Nuclear localization of the actin regulatory protein palladin in sertoli cells
SUMMARY
In the testis, F-actin structures are involved in spermatid nuclear remodeling and cytoplasm reduction, maintenance of the blood–testis barrier, support of the spermatogonial stem cell niche, and release of spermatids into the tubular lumen. To gain a better understanding of actin regulation in Sertoli–germ cell interactions, we investigated the expression of the Palladin (Palld) gene, which encodes a widely expressed phosphoprotein that localizes to actin-rich cytoplasmic structures, including focal adhesions, cell–cell junctions, podosomes, and stress fibers, and serves as a molecular scaffold to bundle actin fibers. In germ cells, PALLD was concentrated along the tubulin- and F-actin-containing cytoplasmic manchette that forms adjacent to the elongating spermatid nucleus during spermiogenesis. To our surprise, PALLD relocated from the cytoplasm to the nucleus of Sertoli cells in the juvenile testis, coincident with the onset of puberty, and this localization was maintained in the adult. We provide evidence that the 140 kDa isoform of PALLD predominates in Sertoli cells, and that it is apparently cleaved, with the C-terminus localizing to the nucleus while the N-terminus remains cytoplasmic. We investigated the nuclear localization of the C-terminus of PALLD and found that it is regulated by a putative nuclear export signal. These results provide the foundation for future work employing Sertoli cell- and spermatid-specific Palld-knockout mice to study diverse roles of PALLD as both a nuclear-actin regulatory protein and as a potential regulator of manchette formation during spermatogenesis. Mol. Reprod. Dev. 80: 403–413, 2013. © 2013 Wiley Periodicals, Inc.
Abbreviations
-
- AR
-
- androgen receptor
-
- dpp
-
- days postpartum
-
- NES
-
- nuclear export signal
-
- Palld
-
- Palladin
INTRODUCTION
Male gametes develop within the seminiferous epithelium in intimate contact with somatic Sertoli cells, under the influence of a combination of paracrine and endocrine signals. Starting at puberty in the second week of life in the mouse, Sertoli cells become terminally differentiated and physically span the seminiferous epithelium to prepare for their eventual support, at male sexual maturity, of germ cell development. At the basal aspect of the tubule, they provide the stem-cell niche where spermatogonia asynchronously divide to give rise to spermatocytes that will enter meiosis (Oatley and Brinster, 2012). Following meiosis, spermatids undergo spermiogenesis, a series of complex morphological changes that result in the highly specialized spermatozoon. Sertoli cells intimately participate in the nuclear remodeling, flagellum formation, cytoplasm reduction, and eventual release of spermatids into the tubular lumen (Griswold, 1998; Russell, 1979b).
Actin is abundant in all cells, and it is primarily known as a structural cytoplasmic protein, contributing to cellular shape, motility, and polarity, as well as providing the “tracks” upon which cellular cargo is transported. The dynamic growth and shrinkage of filamentous (F)-actin is orchestrated by the complex interplay of combinations of multiple actin-regulatory proteins. These proteins regulate filament nucleation, localization, branching, and severing, as well as promote or inhibit loss or addition of globular (G)-actin monomers (dos Remedios et al., 2003). Specialized F-actin-rich structures, which are critical for spermatogenesis, begin to develop at discrete sites in the seminiferous epithelium. At the basal aspect of the tubule, preleptotene spermatocytes must cross the blood–testis barrier, an actin-based tight-adherens junction between adjacent Sertoli cells that functionally separates the basal and adluminal compartments of the seminiferous tubule. This apical, ectoplasmic specialization is a heterotypic adherens junction underlain by bundles of F-actin that are thought to position and retain spermatids in the seminiferous epithelium prior to spermiation (reviewed in Vogl et al., 2008). In spermatids, the formation of the unique F-actin-containing tubulobulbar complexes and manchette are critical for the proper development of spermatids prior to their release into the lumen at spermiation (Russell, 1979a, 1979b; Kierszenbaum and Tres, 2004; Kierszenbaum et al., 2007; D'Souza et al., 2009). The nucleus does not appear to contain F-actin, but it is now clear that abundant G-actin monomers are present in this compartment, although their role there remains unclear (reviewed in Zheng et al., 2009; Olson and Nordheim, 2010; Visa and Percipalle, 2010; de Lanerolle and Serebryannyy, 2011).
