Changes in electrophysiological findings suggestive of demyelination following Guillain-Barré syndrome: A retrospective study
Abstract
Introduction/Aims
Acute inflammatory demyelinating polyradiculoneuropathy (AIDP) is the most common form of Guillain-Barré syndrome (GBS) in Western countries. However, electrophysiological descriptions of changes in abnormalities suggestive of demyelination after an AIDP episode are rare. We aimed to describe the clinical and electrophysiological features of AIDP patients after the acute episode, to investigate changes in abnormalities suggestive of demyelination and to compare with electrophysiological features of chronic inflammatory demyelinating polyradiculoneuropathy (CIDP).
Methods
We reviewed the clinical and electrophysiological characteristics of 61 patients followed at regular intervals after the AIDP episode.
Results
We detected early electrophysiological abnormalities from the first nerve conduction studies (NCS) performed before 3 wk. Abnormalities suggestive of demyelination worsened on subsequent examinations. This worsening continued after more than 3 mo of follow-up for some parameters. We also found the persistence of abnormalities suggestive of demyelination for long periods after the acute episode, beyond 18 mo of follow-up, despite clinical improvement in most patients.
Discussion
In AIDP, NCS findings continue to worsen several weeks or even months after the onset of symptoms, and “CIDP-like” abnormalities suggestive of demyelination may persist for a long period of time, in contrast to the existing literature and the usually favorable clinical course. Thus, the discovery of conduction abnormalities on NCS performed long after an AIDP should always be interpreted according to the clinical context and not systematically lead to a diagnosis of CIDP.
Abbreviations
-
- AIDP
-
- acute inflammatory demyelinating polyradiculoneuropathy
-
- CIDP
-
- chronic inflammatory demyelinating polyradiculoneuropathy
-
- CMAP
-
- compound muscle action potential
-
- CSF
-
- cerebrospinal fluid
-
- DL
-
- distal latency
-
- EFNS
-
- European Federation of Neurological Societies
-
- GBS
-
- Guillain-Barré syndrome
-
- IVIg
-
- intravenous immunoglobulins
-
- MCV
-
- motor conduction velocity
-
- NCS
-
- nerve conduction studies
-
- PE
-
- plasma exchange
-
- PNS
-
- Peripheral Nerve Society
-
- TRF
-
- treatment-related fluctuations
1 INTRODUCTION
Acute inflammatory demyelinating polyradiculoneuropathy (AIDP) is the most common form of Guillain-Barré syndrome (GBS) in Western countries.1 The course of GBS is well defined, with a progressive phase lasting less than 4 wk, followed by a stable phase, and a variable recovery phase.2 The course of AIDP differs from that of chronic inflammatory demyelinating polyradiculoneuropathy (CIDP), which may relapse or progress continually over a period of more than 8 wk.3
The distinction between AIDP and CIDP is crucial for therapeutic management, but may be difficult, as some cases of CIDP may begin with acute paralysis, and some cases of AIDP may develop treatment-related clinical fluctuations (TRF) or relapses.4 Biological parameters are of little use for differentiating between the two, as protein levels in cerebrospinal fluid (CSF) may be elevated and anti-ganglioside antibodies may be present in both conditions. Several authors have investigated clinical characteristics that could potentially be used to guide diagnosis: deterioration more than 8 wk after onset or more than three times, a mild motor deficit, and an absence of cranial nerve dysfunction seem to be more suggestive of CIDP.4, 5 However, none of these features can provide a diagnosis that is certain.
In this context, performing serial nerve conduction studies (NCS) may provide a profile of acute abnormalities suggestive of demyelination that would be useful for the retrospective diagnosis of GBS in difficult situations.6, 7 The long-term electrophysiological course of GBS has been reported in a few studies, most of which were published before the most recent definitions of AIDP. One study in 1985 reported a worsening of motor and sensory values during the progressive phase, with a time lag between clinical worsening and worsening on NCS. The authors reported a nadir of motor values 3 wk after symptom onset and a nadir of sensory values at 4 wk, with values subsequently improving and tending to normalize.8 However, these findings are not consistent with those of some other publications or with our observations in daily practice. Indeed, electrophysical abnormalities persist well beyond the acute episode in many patients.9-12
In this study, we investigated the long-term changes in clinical and electrophysiological characteristics of a group of patients followed after an AIDP episode, focusing, in particular, on abnormalities suggestive of demyelination, to improve our understanding of the natural course of electrophysiological changes in AIDP and to provide data that may improve the neurological management of these patients.
