MRI methods for the evaluation of high intensity focused ultrasound tumor treatment: Current status and future needs
Abstract
Thermal ablation with high intensity focused ultrasound (HIFU) is an emerging noninvasive technique for the treatment of solid tumors. HIFU treatment of malignant tumors requires accurate treatment planning, monitoring and evaluation, which can be facilitated by performing the procedure in an MR-guided HIFU system. The MR-based evaluation of HIFU treatment is most often restricted to contrast-enhanced T1-weighted imaging, while it has been shown that the non-perfused volume may not reflect the extent of nonviable tumor tissue after HIFU treatment. There are multiple studies in which more advanced MRI methods were assessed for their suitability for the evaluation of HIFU treatment. While several of these methods seem promising regarding their sensitivity to HIFU-induced tissue changes, there is still ample room for improvement of MRI protocols for HIFU treatment evaluation. In this review article, we describe the major acute and delayed effects of HIFU treatment. For each effect, the MRI methods that have been—or could be—used to detect the associated tissue changes are described. In addition, the potential value of multiparametric MRI for the evaluation of HIFU treatment is discussed. The review ends with a discussion on future directions for the MRI-based evaluation of HIFU treatment. Magn Reson Med 75:302–317, 2016. © 2015 Wiley Periodicals, Inc.
INTRODUCTION
Thermal ablation using high intensity focused ultrasound (HIFU) allows for noninvasive tumor treatment by selective tissue heating. The first therapeutic trial with focused ultrasound beams dates back to the 1940s 1. The therapeutic application of focused ultrasound was initially restricted to the treatment of neurological disorders, such as Parkinson's disease. The Fry brothers were the first to report successful HIFU-induced local lesions in the central nervous system 2. Tumor treatment using HIFU was introduced in the 1970s 3. Since then, major technical advancements in HIFU set-ups have accelerated the clinical introduction of cancer therapy using HIFU. Nowadays, HIFU is applied for the treatment of a large range of tumor types, including uterine fibroids 4, 5 and prostate 6-9, breast 10, 11, liver 12-14, and bone 15 tumors. Particularly for treatment of malignant lesions, it is of key importance that the HIFU procedure can be accurately planned, monitored and evaluated.
MR-guided HIFU treatment allows for accurate, dynamic assessment of temperature by proton resonance frequency shift-based thermometry 16. The dynamic temperature profile can be used to determine the thermal dose that was delivered to the tissue. A thermal dose of 240 equivalent minutes (EM) is generally considered as the threshold for complete cell death. However, the thermal sensitivity may vary between species and organs, which suggests that the thermal dose threshold is dependent on tissue type 17, 18. In addition, the effects of HIFU are not only based on heating. Depending on the settings, mechanical effects, such as cavitation and radiation forces, may also induce damage 19.
Apart from direct effects of the combined thermal and mechanical damage to the tissue, such as those leading to coagulative necrosis, HIFU also causes initially sublethal tissue changes that may become fatal at a later time point after treatment 20. Therefore, for complete assessment of the success of the HIFU procedure, extensive evaluation of the intervention should be performed next to treatment monitoring. MRI offers a large variety of distinct contrast mechanisms that are sensitive to different effects of the HIFU treatment. While several of these methods seem promising regarding their sensitivity to HIFU-induced changes, there is ample room for improvement of MRI-based HIFU treatment evaluation. In this review article, we will describe the major effects of thermal treatment with HIFU and how MRI could detect these. Specifically, for each treatment effect MRI methods that are potentially sensitive to this particular effect are discussed in terms of their sensitivity to relevant tissue responses and clinical utility. At the end of the article, future directions with respect to MRI methods for the evaluation of HIFU treatment are discussed.
BASIC PRINCIPLE OF HIFU TREATMENT
During the HIFU procedure, ultrasound waves generated by a therapeutic transducer are focused into a very small volume, which greatly increases the intensity of the sound waves and thereby induces energy deposition in the target tissue. The energy that is absorbed by the tissue in the focal point of the transducer generates heat that can cause immediate coagulative necrosis in the tissue. Typically, HIFU ablation treatment schemes are designed in such way that the tissue temperature rapidly increases to approximately 60 °C 21. As stated above, next to thermal effects, HIFU may, depending on the settings, have a substantial mechanical influence on the tissue. One of these mechanical effects is cavitation, during which small gas bubbles in the tissue will start to grow and oscillate under the influence of the varying acoustic pressure during HIFU sonication. During inertial cavitation, these oscillating bubbles can eventually collapse, which leads to cell destruction and high local temperatures. Stable cavitation can lead to microstreaming, i.e., rapid movement of fluid around the oscillating bubble. High shear forces resulting from microstreaming can induce disruption of cell membranes 22. Another mechanical effect of HIFU involves the generation of acoustic radiation forces, which are developed when an ultrasound wave is either absorbed or reflected. When the reflecting/absorbing medium is solid (e.g., tissue), the force pressing against the medium produces a so-called “radiation pressure” 22. This pressure can induce local displacements of tissue in the focal point of the transducer 19, 23. Repetitive displacements may lead to structural damage in the tissue, through the development of local strains 24.
The focal point of the HIFU transducer can, either manually or electronically, be steered to allow for volumetric ablation of the tumor. The ablated lesion present after HIFU treatment generally consists of three zones: the central zone located in the target volume that undergoes immediate coagulative necrosis; the peripheral zone that was subjected to hyperthermia due to thermal conduction from the central zone; and the surrounding tissue that was not affected 20. A schematic drawing of the different tissue zones after HIFU treatment is shown in Figure 1.
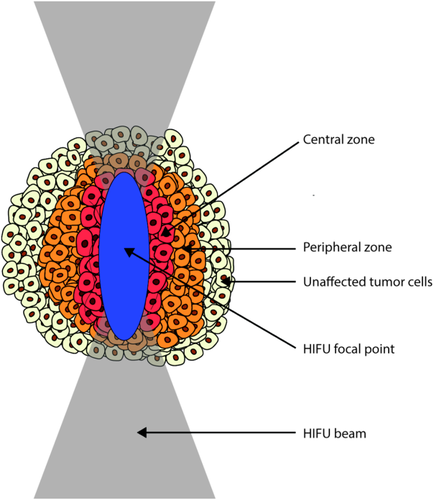
Schematic representation of the presence of a central zone of coagulative necrosis, a peripheral zone and a zone of unaffected tumor tissue after HIFU treatment of tumor tissue. Note: the dimensions of the tumor cells and the HIFU focal point are not to scale in this drawing.
REQUIREMENTS FOR MRI-BASED EVALUATION OF HIFU TREATMENT
MRI biomarkers for the evaluation of tumor therapy, such as HIFU, need to be rigorously evaluated before clinical adoption. Three essential steps for the evaluation of a quantitative imaging biomarker were recently described by Abramson et al 25.
Briefly, in the first step, named analytical validation, the measurement of the biomarker should be evaluated in terms of accuracy, precision and feasibility. This step typically consists of multiple (pre)clinical studies in which the particular biomarker is measured to assess its repeatability and reproducibility.
