Cardiopulmonary dysfunction in patients with limb-girdle muscular dystrophy 2A
Funding: This work was partly supported by research on intractable diseases by Health and Labor Sciences Research grants, Comprehensive Research on Disability Health and Welfare grants, Health and Labor Science Research grants, and an intramural research grant (26-7) for neurological and psychiatric disorders from the NCNP.
ABSTRACT
Introduction: Little is known about the frequency of cardiopulmonary failure in limb-girdle muscular dystrophy type 2A (calpainopathy) patients, although some studies have reported severe cardiomyopathy or respiratory failure. Methods: To clarify the frequency of cardiopulmonary dysfunction in this patient population, we retrospectively reviewed the respiratory and cardiac function of 43 patients with calpainopathy. Results: Nine of the 43 patients had forced vital capacity (FVC) < 80%, and 3 used noninvasive positive pressure ventilation. Mean FVC was significantly lower in patients who were nonambulant and had normal creatine kinase levels. Only 1 patient had a prolonged QRS complex duration. Echocardiography revealed that 1 patient had very mild left ventricular dysfunction. Conclusions: These findings suggest that patients with calpainopathy may develop severe respiratory failure, but cardiac dysfunction is infrequent. Muscle Nerve 55: 465–469, 2017
Abbreviations
-
- CK
-
- creatine kinase
-
- EF
-
- ejection fraction
-
- FVC
-
- forced vital capacity
-
- LGMD2A
-
- limb-girdle muscular dystrophy 2A
-
- NCNP
-
- National Center of Neurology and Psychiatry
-
- NIV
-
- noninvasive positive pressure ventilation
-
- PCR
-
- polymerase chain reaction
-
- VC
-
- vital capacity
Limb-girdle muscular dystrophy 2A (LGMD2A, hereafter referred to as calpainopathy) is an autosomal recessive muscular dystrophy caused by mutations in the Calpain 3 gene (CAPN3, MIM# 114240). CAPN3 is a calcium-activated neutral protease that is specific to mammalian skeletal muscle.1-3 Human CAPN3 has been cloned, its structure determined, and mutations identified in patients with calpainopathy.4 In comparison with dystrophinopathy or other limb-girdle dystrophies such as sarcoglycanopathy, cardiopulmonary involvement in calpainopathy is considered relatively rare.5
Several studies have reported respiratory and cardiac dysfunction in patients with calpainopathy,6, 7 yet no published clinical reviews have reported the frequency of cardiopulmonary dysfunction in this patient population. Groen et al. suggested that, while respiratory function declined over time, it was sufficiently preserved in the late stages of the disease.8 Recent recommendations suggest that early respiratory training9 and medication, such as angiotensin-converting enzyme inhibitors and/or beta-blockers, can effectively delay the progression of cardiomyopathy.10, 11 This study aimed to assess the frequency of cardiopulmonary involvement and identify factors that contribute to respiratory dysfunction in patients with calpainopathy.
MATERIALS AND METHODS
Study Population
Medical charts of 43 patients with calpainopathy who visited the National Center Hospital of National Center of Neurology and Psychiatry (NCNP) from July 1979 to September 2014 were retrospectively reviewed. As the study was retrospective, and many of the patients whose data were examined have not returned to our hospital, the Ethics Committee of the NCNP determined that it would be appropriate to publicize the research proposal, particularly with regard to the study objectives and overview on the Ethics Committee's homepage.
Measurements
Patient diagnoses were confirmed by genetic analysis. Analysis of mutations was performed on: (1) genomic DNA and/or (2) mRNA. For DNA, each of the 24 exons of the CAPN3 gene were polymerase chain reaction (PCR) -amplified from white blood cell DNA, followed by sequencing of the coding regions and splice sites. For mRNA, CAPN3 transcripts were reverse transcriptase-PCR-amplified from skeletal muscle RNA, followed by sequencing of the entire coding region. We collected and analyzed data on age at the time of examination, age at onset, disease duration, ambulation, serum CK levels, symptoms, respiratory function (% forced vital capacity [FVC]) in the seated position, and cardiac function (electrocardiogram and echocardiography). As % vital capacity (VC) changed in conjunction with FVC, we only used FVC in respiratory assessments. Decrements in FVC and ejection fraction (EF) were defined as < 80%12 and <55%,13 respectively. Prolongation of QRS complex duration was defined as >110 ms.14
Statistical Analysis
The t-test was used for group comparisons of continuous data, and the Fisher exact test was used for binary data. The correlation of continuous data was assessed using the Spearman method. The Fisher exact test was used to analyze factors that potentially contribute to respiratory dysfunction: age ≥ 65 years (the age at which one is defined as elderly in Japan),15 ambulation, serum CK levels, and disease genotype. All analyses were performed using SPSS for Macintosh (Version 18; SPSS Inc., Chicago, Illinois).