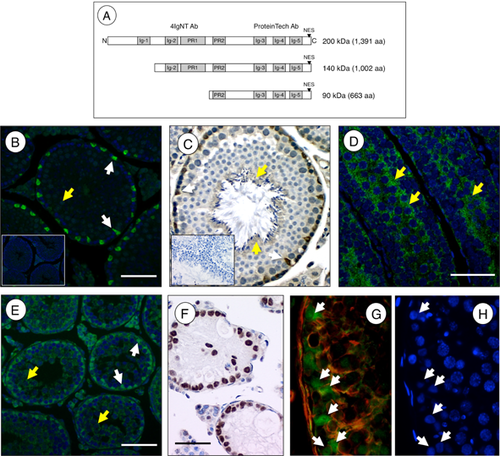
The Palladin (Palld) gene encodes a widely expressed phosphoprotein that localizes to F-actin-rich cytoplasmic structures such as focal adhesions, cell–cell junctions, podosomes, and stress fibers (Parast and Otey, 2000). PALLD is the most widely expressed member of the myotilin/palladin/myopalladin family of proteins, and is detected in both muscle and nonmuscle organs of vertebrate animals (Rachlin and Otey, 2006; Wang and Moser, 2008; Otey et al., 2009). This multidomain protein contains C-terminal immunoglobulin domains and N-terminal proline-rich domains thought to mediate its function as a molecular scaffold, allowing for the recruitment of numerous proteins involved in organizing the F-actin cytoskeleton (Fig. 1) (Parast and Otey, 2000; Dixon et al., 2008; Otey et al., 2009). The importance of PALLD has been shown experimentally using whole-animal knockout as well as cell-culture knockdown and transfection approaches. Palld-null mice die at 12.5 days postcoitum due to exencephaly and other defects (Luo et al., 2005; Liu et al., 2007). In cell-transfection experiments, overexpression of PALLD increased F-actin formation while knockdown resulted in loss of F-actin. The Palld gene encodes multiple tissue-specific isoforms, which are proposed to originate from the utilization of at least three different promoters whose pre-mRNAs may then be subjected to alternative splicing (Rachlin and Otey, 2006; Wang and Moser, 2008). Although PALLD was originally described as a cytoplasmic F-actin binding protein, multiple studies have shown that it can also partially localize to the nucleus in certain cell lines (Goicoechea et al., 2008; Endlich et al., 2009; Jin et al., 2010), where it can bind transcriptional regulators and influence patterns of gene expression (Jin et al., 2010).
Little is known regarding how F-actin-rich structures at the basal and apical aspects of the seminiferous epithelium are established in vivo, or about what specific developmental processes they regulate. In this study, we examined the testicular expression of PALLD as a dominant regulator of the F-actin cytoskeleton, and found that it localizes to nuclei of all Sertoli cells as early as 18-days postpartum (dpp), coincident with the onset of puberty, Sertoli cell differentiation, and formation of the blood–testis barrier. In addition, we discovered that PALLD localized to the manchette, a temporary cytoskeletal structure flanking spermatid nuclei during spermiogenesis. Our findings of nuclear and cytoplasmic localization of PALLD in the seminiferous epithelium indicate diverse roles for this protein in F-actin regulation during spermatogenesis.
RESULTS
PALLD Protein Is Concentrated in the Sertoli Cell Nuclei and in Spermatid Cytoplasm During Spermatogenesis
We performed immunohistochemistry on testis sections using an antibody that recognizes the C-terminus of the 200, 140, and 90 kDa PALLD isoforms. A strong signal for PALLD was found in nuclei of all Sertoli cells in the adult testis (Fig. 1B, C). Cytoplasmic staining for PALLD in germ cells was most evident in condensed spermatids surrounding the tubular lumen (Fig. 1C). There was no detectable seminiferous epithelial, stage-specific expression — as is the case for the androgen receptor (AR), which also localizes to Sertoli cell nuclei (Bremner et al., 1994). To determine when PALLD nuclear localization was first observable, we performed indirect immunofluorescence on juvenile testes at 10, 12, 14, 16, and 18 dpp. Cytoplasmic staining was observed in both Sertoli and germ cells of the 10 dpp testis (Fig. 1D). The percentage of Sertoli cells with nuclear PALLD staining progressively increased over the next few days such that all were positive by 18 dpp (Fig. 1E, data not shown). Development of the seminiferous epithelium involves a number of complex interactions between Sertoli cells and progressively differentiated populations of adjacent germ cells. To determine whether or not the nuclear localization of PALLD was dependent on the presence of germ cells, we performed immunohistochemistry using testis sections from W/WV mice, which lack germ cells due to a naturally occurring mutation in the receptor tyrosine kinase c-kit (Mintz and Russell, 1957; Geissler et al., 1988). PALLD was present in Sertoli cell nuclei in the W/WV testis, revealing that its localization does not require Sertoli-germ cell interaction (Fig. 1F). PALLD was present in Sertoli cell nuclei of the human testis as well (Fig. 1G, H), suggesting a similar role for this actin regulatory protein in humans.