2 METHODS
We retrospectively reviewed all the records of patients admitted to the neurology department or intensive care unit of Raymond-Poincaré Hospital (Garches) for suspected GBS between September 2012 and December 2019. All patients were examined by a physician with expertise in peripheral nervous system disorders. GBS was diagnosed according to the usual (Asbury and Brighton) criteria except for areflexia, the initial absence of which did not rule out the diagnosis if all the other elements of the clinical and electrophysiological presentations were highly suggestive of GBS.2, 13 Clinical, biological, and electrophysiological data were collected and diagnosis was confirmed by follow-up.
We reviewed the neurological examination findings, the duration of the progressive phase, therapeutic management and functional status at nadir and at the end of follow-up as a function of GBS disability score (see Table S1).14
Electrophysiological studies were performed in the electrophysiology laboratory of the hospital by three practitioners using the same protocol: all the reported parameters were obtained for recordings on the left side, unless the right side was more clinically involved; for each patient, the same nerves were then studied by sequential NCS. The temperature of the limbs was not systematically checked. We focused principally on motor conduction studies of the median, ulnar, common peroneal, and tibial nerves using standard methods described elsewhere.15, 16
For each nerve, we assessed the mean value and standard deviation of distal latency (DL) and motor conduction velocity (MCV) at each time point, the mean change in individual DL and MCV values between consecutive time points and the proportion of worsening values between consecutive time points. We also reported any increase in latency or the absence of F waves, temporal dispersions of motor potential (defined as a compound muscle action potential [CMAP] duration increase of more than 30% between distal and proximal stimulation), and conduction blocks (defined as a loss of amplitude of more than 30% between distal and proximal stimulation). For all conduction parameters, we assessed the proportion of abnormal values suggestive of demyelination for each time point, using the demyelination criteria proposed by the French CIDP study group (see Table S2).17 For the diagnosis of CIDP, we also applied the electrophysiological European Federation of Neurological Societies - Peripheral Nerve Society (EFNS-PNS) criteria to the results of our patients, with the diagnosis of CIDP considered “definite” if one of these criteria was fulfilled (see Table S3).18
We investigated the correlation between electrophysiological parameters and clinical status at the last reported clinical assessment, based on GBS disability score. We used two markers of persistent demyelination: the presence of at least one conduction abnormality suggestive of demyelination, and fulfillment of the EFNS-PNS criteria for definite CIDP. We also investigated the relationship between distal motor nerve CMAP amplitude in the median, ulnar and tibial nerves at 1 y and clinical disability.
We reviewed the NCS results available for six periods of interest after symptom onset: less than 21 days, between 21 days and 6 wk, 3 (± 1) mo, 6 (± 1) mo, 12 (± 1) mo, and between 18 and 24 mo. Patients were included if they had NCS results for at least two of the periods of interest. We collected the NCS data recorded up to May 2020.
We excluded patients with atypical clinical or biological features: a progressive phase lasting more than 28 days, a white cell count of more than 50 per mm3 in CSF, a hematological disorder, or excessive alcohol consumption. We excluded axonal GBS and Miller Fisher syndrome.
3 RESULTS
We reviewed 148 medical records for consecutive patients with GBS. After considering the exclusion criteria, we studied the clinical and electrophysiological features of the remaining 61 patients. The detailed study flowchart is presented in Figure 1.
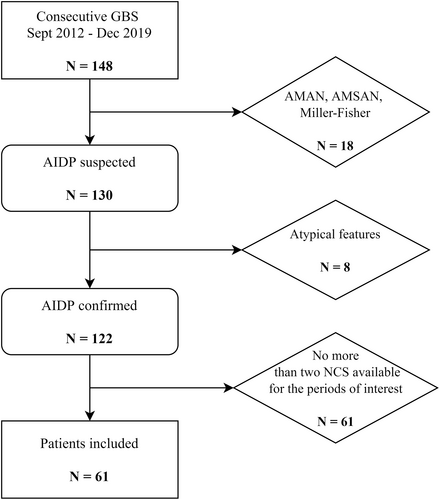
The median age of the patients was 47 y (range: 15–83 y), including 60.7% men. Three patients (4.9%) had already had a GBS episode and were, therefore, presenting relapses at the time of the study. The principal clinical characteristics of our patients are presented in Table 1.