RESULTS
General Characteristics
Data from a total of 43 Japanese patients from 41 families (23 men and 20 women) were analyzed. Mean age at data collection was 47.4 ± 21.2 years, and disease duration from onset to examination was 29.2 ± 18.5 years (Table 1). Twelve patients had consanguineous parentage, 30 were ambulant (9 with and 21 without assistive devices), 12 were nonambulant, and no information regarding ambulation was available for 1 patient. Eleven patients were aged ≥65 years. There were 19 wheelchair users. Seven patients had CK elevations before onset. Ankle joint contracture and/or equinus foot, calf hypertrophy, and muscle pain were noted in 25, 13, and 12 patients, respectively. Mean age at onset was 18.0 ± 12.3 years (range, 2–52 years). Symptoms at onset (other than asymptomatic high CK levels or laboratory test abnormalities) included running or walking difficulty, ankle joint contracture/equinus foot, myalgia, difficulty climbing stairs, falls, difficulty bowing, easy falling, lordosis, difficulty in pedaling a bicycle, and difficulty in lifting the ankles (13, 8, 6, 5, 5, 2, 1, 1, 1, 1, and 1 patients, respectively). Mean serum CK was 1,492.0 ± 1,603.6 IU/L (range, 10–5,545 IU/L).
| n (%) | Mean | SD | Minimum | Maximum | |
|---|---|---|---|---|---|
| Age (years) | 43 (100) | 47.4 | 21.2 | 9 | 84 |
| Men | 23 (53.5) | ||||
| Age at onset (years) | 43 (100) | 18.0 | 12.3 | 2 | 52 |
| Age at pulmonary function tests (years) | 36 (83.7) | 47.2 | 20.9 | 10 | 83 |
| Duration from onset to pulmonary function tests (years) | 43 (100) | 29.2 | 18.5 | 0 | 73 |
| Age at electrocardiogram (years) | 39 (90.7) | 44.6 | 20.9 | 8 | 83 |
| Age at echocardiogram (years) | 22 (51.1) | 44.2 | 22.4 | 9 | 83 |
| Non-ambulant | 12 (27.8) |
Genetic Mutations
CAPN3 gene analysis identified point mutations and/or deletions in all patients. Of the 43 patients, 18 were homozygotes, and 25 were compound heterozygotes (Supplementary Table S1, available online). The most common mutation was c.1795dupA (Supplementary Table S2). Onset tended to be earlier (age, 11.1 ± 5.2 years) in patients with 2 null mutations (nonsense, frameshift, or mRNA null mutations) compared with patients with 1–2 missense mutations or small in-frame deletions (19.3 ± 13.0 years; P = 0.007), although ambulation, VC, FVC, or CK did not differ significantly.
Respiratory Function
Of the 43 patients, 36 underwent pulmonary function tests. Two patients with respiratory dysfunction had a past history of tuberculosis. Their chest CTs showed apex pleural thickening, but no lung field lesions. Mean FVC was 92.6 ± 26.0 (range, 20.7–129.5). FVC was significantly correlated with disease duration (ρ = 0.657; P < 0.001; Fig. 1), age (ρ = 0.422; P = 0.101), and CK levels (ρ = 0.554; P = 0.001). Significant factors identified by Fisher exact tests included age, nonambulatory status, and normal CK levels (Table 2).
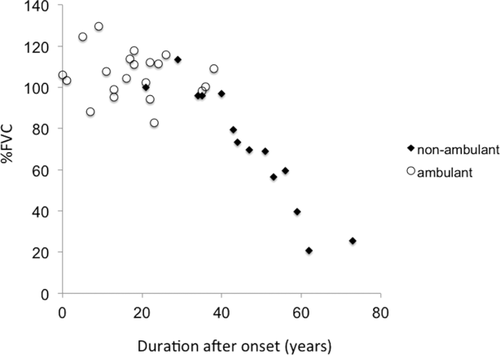
FVC was significantly correlated with disease duration (ρ = 0.657; P < 0.001). FVC of nonambulant patients (black diamonds) was markedly decreased in comparison with ambulant patients (white circles).