Subcellular Localization of PALLD in the Adult Testis
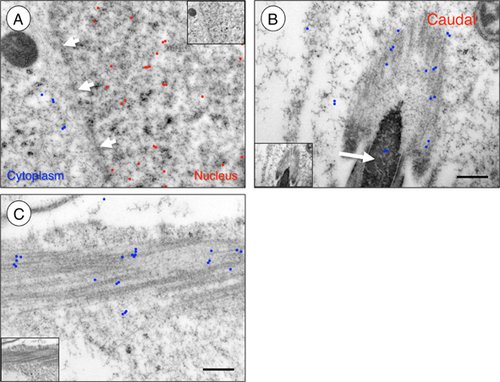
We next utilized immunogold transmission electron microscopy to determine whether PALLD was distributed in random or nonrandom patterns or found in association with specific nuclear structures in adult Sertoli cells. We detected at least a fivefold enrichment of gold particles (indicating the presence of PALLD) in Sertoli cell nuclei as compared to the adjacent cytoplasm (Fig. 2A). We found that PALLD was randomly distributed within Sertoli cell nuclei, and we did not detect increased labeling near structures such as the nucleolus or nuclear membrane. We also did not observe clustering or linear patterning, which might suggest an interaction with the nucleoskeleton (Fig. 2A, and data not shown). No signal was detected in the absence of primary antibody (data not shown). Upon closer examination, we found PALLD clustered along the spermatid manchette (Fig. 2B, C), a transient cytoplasmic F-actin and tubulin-containing structure that forms adjacent to the spermatid nucleus during spermiogenesis (Kierszenbaum and Tres, 2004), but we did not detect concentrated PALLD at either the basal or apical ectoplasmic specializations.
Determining the Predominant Palld Isoform in the Testis
Analysis of expressed sequence tags in the Unigene database representing 5′ sequences of Palld suggested that transcription initiated from three unique promoters, and that resulting transcripts might be alternatively spliced to create multiple distinct isoforms, which appear to be expressed in cell type-specific patterns (Rachlin and Otey, 2006; Wang and Moser, 2008). As a first step to identify which isoform(s) are localized within Sertoli cell nuclei, a Northern blot was performed with total RNA isolated from 10, 18, and >60 dpp (adult) testes. We detected a major ∼4.5 kb band at all stages, similar to what was seen in previous reports (Parast and Otey, 2000; Mykkanen et al., 2001), although additional bands were present in the adult testis at ∼7 and 3.5 kb (Fig. 3B). Since PALLD is nuclear in Sertoli cells in both the 18 and >60 dpp testis, it appears that the common ∼4.5 kb message encodes the major isoform in Sertoli cells. We also detected faint smaller bands in the adult testis, but it is unclear which isoforms they encode.
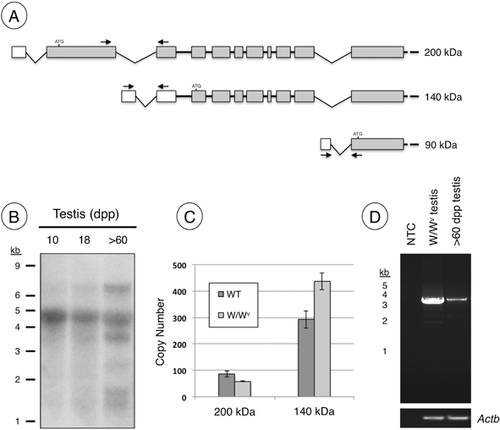
We used RT-PCR to verify the results of the Northern blot, which suggested the ∼4.5 kb band represented the message encoding 140 kDa isoform in the testis. Since the W/WV testis lacks germ cells, and contains Sertoli cells with prominent PALLD nuclear localization (Fig. 1F), we amplified cDNA from this tissue. Upstream primers were designed in the predicted 5′-untranslated regions (UTRs) (Mykkanen et al., 2001; Rachlin and Otey, 2006; Wang and Moser, 2008) of the mRNAs encoding the 90, 140, and 200 kDa isoforms (Fig. 3A). Despite multiple attempts using different primer combinations and various PCR conditions, we were unable to amplify sequences representing the 90 kDa Palld isoform from the testis (data not shown). Therefore, we focused our efforts on measuring the abundance of messages encoding the 140 and 200 kDa isoforms. Using qRT-PCR, we assessed copy number for messages encoding both the 140 and 200 kDa isoforms in cDNA from adult and W/Wv testes. We found that those representing the 140 kDa isoform were more abundant in both (Fig. 3C). We then verified that both primer sets amplified with similar efficiency by amplifying increasing amounts of DNA representing each Palld isoform, and the slopes from each titration were nearly identical (data not shown).