In the second step called qualification, the predictive value of the biomarker is assessed. The biomarker should ideally serve as a surrogate for a clinical end-point, which means that the effect of the treatment on the biomarker should correlate with clinical outcome. In the context of the present review article, the biomarkers should thus be sensitive to specific HIFU treatment effects to allow for accurate noninvasive assessment of the tumor tissue status after HIFU, which may be reflective of clinical outcome. The sensitivity of a biomarker for the detection of the effects of HIFU is related to the magnitude of the HIFU-induced change on the parameter with respect to natural variation of the parameter within the tumor and the signal-to-noise ratio (SNR) of the experiment.
Ideally, the MR protocol should be highly sensitive to acute tissue changes to allow for accurate evaluation of HIFU treatment effects directly after the intervention. If residual viable tumor tissue is detected, immediate re-treatment could be performed, which would minimize the number of required hospital visits and maximize long-term outcome for the patient. Next to HIFU, other methods, such as chemotherapy or radiotherapy, could be used as adjuvant therapy to treat residual tumor tissue after HIFU 20. Extensive MRI evaluation of the HIFU treatment would allow for localization and characterization of the residual tumor tissue and thereby aid in the planning of the adjuvant therapy.
The clinical usage of the biomarker is assessed in the third step of biomarker evaluation. This part addresses for example practical issues associated with clinical introduction, such as scan time and spatial resolution. Especially if treatment evaluation is performed directly after the HIFU ablation, the time duration of the MRI protocol should ideally not exceed 10 min. The spatial resolution should be high enough to allow for the detection of residual, viable tumor tissue. However, MRI—and virtually all other medical imaging techniques—are not sensitive enough to detect single cancer cells. HIFU treatment protocols for malignant tumors often include a treatment margin (∼10 mm) 26 around the MRI-visible tumor to account for the presence of occult, MRI-invisible tumor cells around the primary tumor. To facilitate accurate detection of residual tumor tissue, the spatial resolution should be substantially lower than the treatment margin. In that respect, a spatial resolution of approximately 1–2 mm would be advisable.
MRI-DETECTABLE HIFU TREATMENT EFFECTS
In the next paragraphs, the acute and delayed treatment effects in the central and peripheral zones are described. MRI methods that could provide imaging biomarkers for these treatment effects are discussed. In addition, challenges regarding clinical introduction of these methods for HIFU treatment evaluation, based on the above-mentioned requirements, will be addressed. An overview of the acute and delayed treatment effects and the MRI methods that have been or could be used to detect these is given in Table 1.
| Acute treatment effects | Delayed treatment effects | ||||
|---|---|---|---|---|---|
| Effect | MRI method | Findings | Effect | MRI method | Findings |
| Protein denaturation and aggregation | MT imaging | MTR ↑33-35 | Inflammatory response and edema formation | Macrophage imaging with nanoparticles | - |
| APT imaging | APTw signal ↓ 38 | ||||
| T1ρ mapping | T1ρ ≈ 49 | ||||
| MR elastography | Stiffness ↑ (ex vivo) 51, 52 and ↓ (in vivo) 52 | T2w imaging | Edema extent ↑ in first 2 days, then ↓ 110 | ||
| CE-MRI upon | Signal intensity ↑ 53 | DCE-MRI | Ktrans ↑ 91 | ||
| MS-325 injection | T1ρ mapping | T1ρ ↓ 49 | |||
| Damage of cellular and nuclear membranes | ADC mapping | ADC ↑ 33, 57, 58 and ↓ 61-64 | Ischemia and latent cell death due to vascular damage | (D)CE-MRI | Presence NPV 79, 80, 118, 119 |
| 23Na MRI | Signal intensity ↑ 62 | BOLD MRI | - | ||
| ADC mapping | ADC ↑ 33, 65 | ||||
| Hyperpolarized 13C | - | ||||
| Halted metabolism | 1H MRS | [choline+creatine]/citrate ↓ 71 | |||
| Hyperpolarized 13C | - | ||||
| Vascular collapse and hemorrhage | CE-MRI | Presence NPV 4, 7, 58, 62, 65, 77-79, 81-87 | Apoptosis | ADC mapping | ADC ↑ 33, 65 |
| DCE-MRI | Ktrans ↓ 27, 80, 90, 91, ve ↓ and ↑ 27, 80, 91 | Hyperpolarized 13C | - | ||
| T1w imaging | Signal intensity ≈ 57, 58 and ≈/↑ 97, 98 | ||||
| T1 mapping | T1 ↓ 33 | ||||
| T2w imaging | Signal intensity ≈ 7, 83, 97, 98 | ||||
| T2 mapping | T2 ↑ 58 and ≈ 33, 58 | ||||
| Hyperemia | CE-MRI | Enhancing rim 7, 58, 65, 81-87 | |||
| DCE-MRI | Ktrans ↑, ve ↑ 27 | ||||
| IVIM | - | ||||
- ↑ and ↓ indicate an increase and decrease in the measured parameter after HIFU treatment, respectively. ≈ indicates an absent or heterogeneous change in the parameter. - indicates that there are no studies published (yet) in which the referred MRI method was used to evaluate thermal treatment. Abbreviations: ADC, apparent diffusion coefficient; APT, amide proton transfer; BOLD, blood oxygen level-dependent; CE-MRI, contrast-enhanced MRI; DCE-MRI, dynamic contrast-enhanced MRI; IVIM, intravoxel incoherent motion; MT(R), magnetization transfer (ratio); NPV, non-perfused volume. References to papers are indicated in parentheses.
Acute Treatment Effects
A schematic overview of the acute HIFU treatment effects, for which MRI might provide imaging readouts is given in Figure 2 for the central and the peripheral zone. In the central zone, direct cell death occurs due to a combination of different effects, including protein denaturation, loss of cell membrane integrity, structural changes inducing mitochondrial dysfunction and inhibition of DNA replication 20. The major acute effects that are potentially detectable by MRI are protein denaturation and aggregation, damage of cellular and nuclear membranes, halted metabolism and vascular collapse and hemorrhage 20, 27, 28. In the peripheral zone, there could be a temporarily increased blood flow (hyperemia) and halted/impaired metabolism due to hyperthermic conditions 20. Furthermore, the permeability of the vascular endothelium could be altered in this area, because hyperthermia can induce reversible morphological changes to the endothelial cell cytoskeleton, which may result in changes in the endothelial cell lining 29.
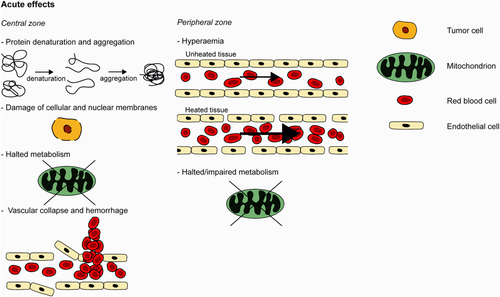
Schematic drawing of acute HIFU treatment effects in the central and peripheral zone. See text for detailed explanation.
Protein denaturation and aggregation
The high temperatures reached during HIFU treatment lead to instant protein denaturation in the central ablation zone. In addition, protein aggregation may eventually occur due to interactions between hydrophobic sites on the unfolded proteins 30, 31. Extensive protein denaturation is immediately cytotoxic and leads to coagulative necrosis 20.