| N (%) | |
|---|---|
| Weakness | 55 (90.2) |
| Lower limbs | 54 (88.5) |
| Proximal | 45 (73.8) |
| Distal | 38 (62.3) |
| Upper limbs | 45 (73.8) |
| Proximal | 27 (44.3) |
| Distal | 40 (65.6) |
| Axial | 20 (32.8) |
| Sensory symptoms and signs | 54 (88.5) |
| Paresthesia | 53 (86.9) |
| Sensory loss (touch or vibration) | 42 (70) |
| Gait | |
| Ataxia | 36 (61) |
| Areflexia | 53 (88.3) |
| Dysautonomia | 19 (31.1) |
| Bladder dysfunction | 15 (24.6) |
| Cardiovascular dysfunction | 4 (6.6) |
| Cranial nerve dysfunction | 35 (57.4) |
| Ophthalmoplegia | 3 (4.9) |
| Ptosis | 1 (1.6) |
| Facial paralysis | 24 (39.3) |
| Bulbar signs | 20 (32.8) |
| Respiratory impairment | 13 (21.3) |
| Mechanical ventilation | 9 (14.8) |
In the vast majority of cases, treatment was administered during the acute phase (89.8%) and usually consisted of intravenous infusions of immunoglobulins (IVIg, 79.2% of treated patients). The six patients not treated during the acute phase were either diagnosed late or had mild and stable symptoms. Six patients received a second course of treatment (10.2%), which in all cases was IVIg: three had received IVIg and three had undergone plasma exchange (PE) as the first-line treatment. The reasons for administering a second course of treatment were TRF (four patients), insufficient improvement at 4 mo with persistent conduction blocks (one patient), and a new motor impairment at 4 mo that was considered to constitute an early relapse (one patient).
Mean GBS disability score at nadir was 3.4, with a loss of walking ability (score greater than or equal to 4) in more than half the patients (51.9%). At the end of follow-up, the mean GBS disability score was 1.3. However, many patients had persistent disabilities: 34.4% were still unable to run and only 19.7% of patients had returned to their usual state. None of the patients died.
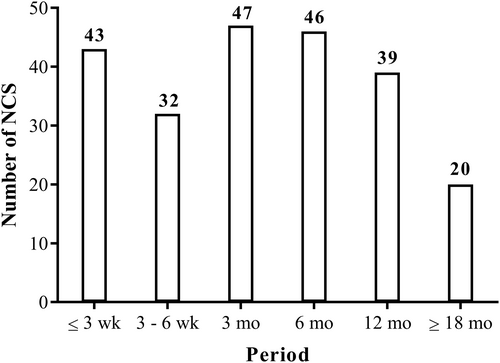
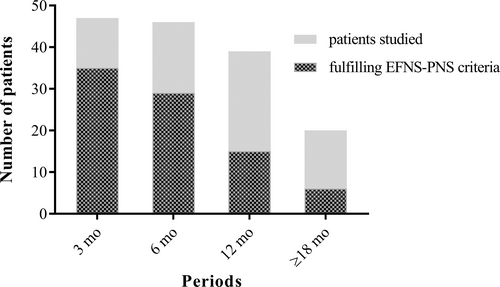
In total, data from 227 NCS were collected for the six periods of interest. The number of NCS available for each period is shown in Figure 2. Most of the patients had data for four NCS over the periods of interest. The median duration of follow-up was 12 mo (range: 3–24 mo).
3.1 Mean values of DL and MCV (Figure 3A,B)
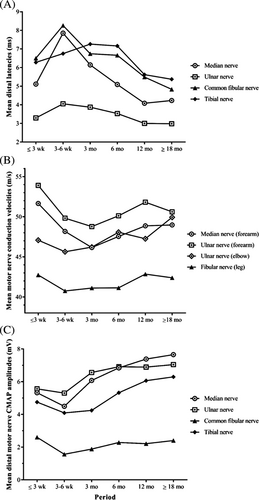
DL values improved after 6 wk for all nerves other than the tibial nerve, for which continued worsening was observed on the third NCS (performed at 3 mo). Mean MCV decreased up to the third NCS (performed at 3 mo) for the median and ulnar nerves (forearm), then gradually improved for the upper limbs, but remained roughly constant for the common fibular nerve.
3.2 CMAP amplitudes for motor nerves (Figure 3C)
More than half the cases displayed a decrease in CMAP amplitudes on the initial NCS. For upper limbs, mean amplitudes initially decreased and then improved markedly from 3 mo onward, with normal values achieved by the end of follow-up in most cases. For lower limbs, amplitudes remained abnormally low in more than 60% of cases at the end of follow-up.