We next determined the sequence of the message encoding the 140 kDa isoform from the testis. Using primers designed at the start and stop codons of the 140 kDa isoform, we amplified cDNA of the predicted size from adult W/Wv and wild-type testes. We generated a single ∼3 kb product (Fig. 3D), which was purified and sequenced (GenBank accession JX477684).
Western Blot Reveals N- and C-Terminal Fragments of PALLD in the Testis
We isolated cytoplasmic and nuclear fractions from 18 dpp testes for Western blot analysis as a biochemical approach to verify the nuclear localization of PALLD. We chose this developmental time point for two reasons: First, PALLD localized to Sertoli cell nuclei (Fig. 1E) as in the adult testis and second, we had consistent cytoplasmic contamination in nuclear fractions isolated from adult testes, likely due to the presence of spermatozoa, which are resistant to the mild lysis conditions required for this assay (and are absent from the 18 dpp testis). Antibodies against established nuclear (H3F3A, AR) and cytoplasmic (TUBB) proteins revealed minimal cross-contamination in extracted fractions (Fig. 4A).
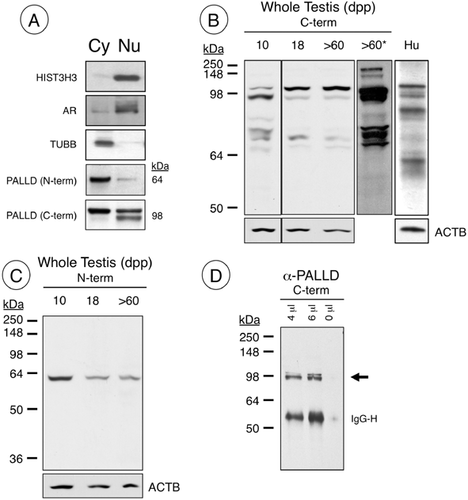
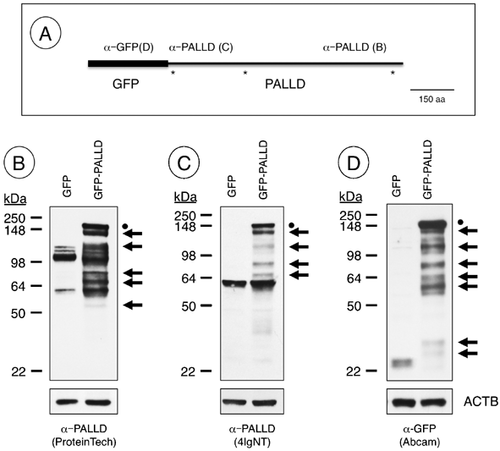
We used an N-terminal antibody (4IgNT, Fig. 1) predicted to bind to the 140 and 200 kDa PALLD isoforms (Rachlin and Otey, 2006) as well as a C-terminal antibody (ProteinTech) that binds to all known isoforms (see Fig. 1A for epitope location). We detected a single band of ∼60 kDa primarily in the cytoplasm using 4IgNT (Fig. 4A). Using the C-terminal antibody, we detected a ∼98 kDa doublet in the nuclear fraction, while the upper band of the doublet was present only in the cytoplasmic fraction (Fig. 4A). This was rather unexpected considering we expected to detect 140 or 200 kDa proteins, but instead detected differing fragments that together approximated ∼140 kDa.
To investigate this further, we performed Western blot analysis using both N- and C-terminal antibodies with whole testis lysates. As observed in the nuclear/cytoplasmic separations (Fig. 4A), the predominant bands detected with the C-terminal antibody (ProteinTech) in testis lysates from 10, 18, and >60 dpp mice were not the predicted 140 kDa, but a doublet around 98 kDa (Fig. 4B). There were 2 faint, larger bands at ∼140 and 200 kDa, both of which were more evident in extended exposures (Fig. 4B). In addition, there was a cluster of faint bands consistently present at 70–80 kDa (Fig. 4B). The overall pattern for adult mouse testis lysates closely resembles those from the human testis (Fig. 4B). We next utilized the N-terminal 4IgNT antibody, and detected a single band at ∼60 kDa that did not match any of the bands detected using the C-terminal antibody (Fig. 4C). We performed immunoprecipitation using the C-terminal antibody, and pulled down the ∼98 kDa doublet from adult testis lysates (Fig. 4D), with a very faint band around 140 kDa, visible only on prolonged exposures (not shown).