Different MRI parameters that are considered to be directly sensitive to the presence of (denatured) proteins have been studied for their suitability for HIFU treatment evaluation. In several studies the effect of HIFU on the magnetization transfer ratio (MTR) was assessed. The MTR is a measure for the level of magnetization exchange between protons of mobile water molecules and protons associated with semisolid macromolecules, such as structural proteins 32. In in vivo magnetization transfer (MT) measurements on mouse subcutaneous tumors 33 an MTR increase was observed directly after HIFU application. Carasso et al developed an MT-based MRI method, named magnetization exchange imaging (MEXI), that is more directly sensitive to the magnetization exchange rate than a conventional MT experiment (34). They observed an increase in the magnetization exchange after HIFU treatment of rat muscle, which may be interpreted as an MTR increase. This apparent MTR increase is consistent with MT measurements after HIFU treatment of ex vivo porcine muscle 35, while an MTR decrease was observed after thermal treatment of different ex vivo tissues 36. The MTR decrease in the latter study was explained by protein denaturation, while the generally observed MTR increase might be caused by increased access of water molecules to semisolid macromolecules due to membrane disruption. The MTR is thus sensitive to multiple HIFU treatment effects and, therefore, not uniquely specific to protein denaturation. This limits the utility of MT imaging for the evaluation of HIFU treatment.
An MRI method that is potentially more specific to protein denaturation is amide proton transfer (APT) imaging 37. APT imaging uses the saturation transfer between water protons and amide protons of mobile protein/peptide backbones. APT imaging is, therefore, more exclusively sensitive to protein content/composition as compared to MT imaging, which reports on macromolecular status in general. The contrast in APT-weighted images is dependent on both the concentration of amide protons as well as the exchange rate. We have previously performed APT imaging to evaluate HIFU treatment of mouse subcutaneous tumors 38. The tumor APT-weighted signal was significantly decreased directly after HIFU treatment compared with baseline measurements. The APT-weighted signal decline was probably associated with HIFU-induced protein aggregation caused by the thermal stress during treatment, which leads to a lower accessibility of water molecules to the amide protons in the aggregated proteins 30, 31. These promising results suggested that APT imaging is indeed sensitive to HIFU-induced protein denaturation/aggregation. Nevertheless, it should be noted that HIFU-induced pH changes in the tissue can also generate contrast in APT-weighted images, because the chemical exchange between amide and water protons becomes slower when the tissue pH decreases 39-41. The hyperthermic conditions in the tissue surrounding the central zone of coagulation can lead to a pH decrease due to lactate accumulation induced by enhanced metabolic activity 42. The possible influence of pH changes on the observed APT contrast after HIFU needs to be investigated to fully assess the potential of APT imaging for the evaluation of HIFU therapy. Despite the inherent need for multiple off-resonance irradiations in APT experiments, recently multiple 3D and multislice APT imaging experiments have been described that allow for large volume coverage with acceptable spatial resolution within a clinically acceptable scan time 43-46. Although the APT effects are generally small (only a few percent of the total signal), technical advancements have led to high-quality APT acquisitions, which for example allow for precise assessment of APT effects in the human breast at 7T with a standard deviation of only 1% and a scan time of 5 min 47. The observed absolute decrease in the APT-weighted signal directly after HIFU treatment was approximately 5% at 6.3T in mouse tumors 38, which suggests that the effect of HIFU treatment on the APT-weighted signal should also be detectable in clinical applications.
An alternative MRI parameter of interest to study protein denaturation is the longitudinal relaxation in the rotating frame (T1ρ). T1ρ is a measure for the decay of magnetization in the transverse plane during a spin-lock pulse that is applied parallel to the magnetization vector. T1ρ is sensitive to interactions between water molecules and macromolecules, predominantly proteins 48, that occur around the carrier frequency of the applied spin-lock pulse. However, in a recent study, we did not observe a difference in tumor T1ρ before and directly after HIFU treatment of subcutaneous mouse tumors, which suggested that T1ρ is apparently not directly sensitive to protein denaturation occurring instantly during HIFU application 49.
An MRI method that has been used to probe HIFU-induced protein denaturation and aggregation indirectly by quantification of HIFU-induced tissue stiffness changes is MR elastography (MRE), which measures tissue stiffness by imaging of the propagation of mechanical waves 50. HIFU treatment introduces major structural changes to tissue and it was hypothesized that particularly protein denaturation and aggregation increase tissue stiffness. Ex vivo MRE studies on thermal treatment of bovine muscle tissue 51 and on HIFU treatment of turkey breast 52 confirmed that tissue stiffness increased after treatment. HIFU treatment of in vivo rat brain rather resulted in tissue softening directly after and up to at least 3 weeks after treatment, which was attributed to the presence of edema. These results suggested that tissue elasticity is more sensitive to edema than to tissue necrosis 52. In addition, the effective spatial resolution of the MRE acquisition is strongly dependent on the frequency of the applied mechanical waves. A sufficiently high spatial resolution can theoretically be achieved by applying high frequency shear waves. However, high-frequency waves are attenuated more rapidly than low-frequency waves and, therefore, cannot reach tumors that are located deeply in the body.
Vogel et al demonstrated that the albumin-binding contrast agent MS-325 may be a suitable MR contrast agent for the characterization of coagulated tumor tissue after laser ablation 53. It has previously been shown that coagulation induces a conformational change to serum albumin, which alters its binding affinity to the contrast agent 54. A pronounced signal intensity increase of up to 40% was observed on T1-weighted images during and after laser thermal treatment of pig muscle in the presence of MS-325, while the signal intensity decreased in the presence of gadolinium (Gd)-DTPA and in absence of contrast agent. This suggests that MS-325 can indeed be used to image tissue coagulation. A potential disadvantage of this method is that it requires presence of contrast agent during treatment, which could induce entrapment of Gd3+ in the tissue with a toxicity hazard and errors in MR thermometry 55, 56.
Damage of Cellular and Nuclear Membranes
The thermal and mechanical effects of HIFU may disrupt the cellular and nuclear membranes. The apparent diffusion coefficient (ADC), which is a measure of the diffusion of water molecules in tissue, could serve as a sensitive readout for membrane damage as this disruption of membranes is expected to increase the ADC. Indeed, an increase in ADC was measured after thermal treatment of various ex vivo tissues 36. The effect of HIFU treatment on the tissue ADC has also been studied quite extensively in vivo, both in preclinical 33, 57-60 and clinical studies 61-65. Similar to the ex vivo study a significant ADC increase was observed instantly after HIFU treatment of subcutaneous mouse tumors 33, 57, 58 and muscle tissue 57. In contrast, a significant decrease in ADC was found directly after treatment of uterine fibroids 61-63 (Fig. 3A). The ADC decline was attributed to cytotoxic edema, which is caused by dysfunction of the cell membrane and is associated with cell swelling 62. However, Pilatou et al reported a heterogeneous change in ADC after HIFU treatment of uterine fibroids 64. While they observed an ADC decrease in 26 fibroids, the ADC was increased in 19 fibroids. Apparently, the change in ADC directly after the HIFU intervention is dependent on tissue type as well as treatment settings. If the combined mechanical and thermal effects are large enough to induce substantial damage to the cell membranes, the ADC will likely increase upon HIFU treatment. However, if the treatment is applied in a more subtle manner, the cell membrane integrity may be maintained, which could allow for the formation of cytotoxic edema and an associated ADC decline. The observed bidirectional effects of HIFU on the ADC could hamper the interpretation of ADC maps early after treatment. Nevertheless, clinical application of ADC measurements for the evaluation of HIFU treatment seems feasible, because diffusion-weighted imaging is already widely applied in clinical studies on the assessment of tumor treatment response with sufficiently high spatial and temporal resolution 66. However, there is a clear need for standardization of ADC measurements as well as for studies that assess the reproducibility of these measurements 67, 68.
