times to muscle biopsy are shown in Table 3. The mean time from the onset of any MCTD-related symptoms to muscle biopsy was 49.8 months in nine patients. The mean time from the onset of muscle weakness to muscle biopsy was 5.5 months in eight patients. The muscle biopsies were taken from a clinically weak muscle. Patient 7 had no muscle weakness and a standard site (the biceps brachii or deltoid muscle) was selected for biopsy. A common reason for muscle biopsy was high CK level. When there were any signs suggestive of MCTD myositis, such as muscle weakness or inflammatory findings in muscle pathology, we considered initiating steroid therapy after performing a muscle biopsy. Prednisolone was initiated at a dose of 0.5–1 mg/kg/day and gradually tapered to approximately 5 mg/day over the course of about 1 year in all patients; in six patients, recurrence of elevated CK levels necessitated the addition of intravenous immunoglobulin (IVIg) or intravenous methylprednisolone (IVMP) 1000 mg/day for 3 days (Table 3). Therapeutic responses were consistently favorable in all patients, and there were no deaths during the observation period, which was an average of 7.3 years (1–21 years) from the time of the muscle biopsy.
3.2 Muscle Pathology
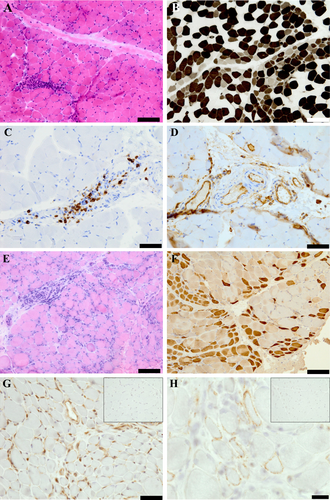
The findings are summarized in Table S1. All nine muscle biopsies demonstrated mild myogenic changes, including increased internal nuclei, myofiber size variability, and connective tissues. Necrotic fibers were found in eight patients, and regenerating fibers were found in all patients. Inflammatory infiltrates were present in 8 patients, predominating in the perivascular region. Other common general findings were moth-eaten fibers and type 2 myofiber atrophy, which were present in 3 and 5 patients, respectively. Perifascicular atrophy (PFA) was present in a single patient (patient 8) (Figure 1A,B). In this patient, perifascicular COX reduction and HLA class I enhancement were also found, nevertheless, MxA positivity was not apparent upon an available small amount of specimen. Although patient 4 did not exhibit typical PFA, there were regional and perimysial clusters of regenerating fibers (Figure 1E,F) and weak MxA positivity in perimysial myofibers and capillaries (Figure 1G). According to immunohistochemical data, HLA class I was upregulated in seven patients. CD20+ predominant B cells were observed in three patients (Figure 1C), whereas CD8+ predominant T cells and CD4+ predominant T cells were observed in one patient each. Inflammatory cell infiltration around nonnecrotic muscle fibers was not found. Positive ICAM-1/VCAM-1 staining of the vascular walls was found in all of seven specimens including vessels (Figure 1D) and that of the perifascicular area was found in patient 8. Although capillary C5b-9 deposition was not apparent in this series, sarcolemmal C5b-9 deposition on perimysial nonnecrotic myofibers was present in patient 4 (Figure 1H).
4 Discussion
Our results provide detailed clinicopathological data on MCTD myositis patients. Although there are previous clinicopathological studies on patients with anti-RNP antibodies positive myositis, this study focused on patients with myositis who met international diagnostic criteria for MCTD. Generally, patients with myositis have proximal limb muscle weakness, however, six out of eight patients in our cohort had atypical weakness in the face, neck, wrist, or fingers. In particular, neck weakness was present in four patients, three of whom exhibited axial myopathy. In a previous study, a patient diagnosed with MCTD exhibited uncommon muscle weakness in facial, neck, and arm muscles [10]. However, no detectable neck weakness was described in 20 patients with U1-RNP-positive myositis in another case series [4]. In patients with myositis that not only test positive for anti-RNP antibodies but also meet diagnostic criteria for MCTD, the pattern of muscle weakness can resemble that of axial myopathy. In addition, four of those patients with atypical weakness distribution required additional intensive treatment (IVMP and IVIg) during the follow-up period, which suggests that patients with muscle weakness beyond the proximal muscles may have higher levels of disease activity.