The 98 kDa Isoform May Be Generated by Proteolytic Cleavage
Since there was a difference in the predicted and observed size of the 140 kDa isoform, we next transfected the mouse Sertoli TM4 cell line with an N-terminal GFP-tagged, 140 kDa Palld expression vector (Fig. 5A). Greater than 70% of cells were transfected, as assessed by fluorescent microscopic detection of GFP expression. Western blot analysis was performed to assess the size of the resultant bands using antibodies against N- and C-termini of PALLD, respectively, and GFP. An expected band was seen at >140 kDa using all three antibodies, representing the recombinant GFP-PALLD. But, a number of smaller fragments were also detected using antibodies against the N-terminus of the recombinant protein (anti-GFP) and the N-terminus (4IgNT) and C-terminus (ProteinTech) of the 140 kDa PALLD isoform, respectively (Fig. 5B–D). The sizes of these fragments depended on the antibody used, but the bands recognized by the N- and C-terminal antibodies were of the sizes anticipated when considering the size and location of the GFP epitope for each PALLD fragment. Similar results were obtained when N-terminal Myc-tagged 140 kDa Palld vectors were transfected into TM4 cells (data not shown). Together, these results suggest that proteolytic cleavage of the 140 kDa isoform at multiple sites (approximated by asterisks, Fig. 5A) is responsible for generating the different bands seen by Western blot.
Nuclear Localization of C-Terminal PALLD in the Absence of a Putative Nuclear Export Sequence
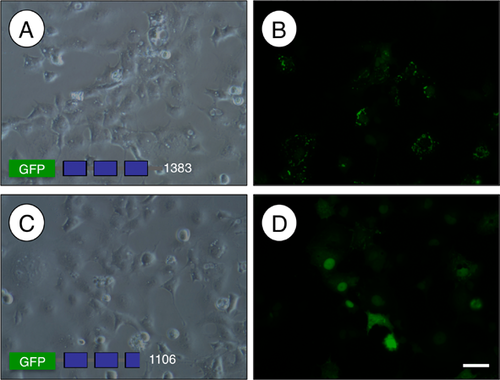
The C-terminus of PALLD (amino acids 618–1,002, NCBI accession JX477684) accumulated in nuclei 24 hr after inhibition of XPO1-mediated nuclear export with Leptomycin B in a kidney podocyte cell line (Jin et al., 2010) and in cultured rat aortic smooth muscle cells (Endlich et al., 2009). Exportin 1 (XPO1) binds to target proteins at leucine-rich nuclear export signals (NES) to mediate their export from the nucleus (Kutay and Guttinger, 2005). We analyzed the PALLD coding sequence, and found an evolutionarily conserved, putative NES (LxLxxxL) with commonly found Glu, Asp, and Ser residues adjacent to it (la Cour et al., 2004). To test if this portion of the C-terminus regulates nuclear export of PALLD, we transfected vectors expressing GFP-PALLD fusion proteins with or without the NES into the TM4 Sertoli cell line and assessed their cellular localization. In the presence of the putative NES, GFP-PALLD was present in cytoplasmic foci around the nucleus (Fig. 6B). In contrast, when amino acids 950–1,002 of the C-terminus containing the NES were deleted, GFP-PALLD localized to the nucleus (Fig. 6D).
DISCUSSION
Sertoli cells terminally differentiate at puberty with the appearance of structures that are essential for spermatogenesis: a highly specialized, F-actin-rich basal blood–testis barrier and an apical ectoplasmic specialization (Vogl et al., 2008; Lie et al., 2010). In this study, we investigated the expression of the actin-regulatory protein PALLD in prepubertal, pubertal, and adult testis. We found that PALLD localized along the manchette in elongating spermatids, and that the C-terminus of the 140 kDa isoform predominates in Sertoli cells, where it is localized in nuclei and is potentially generated by cleavage of the full-length protein into N- and C-terminal fragments. We also provide evidence that this cellular localization of the C-terminus is mediated through a C-terminal NES in a Sertoli cell line.
The staining for PALLD was most intense in Sertoli cell nuclei after 18 dpp, but it was also present in the cytoplasm of both Sertoli and germ cells. Therefore, it was not surprising that Western blot analyses using separated nuclear and cytoplasmic lysates from whole testis revealed PALLD in both compartments. It was unexpected that both bands of the doublet at ∼98 kDa detected by our C-terminal antiserum were found in the nucleus, while only the upper band was present in the cytoplasm. Interestingly, the smaller band was found constitutively in the nucleus, suggesting that the upper band may shuttle between the two compartments. It is unclear at this point whether the size difference between the two bands in the doublet is due to posttranslational modification or to the potential cleavage of a portion of the upper band, but it is tempting to speculate that the constitutively nuclear, smaller band may lack the most C-terminal NES.
In addition to its concentration in the adult testis in Sertoli cell nuclei, we found PALLD localized along the manchette, which extends caudally from the elongating spermatid nucleus. The manchette is predicted to participate in condensation of the spermatid nucleus, sperm head shaping, vesicle transport, and/or flagellum formation; these roles have been proposed based on ultrastructural characterization and localization of various proteins (Kierszenbaum and Tres, 2004). PALLD localization does appear to exhibit some periodicity, with approximately evenly spaced clusters of signal. This staining resembles that for ACTR1/ARP1 (Fouquet et al., 2000), an actin-related protein (ARP) that is an integral part of the dynactin complex involved in organelle transport along microtubules (Lees-Miller et al., 1992; Schroer, 2004). We hypothesize that cytoplasmic PALLD in spermatids may interface with this complex to regulate transport of essential molecules down the length of the developing flagellum.