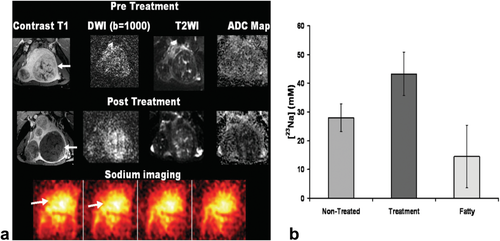
A: Contrast-enhanced T1-weighted image, diffusion-weighted image, T2-weighted image and quantitative ADC map of a uterine fibroid before and after HIFU treatment. Lack of perfusion and a decreased ADC were observed after therapy. At the bottom of the figure, sodium MR images obtained after treatment are displayed. B: Sodium concentrations obtained from sodium MR acquisitions in nontreated uterine fibroid, treated fibroid and fatty tissue, showing an increase in sodium concentration in the treated fibroids compared with nontreated fibroids. Adapted with permission from Jacobs et al 62.
An alternative MRI method that might report more directly on cell membrane dysfunction is sodium MRI, which allows for a quantitative measurement of the sodium concentration in tissue. Cells in healthy tissue maintain a large sodium concentration gradient (high extracellular concentration versus low intracellular concentration), which is mainly controlled by the sodium-potassium pump 69. Alterations in cell membrane integrity may lead to failure of the cells to maintain this sodium concentration gradient. In addition, the performance of the sodium-potassium pump may be affected by the halted metabolism after HIFU treatment, because the exchange of sodium and potassium across the cell membrane is energy-dependent. As a consequence of cell membrane damage, there will be an inflow of sodium ions from the extracellular space and the blood pool, resulting in higher sodium concentrations in the affected tissue. This increased sodium concentration can potentially be detected with sodium MRI. Jacobs et al have performed preliminary sodium MRI measurements immediately after HIFU treatment of uterine fibroids 62. They observed a significant 50% increase in MRI-derived sodium concentrations in the treated uterine fibroids compared with nontreated tissue (Fig. 3), which suggested that the HIFU-induced loss of cell membrane integrity can indeed be probed with sodium MRI. Nevertheless, direct clinical application of sodium MRI for the assessment of treatment response is hampered by the typically observed low SNR in sodium MRI experiments, caused by low sodium concentrations in biological tissue, the intrinsically low sensitivity of MRI to sodium and the short T2 of sodium 69.
Halted Metabolism
The protein denaturation in the central zone of coagulation directly affects the cellular metabolism, which depends on enzymatic activity. In addition, the hyperthermic conditions in the peripheral zone may partly impair the metabolism in that zone, because exposure to temperatures of approximately 43 °C has been shown to promote the leakage of protons through the inner mitochondrial membrane, which induces mitochondrial dysfunction 20, 70. Changes in metabolism may be probed by MR spectroscopy (MRS). It has previously been shown that 1H MRS is a potentially suitable method to detect residual tumor tissue at several months after HIFU treatment of prostate cancer 71. Due to the difference in metabolism, successfully treated tumor tissue generally has a lower [choline+creatine]/citrate ratio than residual tumor tissue, determined by proton MRS. Although there are no reports on 1H MRS directly after HIFU treatment, it is expected that this technique is also suited for the direct assessment of HIFU therapy, because the metabolic changes occur instantly during treatment. Another metabolite of interest that can be measured by 1H MRS is lactate. As opposed to healthy cells that metabolize glucose by means of the citric acid cycle and subsequent oxidative phosphorylation, glucose in highly proliferating tumors is primarily metabolized by aerobic glycolysis which produces lactate 72, 73. Lactate may thus be a suitable biomarker to distinguish between metabolically arrested treated tumor tissue and normally metabolizing residual tumor tissue after HIFU treatment, provided that lactate present in the tumor tissue before treatment does not become entrapped in the coagulated tissue. However, clinical utility of 1H MRS for the measurement of lactate is hampered by low lactate concentrations, even in high-grade prostate tumors 74. For detection of such low concentrations, a relatively coarse spatial resolution of this particular MRS method, as well as for MRS methods in general, is often unavoidable, which would limit the sensitivity of MRS for the detection of local spots of residual tumor tissue after HIFU treatment.
A promising technique for sensitive detection of tumor-associated metabolic activity is hyperpolarized 13C MR. Hyperpolarization of 13C-enriched substrates alters the Boltzmann distribution of spins, which drastically (>104 fold) increases the sensitivity for detection of these substrates and the products of metabolic conversions 75. Because of the enormous increase in signal as compared to conventional MRS methods, hyperpolarized 13C MR experiments, especially if combined with fast imaging read-outs, can be performed with relatively high spatial resolution (1–5 mm2) and fast acquisition time (order of seconds) 75. Recently, Rodrigues et al have shown that hyperpolarized 13C MR allows for real-time imaging of the glycolytic flux of injected 13C-labeled glucose into lactate 76. This technique may become very suitable for the assessment of early tumor response to various treatments, including HIFU therapy. There are several challenges to overcome before large-scale clinical introduction of hyperpolarized MRI, such as the need for in-house polarizers and standardization of protocols. In addition, the polarization life-time of the injected agents should be extended to enhance clinical usage of the technique 75.
Vascular Collapse and Hemorrhage
Similar to tumor cells, tumor blood vessels undergo coagulative necrosis during thermal ablation. Histological analysis of HIFU-treated solid malignancies in patients confirmed severe damage to the tumor vasculature 28. This vascular damage causes impaired blood flow and potentially hemorrhage. For the identification of the impaired blood flow commonly contrast-enhanced MRI (CE-MRI) is used, which is by far the mostly used MR method for the evaluation of HIFU treatment. The CE-MRI methods generally consist of two T1-weighted acquisitions, one before and one after injection of a low-molecular weight Gd-based contrast agent. The region of coagulated tissue is then characterized by a lack of signal enhancement after injection and referred to as the nonperfused volume (NPV). In several studies, the NPV was quantitatively compared with the estimated treatment volume derived from the temperature maps and to the necrotic volume from histopathology. Directly after HIFU treatment, the NPV was generally larger than the estimated treated volume 4, 64, 77-79, probably caused by destruction of large blood vessels that perfuse tissue outside the HIFU-treated volume. Histopathological analysis of HIFU-treated rabbit tumors showed that the NPV underestimated the extent of necrosis early (up to 3 days) after treatment 79. In contrast, in our recent study on dynamic contrast-enhanced MRI (DCE-MRI) of HIFU-treated subcutaneous mouse tumors, we observed that the NPV overestimated the amount of necrosis in histology directly after treatment 80. These findings suggest that care must be taken with the interpretation of CE-MRI performed directly after HIFU treatment. In addition, the NPV is often surrounded by an enhancing rim 7, 58, 65, 81-88. An example of a contrast-enhanced T1-weighted image showing an enhancing rim around the HIFU-treated breast tumor is displayed in Figure 4. This enhancing rim has been attributed to hyperemia in the peripheral zone 88, but also to residual tumor tissue. At later time points after treatment, inflammation and fibrotic tumor tissue may contribute to this peripheral enhancement 7. The presence of this enhancing rim is another factor that complicates the interpretation of CE-MRI images obtained after HIFU treatment.