Clinically, despite the high comorbidity rate of SLE and SSc symptoms, no patient had complicated PAH during the follow-up period (7.45 ± 5.67 years) in our study, which may be associated with the favorable prognosis in this series. PAH is the major cause of death in MCTD patients, and in one study, 50 of 280 general MCTD patients (17.8%) had PAH [11]. These 50 patients developed PAH 14.5 ± 3.71 years after diagnosis of MCTD [11]. In other cohorts, 25% to 29% of myositis patients with anti-U1-RNP antibody positivity developed PAH [4, 5]. Therefore, careful monitoring for PAH is necessary even in patients with MCTD myositis.
Regarding the muscle pathology, the specimen of patient 4 showing regional regeneration with MxA positivity, and patient 8 with perifascicular atrophy were classified into the spectrum of DM pathology [12, 13]. In general, the predominant sites of inflammatory infiltrates in the muscle pathology of DM are perivascular and perimysial [14]. Infiltrating inflammatory cells include CD20+ B cells and CD4+ T cells [11] and C5b-9 is deposited on small blood vessels [15]. The above two specimens with DM pathology also demonstrated predominant perivascular infiltration of CD20+ B cells. Although typical C5b-9 capillary deposition for DM was not observed in these specimens, deposits on perifascicular nonnecrotic myofibers were observed in one biopsy. There were previous reports that the pathology of MCTD myositis exhibits PFA or inflammatory infiltrates predominating in the perivascular region [3, 10, 16]. Although the detailed immunohistochemical features of the two specimens were not homogeneous, the findings overlapping with the pathology of DM suggest the involvement of humoral immunity and underlying vasculopathy in MCTD.
A review of the literature (Table 4) found that 4 of 7 (57%) and 35 of 42 (83%) myositis patients with anti-U1-RNP autoantibodies were described as having biopsies showing a necrotizing pattern [4, 5]. In our cohort, necrotic and regenerating fibers were indeed observed in all patients, although the number of necrotic fibers in 7 non-DM patients was less than expected in IMNM. There was also relatively frequent inflammatory cell infiltration. Accordingly, it was difficult to fit these patients into typical IMNM [17]. However, the possibility of a milder or inactive form of IMNM remains in situations where IMNM-specific histological markers have not been found.
| Lundberg et al. [16] | Vianna et al. [3] | Tasca et al. [10] | Casal-Dominguez et al. [4] | Wesner et al. [5] | Our study | |
|---|---|---|---|---|---|---|
| Number of patients | 7 MCTD | 14 MCTD | 1 MCTD | 7U1-RNP + (Data not shown how many of these cases meet the criteria for MCTD) | 46 U1-RNP + (37/46 MCTD) | 9 MCTD |
| Inflammatory cell infiltrate | 7/7 (100%) | 14/14 (100%) | 1/1 (100%) | NA | NA | 8/9 (89%) |
| Predominant infiltrate region and inflammatory cell | Perimysium 7/7 |
CD4, CD20 cell in perivascular CD8 cell in endomysium |
CD4 cell in perivascular |
Perimysium 2/3 (67%) Perivascular 3/7 (43%) |
Perivascular 16/42 (38%) |
Perivascular 8/8 (100%) CD20 predominancy 3/8 (38%) CD4 predominancy 1/8 (13%) CD8 predominancy 1/8 (13%) |
| Nonnecrotic muscle fibers surrounded by inflammatory cell | NA | Some | NA | 0 | NA | 0 (0%) |
| MHC class I | 7/7 (100%) |
Invaded fibers 4/14 (29%) Sarcolemmal membrane 2/14 (14%) |
Sarcolemmal membrane 1/1 (100%) | NA |
Muscle fibers 36/42 (85%) Diffuse 27/42 (64%) Scattered 6/42 (14%) Peri-fascicular enhance 3/42 (7%) |
7/9 (78%) |
| Perifascicular atrophy | 1/7 (14%) | NA | 1/1 (100%) | 0 | NA | 1/9 (11%) |
| Necrotic fiber | 5/7 (71%) | Some | 0 | 4/7 (57%) | 35/42 (83%) | 8/9 (89%) |
| Regenerating fiber | 2/7 (29%) | NA | Few | 1/3 (33%) | 9/9 (100%) | |
| MAC or C5b-9 | NA |
Vessels 14/14 (100%) Invaded fibers 4/14 (29%) |
Small vesseles and capillaries 1/1 (100%) | NA | Sarcolemmal deposits 20/38 (53%) |
Perimysial vessels 6/9 (67%) Perimysial non-necrotic fibers 1/9 (11%) |
| ICAM-1 & VCAM-1 | NA |
Vessels 14/14 (100%) Invaded fibers 5/14 (36%) & 1/14 (7%) |
Vessels weakly positive for VCAM | NA | NA |
Vascular wall 7/7 (100%) Perifascicular area 1/8 (13%) |
| MxA | NA | NA | NA | NA | NA |
Vascular wall 5/8 (63%) Perimysial fiber 1/8 (13%) |
- Abbreviations: ICAM, intercellular adhesion molecule; MAC, membrane attack complex; MCTD, mixed connective tissue disease; MHC, major histocompatibility complex; MxA, myxovirus resistance protein A; NA, not available; VCAM, vascular cell adhesion molecule.