We determined that the mRNA encoding the 140 kDa PALLD isoform was most abundant in Sertoli cells in the testis, although we did not detect bands of the expected sizes on Western blots of testis lysates. Using the C-terminal PALLD antiserum (ProteinTech), we detected a doublet at ∼98 kDa that was present in the nucleus and cytoplasm. The N-terminal PALLD antiserum (4IgNT), on the other hand, detected a single band at ∼60 kDa that predominated in the cytoplasm. We wondered whether these two distinct bands representing the respective PALLD C- and N-termini originate from the translation of distinct messages or from the production of one polypeptide that is cleaved in vivo. Much of our data support the cleavage model: (1) Neither the C- or N-terminal antisera identified predominant bands at 140 kDa, although our Northern blot, RT-PCR, and qRT-PCR results indicated that it is the most abundant message; (2) When added together, the N- and C-terminal pieces approximated 140 kDa; and (3) When expressed as a recombinant GFP fusion protein in TM4 cells, the 140 kDa isoform generated multiple fragments instead of one discrete band. There is precedence to support the idea that proteolytic cleavage could generate multiple isoforms of PALLD in differentiated Sertoli cells. Two independent groups found that the F-actin binding protein FLNA was cleaved by calpains into 190, 100, and 90 kDa fragments (Fox et al., 1983; Gorlin et al., 1990). The C-terminal fragment of FLNA then bound to the AR in a ligand-independent manner, repressed AR-mediated transactivation (more than full-length), and was required for AR nuclear translocation (Ozanne et al., 2000; Loy et al., 2003). In addition, it was previously shown that the general protease inhibitor α2-macroglobulin prevented the cAMP-mediated reorganization of the Sertoli cell actin cytoskeleton (Tung et al., 1993). So perhaps the most important aspect of Palld expression and regulation in the testis is not which isoform is transcribed, but where and how the protein is cleaved by specific proteases.
There are multiple N- and C-terminal motifs present in the 140 kDa PALLD protein. In the N-terminus, proline-rich domains bind to a number of important signaling and cytoskeleton-modifying proteins, including EPS8, PFN1, LASP1, and VASP (Boukhelifa et al., 2006; Goicoechea et al., 2006, 2008; Rachlin and Otey, 2006), while the C-terminus contains three immunoglobulin-like domains capable of binding and bundling F-actin (Dixon et al., 2008; Otey et al., 2009). The separation of the potential factor (N-terminus) and F-actin (C-terminus) binding functions of PALLD might play an important role during Sertoli cell maturation and development of the specialized Sertoli cell cytoskeleton. The generation of Sertoli cell- and spermatid-specific Palld knockout mice will clarify the dual roles of PALLD in the testis; such results will have implications for understanding the role of nuclear and cytoplasmic actin regulatory protein function in diverse cell types.
MATERIALS AND METHODS
Testicular Tissue Collection
Tissues from CD-1 mice were utilized for all of the developmental studies of endogenous PALLD expression. The day of birth was designated 0 dpp. All procedures were performed in accordance with the National Research Counsel Guide for the Care and Use of Laboratory Animals, and were approved by the East Carolina University Animal Care and Use Committee (AUP# A178). Paraffin-embedded testis sections from adult W/Wv mice were a gift from Dr. E. M. Eddy, National Institute of Environmental Health Science/National Institutes of Health (NIEHS/NIH). Human testicular tissue was from an 18-year-old, apparently healthy black male who suffered scrotal trauma, necessitating an orchiectomy at Pitt County Memorial Hospital (now Vidant Medical Center). The East Carolina University Institutional Review Board approved our protocol (UMCIRB# 10–0627) for collection and utilization of human testicular tissue.
Immunohistochemistry and Indirect Immunofluorescence on Paraffin Sections
Experiments were repeated at least three times using sections from human and mouse testis samples. For mice, at least two gonads were analyzed from different animals. Tissues were collected and fixed overnight at 4°C in 4% paraformaldehyde (PFA), pH 7.4, washed three times in phosphate-buffered saline (PBS), dehydrated through an ethanol series, embedded in paraffin, and cut into 6-μm sections using standard procedures.