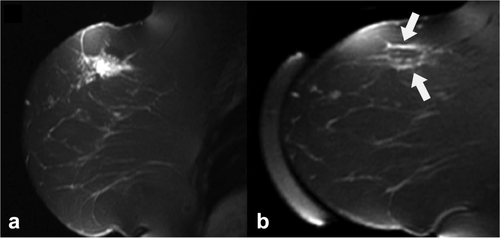
Sagittal contrast-enhanced T1-weighted image of a differentiated invasive ductal carcinoma before (a) and 3 days after (b) HIFU treatment. After treatment, a rim of contrast enhancement (white arrows) was observed around the treated lesion. Adapted with permission from Zhou 88.
DCE-MRI may have additional value for the evaluation of HIFU treatment compared with conventional CE-MRI. In DCE-MRI experiments the signal intensity is measured dynamically before, during and after injection of a contrast agent. Quantitative analysis of the DCE-MRI data can be performed by pharmacokinetic modeling. With the widely applied Standard Tofts model the transfer constant Ktrans and the extravascular extracellular volume fraction ve can be estimated 89. A few studies have performed pharmacokinetic modeling of DCE-MRI data from HIFU-treated tissue. A significant decrease in tumor Ktrans was reported after HIFU treatment of subcutaneous rat tumors 90. Cheng et al performed an extensive analysis of the Ktrans and ve values in HIFU-treated rabbit skeletal muscle tissue 27, 91 (Fig. 5). After HIFU, the central zone was characterized by low Ktrans and low ve values. Adjacent to this zone, a region of low Ktrans and high ve was observed, which was associated with structural disruption, vascular congestion, hemorrhage and vacuolation. A tissue region located further away from the central zone was characterized by higher Ktrans and ve, caused by edema, hyperemia, mild inflammation, and increased vascular permeability. We have recently performed cluster analysis of Ktrans and ve values after HIFU treatment of subcutaneous mouse tumors and observed similar regions with low Ktrans and either low or high ve around the nonperfused volume 80. These studies show that extensive pharmacokinetic analysis of DCE-MRI data gives improved insights in the vascular effects of HIFU treatment. Quantitative DCE-MRI measurements could for example give more information about the characteristics of the above-mentioned enhancing rim, which would increase the usefulness of contrast-enhanced MRI for the evaluation of HIFU treatment. Quantitative DCE-MRI is regularly performed in clinical studies. Due to hardware limitations, there is a trade-off between the temporal resolution (i.e., the number of dynamic scans per time unit), spatial resolution, and SNR of DCE-MRI acquisitions 92. Reliable absolute quantification of the pharmacokinetic parameters needs accurate knowledge of the arterial input function (AIF) 93. However, extraction of the AIF from MRI experiments is often not feasible in practice because of the absence of a large artery in the field-of-view 94. Nevertheless, there are several methods for AIF determination without the need of direct measurement of the AIF 95, 96.
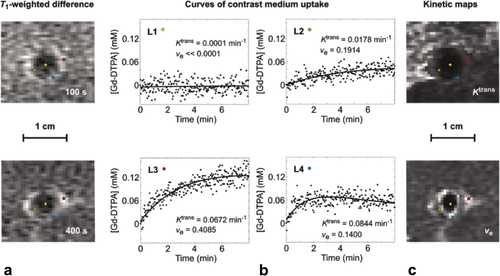
Dynamic tissue contrast agent (Gd-DTPA) concentration curves derived from DCE-MRI measurements performed after HIFU treatment of rabbit muscle tissue. a: Contrast-enhanced T1-weighted difference images at 100 and 400 s after bolus injection of Gd-DTPA. b: Dynamic Gd-DTPA concentration curves at select locations L1–L4 (indicated with the colored dots in the left and right images). The experimental data (dots) were fitted to the Tofts model (solid lines). The estimated parameter values for Ktrans and ve are shown bottom right in each graph. c: Ktrans and ve parameter maps. Adapted with permission from Cheng et al 27.
In addition to contrast-enhanced MRI, several intrinsic contrast MRI parameters are sensitive to HIFU-induced changes to the tissue vasculature. For instance, the HIFU-induced damage to blood vessels can lead to hemorrhage that is associated with an increase in the concentration of paramagnetic deoxygenated hemoglobin, which influences the tissue T1 and T2 relaxation times. T1- and T2-weighted imaging have been used extensively for the evaluation of HIFU treatment. However, no consistent results have been reported. In preclinical studies of HIFU treatment of muscle 57 and subcutaneous tumors 58 no significant treatment-induced change in the T1-weighted signal intensity was observed. In two clinical studies of HIFU treatment of uterine fibroids 97 and pancreatic cancer 98, the treated lesion appeared hyperintense on T1-weighted images. However, this hyperintense region was heterogeneous 97 and not seen in all cases 98. In our recent study on multiparametric MR analysis of HIFU-treated tumor tissue, we have performed quantitative T1 measurements of subcutaneous mouse tumors before and after HIFU treatment. T1 was significantly lower in HIFU-treated tumor tissue compared with nontreated tumor tissue directly after treatment 33, which is consistent with hyperintensity on T1-weighted images. On T2-weighted images, a heterogeneous appearance of the HIFU-treated tissue was reported after treatment of uterine fibroids 97, prostate 7, 83, and pancreatic 98 tumors. This is in line with our findings in the aforementioned multiparametric study, in which we found a broad range of T2 values in the HIFU-treated tumor tissue 33. Hundt et al observed a significant increase in T2 after HIFU treatment of mouse muscle tissue, while T2 did not change significantly after treatment of subcutaneous tumors 58. The apparently conflicting results on T1 and T2 responses after HIFU treatment are probably caused by the fact that T1 and T2 are sensitive to multiple, possibly counteracting, effects of the HIFU intervention. Next to vascular damage, other HIFU effects such as membrane disruption and protein denaturation/aggregation will affect the general tissue structure and thus T1 and T2, leading to the reported heterogeneous changes in these parameters after HIFU treatment.
Hyperemia
The hyperthermic conditions in the peripheral zone may cause a temporarily increased blood flow (hyperemia). In preclinical tumor models, it is generally observed that the blood flow in this region indeed increases upon heating after which it gradually declines to pretreatment values again. However, in human tumors there seems to be no such clear unidirectional effect of hyperthermia on tumor blood flow 99. Apart from the influence of tumor type, the effect of hyperthermia on blood flow is strongly dependent on the temperature and the time period during which the tissue was exposed to this temperature. Similar to the vascular damage in the central zone, the effect of hyperthermia on the blood flow in the peripheral zone could be probed with (D)CE-MRI. On CE-MRI images, hyperemia can be observed as an enhancing rim around the central ablation volume. However, as discussed earlier, residual tumor tissue could also be the cause of this peripheral enhancement. DCE-MRI may allow for better characterization of hyperemic effects and possibly for quantitative estimates of changes in blood flow and vascular permeability. As indicated above, Cheng et al observed a hyperemic region with high Ktrans and ve around the central ablation volume 27. Quantification of the hyperemic effect may be particularly interesting if one would like to exploit this effect for example for the stimulation of local delivery of chemotherapeutic agents 100, 101. Adjuvant chemotherapy could be performed after HIFU treatment to treat residual tumor tissue present at the ablation margins 102. DCE-MRI has been used previously to measure tumor perfusion after hyperthermia 94. Importantly, the authors noted that for accurate quantification of the change in perfusion after hyperthermia one has to take into account that the AIF also changes due to hyperthermia.