| Respiratory function | ||||
|---|---|---|---|---|
| FVC ≥ 80% | FVC < 80% | P | ||
| Age (years) | ≥65 | 4 | 6 | <0.006 |
| <65 | 23 | 3 | ||
| Gender | Men | 15 | 5 | 1.00 |
| Women | 12 | 4 | ||
| Ambulation status | Ambulant | 23 | 3 | <0.001 |
| Non-ambulant | 0 | 9 | ||
| CK | Normal | 0 | 6 | <0.001 |
| Elevated | 26 | 3 | ||
| Mutation | Both null mutations | 4 | 2 | 0.627 |
| Others | 23 | 7 | ||
Patients with Respiratory Dysfunction
Decreased FVC (<80, range, 20.7–79.5) was observed in 20.9% (9 of 36) of patients (Table 3). All 9 patients were nonambulant, and 6 had serum CK levels within the normal range (Table 3). Among the 9 nonambulant patients, 2 were siblings, and 7 had a homozygous mutation. The mean age of these patients was significantly greater (68.7 ± 11.5 vs. 40.0 ± 17.8; P < 0.001) and mean CK levels significantly lower (229.9 ± 250.4 vs. 1628.7 ± 1486.7; P = 0.001) compared with patients with normal respiratory function. Two patients had a past history of tuberculosis (1 pulmonary, 1 gastrointestinal and spondylitis). Two patients required nocturnal or occasional noninvasive positive pressure ventilation (NIV). One patient visited us due to respiratory failure and died after refusing to use respiratory devices.
| Patient | Age at onset | Disease duration | Age at lost ambulation | FVC | NIV | NIV induction (years) | CK (IU/L) | Genotyping |
|---|---|---|---|---|---|---|---|---|
| 74. W | 12 | 62 | 34 | 20.7 | Continuous | 71 | 104 | p.G233V homozygote |
| 71. W | 14 | 57 | 40 | 59.4 | - | - | 153 | p.G233V homozygote |
| 52. M | 7 | 45 | 25 | 73.4 | - | - | 466* | p.D631G/c.1951delC |
| 56. M | 6 | 50 | 47 | 68.8 | - | - | 797 | c.1524 + 1G>T homozygote |
| 74. W | 15 | 59 | 33 | 39.5 | Nocturnally | 71 | 97 | c.1769insA homozygote |
| 57. M | 13 | 44 | 25 | 79.5 | - | - | 150 | c.1795dupA homozygote |
| 83. M | 10 | 73 | 65 | 25.5 | - | - | 56 | c.1194-9A>G/p.R490W |
| 84. M | 35 | 49 | 81 | 69.6 | - | - | 111 | p.D707G homozygote |
| 72. M | 18 | 54 | 43 | 56.6 | Temporarily | 72 | 236a | G234E homozygote |
- a CK elevated (the upper limit of normal, 283 for men and 163 for women).
Electrocardiogram and Echocardiography
None of the patients had a history of diseases that could affect cardiac function. Electrocardiogram and echocardiography were performed in 39 and 22 patients, respectively. Abnormalities on electrocardiograms were seen in 14 patients, although intraventricular conduction delay (i.e., QRS duration prolongation) was detected in only 1 patient (Fig. 2a). Mean EF was 70.4 ± 9.0 (median, 71.0; interquartile range, 65–71; range, 49–84; Fig. 2b). Echocardiography revealed abnormal findings in 7 patients, although the findings were not clinically significant. Five patients had mild valvular regurgitation.
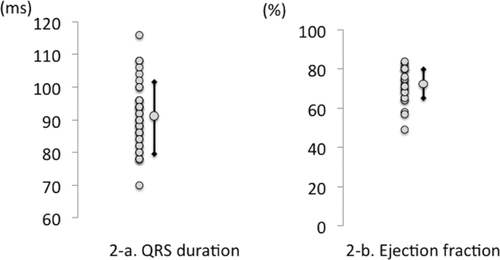
(a) QRS duration. Intraventricular conduction delay (i.e., QRS duration prolongation) was detected in only 1 patient. (b) Echocardiography revealed no clinically significant abnormal findings, and only 1 patient had an EF <50% (46%) (mean EF, 70.4 ± 9.0; median, 71.0; interquartile range, 65–71; range, 49–84).
DISCUSSION
Two major case series have reported the presence of respiratory dysfunction in patients with calpainopathy. Fardeau et al. first reported 23 genetically confirmed calpainopathy patients in the Reunion Islands,16 with a mean disease duration of 13.5 years. The most frequent mutation was 946-1 G>A, with others including R572Q, 1872C>T, 2069delCA, S744G, 2362AG→TCATCG. While respiratory function was examined in 19 of their patients, only 4 showed slight reductions. On this basis, the authors concluded that respiratory impairment was moderate. In another study, Groen et al. reported 85 patients with calpainopathy who were diagnosed by immunoblotting and/or gene analysis.8 They reported that respiratory function declined over time, but it was still well preserved, with overall values up to 30 years after onset of 80% or more for FVC. Only 2 patients exhibited FVC values significantly lower than 80%; 1 patient had a value of 48% 58 years after onset, and the other patient had a value of 62% 13 years after onset. The authors concluded that calpainopathy involves respiratory muscles in later stages, although respiratory failure was not observed in their patient population.8 Results from these 2 important studies, however, may lead to the misconception that respiratory involvement in calpainopathy is quite mild and not clinically significant.