For immunohistochemstry, sections were deparaffinized and rehydrated using standard methods. C-terminal-specific anti-PALLD (1:500, Cat# 10853-1-AP, ProteinTech Group Inc., Chicago, IL) and anti-AR (1:250, sc-816, Santa Cruz Biotechnology, Santa Cruz, CA) were applied to sections in blocking buffer (0.05 M Tris–HCl, 0.15 M NaCl, 0.02% Triton X-100 + 1% BSA, pH 7.6) without antigen retrieval for overnight incubation at 4°C. Primary antibody was omitted in negative controls. Biotinylated secondary goat anti-rabbit antibody (Vector Laboratories, Burlingame, CA) and DAB peroxidase substrate (Vector Laboratories) were used to detect antibody complexes according to manufacturer's recommendations. Sections were counterstained with hematoxylin, mounted with Permount (Sigma-Aldrich, St. Louis, MO), and visualized using a Zeiss Photomicroscope III (Carl Zeiss Microscopy LLC, Thornwood, NY) with a Dage XL16C digital camera and Dage Exponent software, version 1.3 (Dage-MTI, Michigan City, IN).
For immunofluorescence, sections were deparaffinized and rehydrated in PBS, and blocking was performed with 3% BSA + 0.1% Triton X-100 for 1 hr at room temperature. C-terminal anti-PALLD was applied to sections (1:200) in blocking buffer and incubated overnight at 4°C. Following three washes in PBS + 0.1% Triton X-100, Alexa Fluor-488 donkey anti-rabbit IgG (1:500, Invitrogen, Carlsbad, CA) was added for a 1 hr incubation at room temperature. Coverslips were mounted with Vectastain containing 4′,6-Diamidino-2-Phenylindole (DAPI; Vector Laboratories) and images acquired using a Nikon E600 fluorescence microscope with an Orca II CCD camera (Hamamatsu, Bridgewater, NJ) or a Zeiss Axio Observer A1 fluorescence microscope with a Dage XL16C camera (Dage-MTI).
Indirect Immunofluorescence on Frozen Sections
Human testicular tissues were fixed overnight in 4% PFA pH 7.4 at 4°C, and then washed three times in PBS prior to overnight incubation in 30% sucrose solution in PBS. Tissue was then embedded in O.C.T. compound, and 8 μm sections were cut and washed three times in PBS. Following blocking for 1 hr in 3% BSA, C-terminal anti-PALLD (1:200) was added to blocking buffer and incubated overnight at 4°C. Primary antibody was omitted in negative controls. Following three washes in PBS, Alexa Fluor 488 anti-rabbit IgG (1:500, Invitrogen) was added for 1 hr at room temperature. Sections were then mounted and counterstained with Vectastain containing DAPI (Vector Laboratories), and images were acquired using a Nikon E600 fluorescence microscope with an Orca II CCD camera (Hamamatsu).
Immunogold Electron Microscopy
For immunoelectron microscopy, testis tissues (1–3 mm cubes) were fixed for 2–4 hr by immersion in 4% PFA (pH 7.3). Tissues were then dehydrated in a graded series of ethanol, and embedded in L.R. White embedding media (London Resin Company Limited, Berkshire, UK). Eighty-nanometer sections were collected on formar/carbon-coated nickel grids (200 mesh) and incubated in PBS prior to immunostaining. Non-specific binding was blocked by incubation in 5% normal goat serum (NGS)-PBS (12 mM PBS, 0.05% Tween-20) for 1 hr, followed by a brief rinse in PBS. Sections were then incubated without primary antibody (control grids) or with C-terminal anti-PALLD (1:500 in 1% NGS in PBS overnight at 4°C, washed in PBS, and then incubated with 12 nm colloidal gold-goat anti-rabbit (Jackson ImmunoResearch Laboratories, West Grove, PA) solution (1:20 in 1% NGS-PBS) for 1 hr. After washing, sections were incubated in 0.1% glutaraldehyde for 5 min, and then rinsed in water. Sections were viewed with a JEOL 1200 EX electron microscope (Jeol USA, Peabody, MA) at 60-kV accelerating voltage. Images were recorded using an SIS MegaView III CCD camera (Olympus, Tokyo, Japan), and the number of gold particles in each image were counted to approximate the ratio between those in the cytoplasm and the nucleus.