An alternative MRI method that could be used for perfusion measurements is intravoxel incoherent motion (IVIM) imaging, which estimates both tissue perfusion and diffusion based on a series of diffusion-weighted acquisitions with low and high b-values 103, 104. An advantage of IVIM imaging compared with (D)CE-MRI is that it does not require the injection of a contrast agent and, therefore, can be more readily combined with MRI guidance of HIFU and repeated at will to assess the dynamics of perfusion changes. However, the reproducibility of perfusion measurements using IVIM has shown to be low 105. Efforts should be made to allow for more robust acquisition of multiple low b-value images.
Delayed Treatment Effects
Delayed effects occurring at several days after HIFU treatment that are potentially detectable by MRI are illustrated in Figure 6. The HIFU treatment could lead to a systemic inflammatory response and formation of edema in and around the treated tumor. In addition, the vascular damage may result in ischemic conditions in tumor tissue outside the central ablation zone, which could eventually cause latent cell death 20. Heat stress in the peripheral zone during ablation may lead to apoptosis 106.
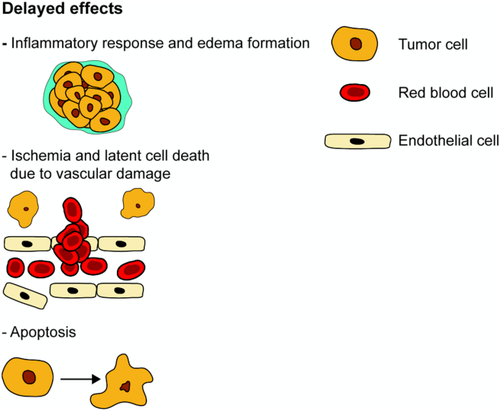
Schematic drawing of delayed HIFU treatment effects. Details are provided in the main text.
Inflammatory Response and Edema Formation
In the process of tissue repair, the inflammatory activity in the ablated lesion will increase in the first days after HIFU treatment. After HIFU ablation of a rabbit tumor, a clear rim of neutrophilic granulocytes and macrophages was observed at day 1 and 3 after treatment. No signs of inflammation were visible in the central region of ablation 79. The macrophages may be visualized with MRI by the injection of nanoparticles, that are passively targeted to macrophages by using the naturally high endocytotic activity of these inflammatory cells. Examples of nanoparticles that have been used for MRI detection of macrophages are iron oxide particles and 19F MRI-detectable perfluorocarbon emulsions 107, 108. Macrophage imaging with nanoparticles has been applied quite extensively in multiple pathologies, including cancer, atherosclerosis, myocardial infarction, and stroke 109, but it remains to be investigated whether this technique is sensitive enough to assess the level of inflammation after HIFU therapy.
While probably not suitable for the direct visualization of inflammatory cells, intrinsic MRI contrast methods could be used for the detection of edema around the central ablation zone. This edematous tissue is associated with the inflammatory infiltration and can be visualized with MRI based on either T2 or ADC contrast mechanisms because of the high-water and low-cellular content in edema. Mórocz et al have followed the development of edema after HIFU ablation of rabbit brain tissue with T2-weighted images. The extent of peripheral edema increased during the first 2 days after treatment, after which it gradually decreased again during the next five days 110. Cheng et al used DCE-MRI to assess delayed effects of HIFU treatment of rabbit thigh muscles. At 40 h and 3 days after treatment, a pronounced ring of inflammatory edema, that was characterized by high Ktrans values, was observed around the central zone of ablation 91.
Apart from the inflammatory response, the HIFU-induced protein denaturation and aggregation may indirectly lead to formation of edema around the ablated tissue. Protein denaturation/aggregation leads to increased exposure of water molecules to hydrophobic protein sites. This can ultimately lead to extrusion of water from the treated tissue, which has been addressed previously in a study on changes in MR properties of various tissues after thermal treatment 36. In our recent study on T1ρ after HIFU treatment of murine tumors, we observed signs for this denaturation-induced extrusion of water 49. While the protein denaturation itself did not lead to direct change in the tumor T1ρ values, at 3 days after treatment a significant approximately 15% decrease in T1ρ was observed in the treated tumors compared with baseline measurements. As indicated above, T1ρ is sensitive to interactions between water molecules and proteins, and consequently dependent on the tissue concentration of these molecules. Because the change in interactions due to protein denaturation apparently did not greatly influence the tumor T1ρ values considering the absence of T1ρ changes directly after HIFU treatment, we hypothesized that the observed delayed decrease in T1ρ was mainly caused by extrusion of water. T1ρ may thus be a suitable biomarker for the detection of delayed HIFU-induced tissue changes. Nevertheless, while T1ρ has shown great clinical promise for the characterization of cartilage 111, 112, multiple clinical trials should be done to assess the repeatability and reproducibility of T1ρ in tumor tissue, because there are only very few cancer studies that use T1ρ 113-116.
Ischemia and Latent Cell Death due to Vascular Damage
The vascular damage induced by the HIFU treatment could lead to diminished oxygen and nutrient delivery to regions of tumor tissue extending beyond the central zone of ablation if the damaged vessels perfused these regions, leading to ischemic conditions in the peripheral zone. In the first days after HIFU treatment, the perfusion can be partially restored due to increased blood supply by peripheral blood vessels and the collateral circulation 106. In addition, HIFU-induced vascular occlusion can be transient and followed by partial reperfusion of the ischemic tumor tissue 117. Similar to early after HIFU, the vascular status of the tumor tissue at a later stage after HIFU treatment can be assessed by (D)CE-MRI. It has been shown that the NPV derived from (D)CE-MRI measurements correlates better with the extent of tissue necrosis at later time points (at least 3 days) after treatment compared with directly after treatment 79, 80. In addition, strong correlations between semiquantitative DCE-MRI parameters and the amount of residual tumor tissue have been reported at 3–14 days after treatment 118, 119. Khiat et al showed that the correlation between semiquantitative DCE-MRI parameters and the amount of residual tumor tissue was highest at least 7 days after treatment 119. The mismatch between the extent of necrosis and the (D)CE-MRI findings early after treatment was explained by the influence of other treatment effects such as hemorrhage, edema, inflammation, and fibrosis on the contrast enhancement patterns. Furthermore, the transient vascular damage and latent cell death could negatively influence the accuracy of (D)CE-MRI for the early evaluation of HIFU.