Contrary to those studies, Pollitt et al. and Urtasun et al. reported that respiratory dysfunction is relatively common in the advanced stages of calpainopathy. Specifically, Pollitt reported that among 9 calpainopathy patients diagnosed by immunoblotting (3 were also genetically confirmed) in a Newcastle cohort, 4 had respiratory dysfunction, and 1 was a compound heterozygote for T566C and G>T exon 18-1 with respiratory insufficiency requiring ventilatory assistance. Moreover, 4 patients with mild respiratory dysfunction under age 40 years had scoliosis, indicating that scoliosis may also influence respiratory function.17 Urtasun reported that FVC<80% was found in 12 of 31 Basque patients and that even patients with normal VC showed a reduction in maximal expiratory pressure, suggesting weakness in the thoracic muscles.18 The most frequent mutations reported in their study were G222R, R489W, R748Q, and 2362AG→TCATCT. We found that patients with calpainopathy suffered from severe respiratory failure, and some required NIV.
Hashiguchi et al. also reported 2 genetically confirmed autopsy cases of calpainopathy. One patient, a 72-year-old man, died of cardiopulmonary failure. The other patient, a 70-year-old man who was homozygous for c.1795dupA (p.T559fs), required NIV, had a markedly dystrophic diaphragm, and died of respiratory failure.7 Similar to our study, that report demonstrated that some elderly patients with calpainopathy are at risk of developing respiratory failure. The c.1795dupA mutation was a common null mutation in our cohort, with 1 NIV patient also harboring the mutation. Although the null mutation and respiratory failure were not significantly correlated, patients with the null mutation had a significantly earlier onset and longer duration than those without it.
One patient had acute respiratory failure in our cohort. Dirik et al. previously reported a 14-year-old boy diagnosed with calpainopathy by immunoblot analysis. He developed cardiopulmonary arrest after acute airway infection,6 and required tracheotomy and nocturnal ventilation. His FVC was 76%, and weakness of respiratory muscles appeared to have caused the respiratory failure. Nonetheless, even those with an early stage of the disease or with mild symptoms should be closely monitored, and efforts should be made toward early detection of respiratory compromise.
We found that Japanese patients with calpainopathy who were aged ≥65 years, were nonambulant, and had normal CK levels were more likely to show respiratory dysfunction. This finding underscores the need to assess respiratory function in patients with these risk factors. As low or normal CK levels in nonambulant aged patients indicate the presence of severe and advanced muscle atrophy, physicians should pay close attention to respiratory risk. We could not find any correlation between respiratory dysfunction and whether patients had 2 null mutations (2 nonsense/splice-site/out-of-frame mutations), although 2 null mutations were associated with a significantly earlier onset. This suggests that genotype is not directly predictive of respiratory function in these patients. However, we should note that our study population is quite small, and in addition, long disease duration resulting in early onset is a risk for respiratory failure.
Most studies, with a few exceptions, have reported the lack of cardiac dysfunction in patients with calpainopathy. Groen et al. reported that cardiac function in 33 patients was normal on electrocardiogram and echocardiography, with the exception of 2 patients who had atrial fibrillation.8 Cardiac dysfunction was infrequent in our case series, which suggests that cardiac involvement is relatively rare in these patients. In mice, calpain 3 transcripts are expressed during cardiogenesis, although its expression decreases as the organ matures.19 The absence in mature cardiomyocytes is a possible explanation for the absence of cardiomyopathy in the majority of patients.
A few case reports have suggested cardiac involvement. For example, Okere et al. reported that a 23-year-old patient with calpainopathy had cardiomyopathy.20 Of the 2 autopsy cases reported by Hashiguchi et al., 1 with an incomplete right bundle branch block showed myocardial atrophy without any other causative disease; however, no degeneration or necrosis was found in the myocardium of this patient. As it stands, we cannot determine whether these cardiac events are related to calpainopathy, or whether they are rare incidental complications. In addition, it is not clear why the prevalence of severe respiratory failure differed by report. One possible explanation is that the common genotyping differed by cohort. Differences in genotyping may have also been due to differences in ethnic groups, size, or proportion of elderly individuals within each cohort. Studies with small sample populations may yield an underestimate of cardiopulmonary involvement. Thus, larger studies, for example, those that use an international registry, as well as clinical outcome studies, are warranted.
In conclusion, as patients with calpainopathy are prone to respiratory dysfunction, respiratory function should be regularly evaluated, especially in older patients in advanced disease stages. Physicians should regularly monitor patient respiratory function with pulmonary function tests to look for early signs of respiratory dysfunction, perform respiratory training, address any airway infections using a mechanical in-exsufflator (MI-E), and induce mechanical ventilation if required, as they do for patients with neuromuscular disease who exhibit respiratory failure. Cardiac involvement, however, could not be determined in this study.