3.3 Changes in DL and MCV (Figure 4)
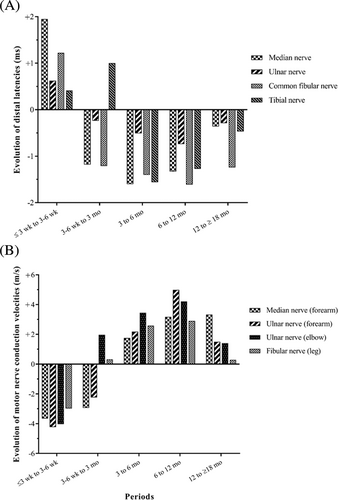
For each patient, we analyzed the change in each parameter between two successive periods, and then averaged these changes. For the tibial nerve, the greater worsening of DL values was over the period between the second and third NCS, and no improvement was observed for this nerve until 3 to 6 mo after symptom onset. We found similar results for MCV values of the median and ulnar nerves in the forearm. We then evaluated the number of patients whose DL or MCV values were worse on the NCS performed at 3 mo than on that for the previous period. Data were available for both these periods for 21 patients, of whom 11 (52.4%) showed a worsening of at least two parameters. A similar evaluation for the subsequent period showed that 11 of 36 patients (30.6%) demonstrated a worsening of at least two conduction parameters at 6 mo.
3.4 Frequency of conduction abnormalities suggestive of demyelination over time (Figure 5)
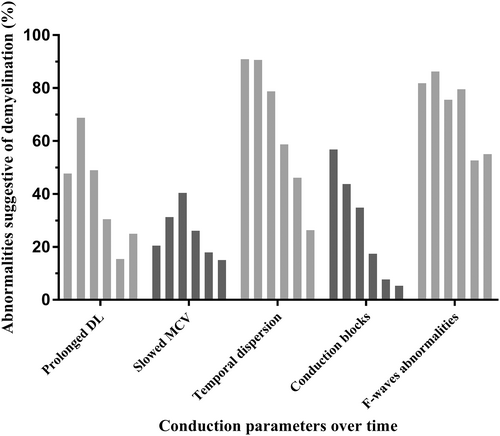
Such abnormalities were frequent from the first NCS, with an evolution depending on the parameter studied. Nevertheless, their frequency remained high in late NCS for several parameters, including a significant increase in DL values in more than 20% of patients at the last NCS, a temporal dispersion in more than a quarter of patients at the last NCS, and at least one persistent conduction block in three patients at 1 y.
3.5 Proportion of patients with NCS findings suggestive of demyelination
Almost all patients (97.7%) demonstrated at least one significant abnormality for at least one nerve on the first NCS. The percentage of patients with at least one abnormality subsequently decreased, but 14 of the 20 patients with data for the last time point (70%) continued to display such abnormalities, corresponding to 23% of the 61 patients in our cohort. We found that 81.8% of patients demonstrated at least two abnormalities suggestive of demyelination on early NCS, with maximal rates at between 3 and 6 wk (90.6%). By the last time point, 45% of the patients studied, corresponding to 14.8% of the 61 patients, continued to show at least two significant abnormalities. In light of these results, we applied the EFNS-PNS criteria used for CIDP diagnosis to our patients. These criteria use a stricter definition of demyelination (Figure 6). We found a high frequency of NCS that met the criteria for “definite” CIDP, even for examinations performed long after the acute episode. Indeed, 1 y after the acute GBS episode, 38.5% of the patients studied continued to meet these criteria, and, more than 18 mo after the acute episode, 30% of the patients studied (corresponding to 9.8% of our 61 patients) still had NCS results compatible with a “definite” CIDP.
3.6 NCS-clinical correlation
We found no correlation between persistence of at least one conduction abnormality suggestive of demyelination or fulfillment of the EFNS-PNS criteria for definite CIDP at 1 y and long-term disability. However, we found a strong correlation between a persistent decrease in CMAP amplitudes and an adverse clinical course with late motor sequelae (p < .0005).
4 DISCUSSION
Our electrophysiological follow-up confirmed the initial time lag in GBS between clinical and electrophysiological worsening, but our results go beyond that, showing some conduction parameters still worsening at 3 mo, and the persistence of demyelination features more than 1 y after the onset of AIDP.
Overall, conduction abnormalities were more frequent and diffuse on the second NCS, performed between 3 and 6 wk after the onset of symptoms. This result seems to be consistent with published findings: the principal previous long-term study by Albers et al. reported that motor conduction parameters reached a nadir 3 wk after symptom onset.8 These data have been adopted as the standard for interpreting NCS results in patients followed after an acute episode of GBS, despite conflicting data obtained in other studies.9-12 However, we found the time to improvement on NCS to be significantly longer than expected, with many conduction parameters continuing to worsen at 3 mo. We avoided bias due to the variability of mean values resulting from the absence of data for some periods for some patients, by studying the individual change in each parameter between two consecutive periods for which it was available, and averaging these changes, which led to the same result. Furthermore, more than half the patients (52.4%) displayed a worsening of electrophysiological results for at least two conduction parameters at 3 mo, and almost a third (30.6%) at 6 mo. Paradoxical electrophysiological worsening is not, therefore, unusual, even months after the acute episode of GBS.