To the best of our knowledge, there are no reports on the direct MR visualization of ischemia after HIFU treatment. Nevertheless, it seems important to detect hypoxic residual tumor tissue, because there may be a risk of metastatic spread induced by hypoxia. Hypoxia could trigger the activation of the hypoxia inducible factors HIF-1α and HIF-2α, which may eventually lead to angiogenesis by increased expression of vascular endothelial growth factor 120, 121 and subsequently induce tumor progression and the development of metastases. Blood oxygen level-dependent (BOLD) MRI may be used to image hypoxia. BOLD MRI has been used quite extensively to assess tumor tissue oxygenation 122. Preliminary data on BOLD MRI after chemotherapy of rat liver tumors 123 and human breast tumors 124 indicate that this technique may provide a suitable biomarker for the assessment of treatment response. While the signal in BOLD MRI mainly depends on blood oxygenation in treatment-naive tumors, it appears that the BOLD signal rather reflects interstitial tissue oxygenation in treated tumors 122. More studies are needed to further interrogate the potential value of BOLD MRI for the assessment of cancer treatment response.
The ADC is a promising MR biomarker for the detection of latent, ischemia-induced necrosis. Cell lysis that is associated with necrosis generally leads to elevated tissue ADC values 66. Indeed, a strongly significant (P < 0.001) ADC increase has been observed at 1 week after HIFU treatment of human liver tumors 65 and at 3 days after treatment of mouse 33 and rat 125 subcutaneous tumors. In the latter study, in which we treated the rat tumors in a clinical 3Tesla (T) MR-guided HIFU system, an average ADC increase of 0.51 × 10−3 mm2/s was observed at 3 days after therapy. A recent clinical study on the reproducibility of diffusion-weighted imaging of breast tissue at 3T reported a confidence interval of the ADC value of 0.04 × 10−3 mm2/s 126, which suggests that diffusion-weighted imaging is sensitive enough to detect HIFU-induced, necrosis-associated changes in the ADC.
Hyperpolarized 13C MR that uses the production of 13C-labeled malate from intravenously injected 13C-labeled fumarate could be another promising method for the detection of the latent ischemia-induced necrosis. The production of labeled malate increases in a binary manner during the onset of necrosis, due to increased access of fumarate to the enzyme fumarase that catalyzes the reaction from fumarate to malate 127. This enhanced access of fumarate is caused by higher permeability of the cellular membrane due to necrosis. It has been reported that the increased production of 13C-labeled malate occurs parallel to the onset of necrosis induced by anti-angiogenic therapy and precedes any change in tumor ADC 128. This suggests that hyperpolarized 13C MR may allow for an earlier assessment of latent cell death after HIFU treatment compared with ADC measurements.
Apoptosis
Instead of necrosis, cells in parts of the peripheral zone may undergo apoptosis after HIFU treatment. It has been reported that the maximum level of apoptosis was reached at 72 h after HIFU treatment of rabbit liver tumors 106. The particular reason for the onset of apoptosis is unknown. Apoptosis may be induced by the release of reactive oxygen species due to either hyperthermic or ischemia-reperfusion processes that can occur due to increased blood flow in the collateral circulation. In addition, apoptosis can be induced by mitochondrial dysfunction 106. Similar to necrosis, apoptosis may be visualized by diffusion-weighted MRI. Apoptosis-related cell shrinkage, cell blebbing and phagocytosis all cause an elevation in the tissue ADC value 66. Next to ischemia-induced necrosis, apoptosis may thus partly explain the observed elevated ADC values at later stages after HIFU treatment 33, 65. Because of the similar effect of apoptosis and necrosis on the tumor ADC, it is difficult, if not impossible, to distinguish these different types of cell death based on diffusion-weighted imaging alone. Hyperpolarized MRI with 13C-labeled fumarate 127 may provide a means to distinguish between these types of cell death, because the increased malate production during necrosis mainly relies on permeabilization of the cell membrane, which does not occur during the early phase of apoptosis.
Multiparametric MRI for the Evaluation of HIFU Treatment
As described above, multiple MRI methods have been used for the evaluation of HIFU treatment, each providing sensitivity to (several) changes after HIFU treatment. Instead of evaluating changes in a single MRI parameter, with multiparametric MRI the effect of the HIFU treatment on a combination of MR parameters can be assessed. Because the different MRI parameters are each sensitive to different treatment effects, a multiparametric MR approach may provide a more complete evaluation of the HIFU intervention. The potential value of multiparametric MRI has recently been addressed in a review on prostate focused ultrasound therapy 6. In several studies, a multiparametric MRI protocol was used for HIFU treatment evaluation. As an example, Jacobs et al evaluated thermal ablation of uterine fibroids with multiparametric MRI that consisted of T2-weighted imaging, ADC mapping, sodium MRI and contrast-enhanced T1-weighted imaging 62 (Fig. 3). Partanen et al performed contrast-enhanced and diffusion-weighted MRI directly after HIFU treatment of canine prostate and subsequently did a correlation analysis between nonviable tumor tissue identified on each of the scans and in histology. Preliminary observations indicated a strong correlation between histology and each of the MRI methods 60.
Instead of assessing changes in the individual parameters of the multiparametric MRI protocol, the information of the different parameters can also be intregrated into a combined, automated analysis. This could potentially allow for automatic segmentation of successfully treated and residual nontreated tumor tissue. A few studies have used such automated analysis of multiparametric MR data for the assessment of HIFU treatment effects. Jacobs et al used a multiparametric analysis using diffusion-weighted and contrast-enhanced T1-weighted images to segment successfully treated and nontreated tissue directly after HIFU treatment of uterine fibroids. Excellent agreement was observed between the segmentations based on both MRI methods 63. We have recently performed cluster analysis on multiparametric MRI data obtained after HIFU treatment of subcutaneous mouse tumors. The clustering algorithm segments the multiparametric data into groups of pixels, i.e., clusters, with similar MR parameter values. We observed that a cluster analysis that combines T1, T2, and ADC maps could accurately distinguish successfully treated tumor tissue from nontreated tumor tissue 33. At 3 days after HIFU treatment, a strong correlation between histology-derived nonviable tumor tissue and HIFU-treated tumor tissue segmented by the cluster analysis was observed, while this correlation was lower early after HIFU treatment (Fig. 7). In a later study, we have performed multiparametric MRI consisting of T1, T2, ADC, APT-weighted signal, and T1ρ mapping to evaluate MR-guided HIFU treatment of rat tumors in a clinical 3T MR-HIFU system. The cluster analysis showed that the extent of nonviable tumor tissue could be accurately determined at 3 days after HIFU treatment when the ADC and APT-weighted signal data were combined 125.