ICAM-1 and VCAM-1 expression in the vascular wall was observed in all seven of our MCTD specimens containing vessels and was especially strong in a single patient with PFA. Since there are few previous reports focused on ICAM-1 and VCAM-1 staining in inflammatory muscle diseases, we further examined ICAM-1 and VCAM-1 staining in two patients each clinically diagnosed with DM, ARS syndrome, IMNM, and sporadic inclusion body myositis (sIBM) in our department (data not shown). Vascular ICAM-1/VCAM-1 was positively stained in one out of two DM, ARS syndrome, and sIBM specimens with VCAM-1 predominance. In IMNM, they were only faintly stained in one patient. In the report analyzing the quantification of protein expression of ICAM-1 in the endomysial vessels, capillary ICAM-1 expression was significantly stronger in DM and ARS syndrome than that in IMNM [18]. In another previous study, all 14 patients diagnosed with MCTD exhibited ICAM-1/VCAM-1 staining in 25%–50% of the vessels, whereas patients diagnosed with DM often showed ICAM-1/VCAM-1 staining in more than 50% of the vessels [3]. These reports along with our results suggest that although vascular ICAM/VCAM overexpression is not necessarily specific to DM or MCTD-related myositis, the positive rate and its degree appear to be higher than in other myositis groups.
Moreover, in our MCTD patients, vascular ICAM-1 staining tended to be more significant than VCAM-1. Vascular ICAM-1 expression predominance in comparison to VCAM-1 is described in juvenile DM corroborating the microvasculature involvement [19]. There is also a report that ICAM-1 is upregulated in human pulmonary artery endothelial cells cultured in the presence of anti-U1-RNP containing IgG from MCTD patients in the experimental setting [20]. Therefore, vascular pathology in which ICAM-1 plays a major role is suspected in MCTD regardless of myositis classification.
5 Conclusion
Atypical myopathic presentations, including facial and neck muscle weakness, are occasionally present in MCTD patients, which may require additional immunotherapy. Muscle pathology revealed DM pathology in 22% of our nine patients. The detailed pathomechanism of vasculopathy has yet to be elucidated and requires the study of additional patients.
Author Contributions
Naohiro Sakamoto: conceptualization, methodology, data curation, investigation, writing – original draft. Rumiko Izumi: conceptualization, methodology, data curation, investigation, writing – original draft, supervision. Naoki Suzuki: conceptualization, investigation, funding acquisition, writing – original draft, methodology, data curation, supervision. Maki Tateyama: conceptualization, investigation, writing – original draft, methodology, data curation, supervision. Masashi Aoki: writing – original draft, supervision, data curation, methodology, conceptualization, investigation.
Acknowledgments
The authors would like to thank Maya Narisawa, Hinako Shigihara, Mai Kakinuma, Yayoi Aoyama, Kazumi Ogawa, and the technical staff of the Department of Pathology, Tohoku University Hospital for their excellent technical assistance and Hideyuki Horikoshi for his advice on the MCTD diagnostic criteria. The authors also thank the Nature Springer Author Service for the English language review.
Ethics Statement
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.