We also looked at changes in NCS values with time after the GBS episode. As expected, these values gradually improved over time. However, we found a high rate of persistent conduction abnormalities suggestive of demyelination. We probably performed late NCS more often in patients with persistent conduction abnormalities, causing a potential follow-up bias, but a large proportion of patients demonstrated abnormalities even if the entire cohort was considered: after 18 mo of follow-up, 23% of our 61 patients continued to display at least one conduction abnormality suggestive of demyelination, and 14.8% had at least two different abnormalities. Some of these abnormalities suggestive of demyelination may instead reflect a process of remyelination, characterized by a shortening of the internodal space and a thinning of the myelin sheath, resulting in a moderate slowing of nerve conduction without clinical manifestations or underlying active demyelination.19 Another explanation may be a secondary axonal loss predominantly affecting the larger, faster nerve fibers, causing a slowing of the estimated speed. Such conduction abnormalities should not lead by themselves to reconsider the initial diagnosis of AIDP if the clinical course is typical.
Conversely, other abnormalities, such as the persistence of conduction blocks in three patients on the NCS performed at 1 y, are more suggestive of an active demyelination process. The presence of such abnormalities raises questions about differentiation from acute-onset CIDP (A-CIDP). Indeed, this disease may begin like GBS, but subsequently progresses toward chronicity: abnormalities suggestive of demyelination on NCS performed long after the onset of symptoms are common in such cases. We therefore applied the EFNS-PNS diagnostic criteria for CIDP to our results. Interestingly, we found that many patients in our cohort met these criteria: 47.5% of our 61 patients met them at 6 mo, a quarter continued to meet them at 1 y, and almost 10% met these criteria more than 18 mo after the GBS episode. It therefore appears justified to continue to monitor patients with such electrophysiological findings, particularly those with poor clinical recovery or reporting fluctuations in their residual symptoms, as slowly progressive CIDP cannot be ruled out as long as such abnormalities persist. It is interesting to note that among the initial 148 medical records, only four patients were ultimately diagnosed with CIDP. These four patients were not included in our analysis because they met our exclusion criteria (two of them had a progressive phase lasting more than 28 days, the other two did not have a sufficient number of NCS available). To date, no other diagnosis of CIDP has been made in our cohort.
Symptoms clearly improved during follow-up, but sequelae persisted, as reported in previous studies:20, 21 34.4% of our patients had significant residual symptoms (GBS disability score ≥2), and 6.6% were still unable to walk at the end of follow-up. Less than 20% of our patients regained their prior functional status, and many reported a persistent psychosocial impact. We found no correlation between the persistence of abnormalities suggestive of demyelination and clinical condition 1 y after the acute episode. Some studies have suggested a possible role for persistent demyelination in the fatigue or slowness sometimes reported long after GBS, but no correlation has ever been demonstrated.22 Conversely, we found a clear correlation between decrease in distal motor nerve CMAP amplitudes and the presence of sequelae. There are essentially two explanations for such decreases: distal conduction blocks or secondary axonal damage, with inconsistent, slower recovery.23 For the upper limbs, we observed an early decrease in distal motor nerve CMAP amplitudes, followed by a rapid improvement. In the lower limbs, these amplitudes normalized slowly, and low values persisted in many patients. This pattern of change over time is consistent with distal conduction blocks in the upper limbs, and axonal loss in the lower limbs. This axonal loss may cause muscle atrophy and poor motor recovery, accounting for the correlation between our results and the clinical outcome of our patients.22
5 CONCLUSIONS
In our study, we demonstrate that significant NCS abnormalities suggestive of demyelination can persist for up to 2 y after the onset of GBS. This emphasizes the importance of associating the interpretation of NCS results with a fine analysis of the chronological changes in symptoms and clinical examination findings during follow-up, before raising the possibility of a chronic form of polyradiculoneuropathy.
AUTHOR CONTRIBUTIONS
Clément Guémy: Conceptualization; investigation; methodology; writing – original draft; writing – review and editing. Marie-Christine Durand: Investigation; methodology. Marion BRISSET: Investigation; methodology. Guillaume Nicolas: Conceptualization; investigation; methodology; supervision; validation; writing – review and editing.
ACKNOWLEDGMENTS
This research received no specific grant from any funding agency.
CONFLICT OF INTEREST STATEMENT
None of the authors has any conflict of interest to disclose.
ETHICS STATEMENT
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. This study was approved by the relevant research ethics committee and informed consent was obtained from the research subjects.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.