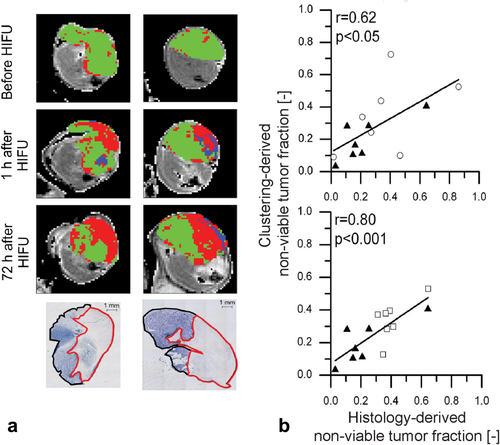
a: Representative results of clustering based on quantitative T1, T2, and ADC data of subcutaneous mouse tumors that were treated partially by HIFU. The cluster results are overlaid on the tumor pixels of axial T2-weighted images. Before HIFU treatment the majority of the tumor was segmented as viable (green) by the cluster analysis, while both at 1 h and at 72 h after HIFU treatment, a distinct region of nonviable (red) tumor tissue was identified by the cluster analysis. The identified nonviable tumor areas corresponded to nonviable (pale) tumor tissue in NADH-diaphorase histology. b: Correlation plots between histology-derived and clustering-derived nonviable tumor fractions at 1 h after HIFU treatment (top) and at 72 h after HIFU treatment (bottom). The symbols ▲, ○, and □ indicate nontreated control animals and HIFU-treated animals that were killed 1 h after treatment and 72 h after treatment, respectively. The correlation was highest at 72 h after HIFU treatment, which indicates that this multiparametric MR analysis is most sensitive to delayed treatment effects. Adapted with permission from Hectors et al 33.
Magnetic resonance fingerprinting (MRF) is a recently introduced MR method that uses a pseudorandom acquisition by dynamic alteration of several MR acquisition parameters 129. The resulting signal evolutions—or “fingerprints”—obtained in the imaging pixels are unique for each tissue type and a function of multiple MR contrast parameters. The fingerprints are subsequently matched to a predefined dictionary of predicted signal evolutions, which allows for the construction of quantitative maps of the MRI parameters of interest. The use of such method may be preferable to a multiparametric MR protocol that consists of a series of acquisitions that measure a single MR parameter, because it seems more time-efficient.
CONCLUSIONS AND FUTURE DIRECTIONS
The clinical introduction of HIFU for the thermal treatment of malignant lesions requires a treatment evaluation technique that can accurately detect residual tumor tissue, and extent of the lesion. This review focused on the different MRI methods, that have been—or could be—used to assess the HIFU treatment effects. Among these methods, CE-MRI is by far the most frequently applied method. However, as indicated in this review, in several studies CE-MRI has been shown to over- or underestimate the treated volume early after HIFU. Therefore, CE-MRI cannot always be considered a reliable MR method for the direct evaluation of HIFU treatment. Another drawback of (D)CE-MRI as evaluation method is that it requires the injection of a contrast agent. If one would want to perform immediate re-treatment when residual tumor tissue was detected, the presence of the contrast agent may introduce errors in the MR thermometry performed during treatment 56. In addition, the Gd-based contrast agent could become entrapped in the treated tumor tissue, possibly leading to tissue retention of (toxic) Gd3+ 55.
Fortunately, MRI offers a wide variety of contrast parameters that are purely based on intrinsic tissue contrast. Most studies on the MRI-based evaluation of HIFU treatment have focused on acute treatment effects. However, the MRI findings directly after treatment are often conflicting and strongly dependent on tissue type. These mixed MRI results are probably partly caused by the large number of changes that occur during treatment that have different effects on the MR contrast parameters. Some of these changes are transient (e.g., hyperemia and vascular occlusion), which may explain that the reports on treatment assessment a few days after treatment seem to be more consistent. In addition, apart from detection of direct coagulative necrosis, immediate assessment of the therapy response should ideally have a prognostic value, because latent cell death can occur in the first days after treatment. If this latent cell death can be predicted based on MRI results obtained directly after therapy, over- and undertreatment could be prevented. This would require the use of MRI methods that are sensitive to precursors of latent cell death, such as ischemia. As indicated earlier, examples of potentially ischemia-sensitive MRI methods are BOLD MRI and APT imaging.
A multiparametric MRI approach enhances the sensitivity of MRI to HIFU-induced necrosis. A convincing indication for this was found in our recent study in which we observed that the correlation between nonviable tumor tissue in histology and MRI was higher when a multiparametric MR analysis, that combined the T1, T2, and ADC data, was performed instead of analysis based on a single MR parameter 33. The sensitivity of the proposed multiparametric analysis to early changes after treatment could potentially be increased by inclusion of MR methods that are more sensitive to acute effects, such as 23Na MRI, APT imaging, or hyperpolarized 13C MR.
A potential drawback of the use of a multiparametric MRI protocol is that the need for assessment of multiple MRI parameters could lead to long acquisition times, which are generally unacceptable in clinical practice. However, the acquisition times of the individual MRI scans could be substantially reduced by acceleration techniques such as compressed sensing 130 and parallel imaging 131, 132. In addition, integration of the multiparametric protocol into a MRF acquisition could considerably shorten the acquisition time. Multiparametric protocols consisting of acquisitions that measure different nuclei can be made practically applicable by using multinuclear coil designs. Coil setups that allow for combined 1H and 23Na 133 or 13C 134 acquisitions within the same experiment without the need for moving the RF coil or subject have been described previously.
In addition to the studies reviewed in this article in which the MRI methods for assessment of treatment success were performed after the thermal ablation, there are a few reports on methods in which the MRI parameter of interest is acquired during the treatment, interleaved or simultaneous with the temperature maps. An MR method that allows for simultaneous T1 and temperature mapping was developed and used to monitor the release of MR contrast agent from thermosensitive liposomes during HIFU-induced hyperthermia 135. Plata et al showed preliminary results on interleaved ADC and temperature measurements during HIFU treatment of canine prostates 136. A marked decrease in ADC was observed during treatment. Peng et al performed simultaneous MT and temperature measurements during HIFU ablation of ex vivo porcine muscle tissue. A pronounced increase in the tissue MTR was noted during sonication 35. These reports on simultaneous temperature monitoring and treatment assessment seem promising, because they give early insights in the success of the treatment. However, care should be taken with the interpretation of these measurements. Because many MRI parameters, including the T1, ADC, and MTR, are strongly sensitive to temperature, prior knowledge on the exact influence of temperature on the parameter of interest is needed to correct the observed parameter changes for the temperature elevations during treatment.
Importantly, the influence of HIFU treatment on a certain MRI contrast parameter may be partly dependent on the tumor type, but also on the HIFU treatment settings that vary largely between different sites. Standardization of these settings, or more extensive reporting of the exact ultrasound exposure conditions as addressed recently by Ter Haar et al 137, would give a clearer understanding of the biological effects of HIFU treatment as well as the influence of these effects on the different MRI contrast parameters. In addition, extensive histological validation of the MRI findings in multiple tumor types is necessary to assess whether the measured responses in MRI parameters are indeed reflective of the extent of tissue damage induced by the HIFU treatment.
In conclusion, multiple MRI methods have been used for the evaluation of HIFU treatment. Several MR contrast parameters have shown sensitivity to HIFU-induced tissue changes. In addition, multiparametric MRI analysis that combines the data of several MRI parameters seems promising for accurate and automatic segmentation of nonviable and viable tumor tissue after thermal ablation with HIFU. As a next step, these MRI methods should be used in a larger variety of tumor types to fully assess their suitability as a biomarker for HIFU treatment evaluation. Studies along these lines, both in the preclinical and the clinical setting, are critically needed to define the ultimate role of MRI for the evaluation of HIFU-based thermal therapy of cancer.
ACKNOWLEDGMENT
The work in the authors’ laboratories on the topic of this review was supported by the Center for Translational Molecular Medicine (VOLTA).