RNA Isolation and Quantitative (q)RT-PCR and RT-PCR
Total RNA was isolated from tissues using the RNeasy kit (Qiagen, Valencia, CA) according to manufacturer's instructions, and then quantitated by UV spectroscopy. For qRT-PCR, 100 ng of RNA was reverse-transcribed and amplified in triplicate using iScript One-Step RT-PCR kit with SYBR green (Bio-Rad Laboratories, Hercules, CA), according to manufacturer's instructions, on a Bio-Rad iQ5 real-time PCR detection system (Bio-Rad Laboratories). Primer pairs used were as follows: 200 kDa isoform [Genbank Accession AK052489 (Rachlin and Otey, 2006)] (5′-ACTGAGGAGCCAAGAAGTTGCTGA and 5′-CTCAAAGGCCTCAGCAATGACCAA), 140 kDa isoform (Genbank Accession JX477684) (5′-TACAGAGCGTATTTGGTGCCCAGT and 5′-TCAAAGGCCTCAGCAATGACCAAG), and the 90 kDa isoform [Genbank Accession BQ927960 (Rachlin and Otey, 2006)] (Forward: 5′-ATGCTTTGATACTCAGGAGGACCC and 5′-AGGAGCCCTCGACACCCA and Reverse: 5′-TGTTCCAGGCGCACTTGGTTC). All primers were designed to amplify across an intron/exon junction to avoid the possibility of genomic DNA amplification giving erroneous results. Each sample was amplified in triplicate, and each reaction repeated at least twice. For RT-PCR, 50 ng of cDNA were amplified with LA Taq (Takara Bio, Shiga, Japan) using manufacturer's recommendations using primers at the 5′- and 3′-end of the 140 kDa isoform (respectively, 5′-TGGCTCAGACAGCACATCTGCAGAG and 5′-TTAAACGAGTTCTGTCCTGCTCTGTTG).
Ct values were normalized to Rpl19 (60S ribosomal protein L19), and relative mRNA levels were calculated by using the delta-delta Ct (ΔΔCt) method. Student's t-test was used for all statistical analyses, and significant differences are indicated with an asterisk (P < 0.05).
Nuclear and Cytoplasmic Fractionation and Western Blot Analysis
Cytosolic and nuclear fractions from 18 dpp testes were isolated using the NE-PER Nuclear and Cytoplasmic Extraction Kit (Thermo Fisher Scientific, Waltham, MA), as per the manufacturer's instructions with minor modifications. Briefly, 100 mg of testes were homogenized in CER I solution at 20,000 rpm for 30 sec. Once the cytosolic fraction was isolated, the nuclear fraction was briefly centrifuged, and the supernatant was removed using an insulin syringe to reduce cytoplasmic contamination. Nuclear pellets in NER solution were subjected to a 10-sec burst of sonication to disrupt nuclear membranes, and isolated fractions were quantitated using the BCA assay (Thermo Fisher Scientific), then stored at −80°C prior to use. Fifteen micrograms (for anti-TUBB and anti-H3F3A) or 25 μg (for anti-AR and anti-PALLD) of protein from each fraction was separated by polyacrylamide gel electrophoresis prior to Western blot analysis using standard methods. Primary antibodies used were: C-terminal anti-PALLD (1:1,000); N-terminal anti-PALLD (1:1,000); 4IgNT (Rachlin and Otey, 2006); anti-H3F3A (1:1,000, Cat #05-928, Millipore, Billerica, MA); anti-TUBB (1:10, kind gift from Dr. Phil Pekala, East Carolina University); anti-AR (1:250, sc-816, Santa Cruz Biotechnology); and anti-GFP (1:2,000, ab290, Abcam, Cambridge, MA). HRP-conjugated secondary antibodies (Cell Signaling Technology, Danvers, MA) were used at 1:5,000. Detection was performed using enhanced chemiluminescence LumiGlo (Thermo Fisher Scientific).
Northern Blot
Total RNA was isolated from testes at 18 and >60 dpp using Trizol reagent (Invitrogen), according to manufacturer's instructions. Five micrograms of RNA and 5 µg Millennium Markers ladder (Life Technologies, Grand Island, NY) were denatured, separated on a MOPS gel, and transferred to a nylon membrane (GE Healthcare Biosciences, Pittsburgh, PA). Hybridization was performed using a 32P-lableled antisense riboprobe corresponding to Palld nucleotides 2,370–2,709 (Genbank Accession JX477684). Following stringency washes to remove unbound probe, the membrane was exposed to autoradiography film for 48 hr.
Vectors, Cell Culture, and Transfection
The coding sequence of the 140 kDa Palld cDNA was amplified using LA Taq (Takara Bio), according to manufacturer's instructions, with the following primers: 5′-ATGCCACAAGCTCAGAAGAAAACAACG and 5′-TCACAGGTCTTCACTTTCTACCAAGC. The amplified product was cloned into pEGFP-C1 (Clontech Laboratories, Inc, Mountain View, CA) to create an N-terminal Gfp-Palld fusion vector. Plasmids were sequence-verified prior to transfection. TM4 cells were purchased from ATCC (Manassas, VA), and grown in Ham's F-12 with 5% horse serum and 2.5% FBS (Invitrogen) at 37°C in 95% air with 5% CO2. Transfections were performed with Lipofectamine 2000 (Life Technologies) using the manufacturer's recommendations.
ACKNOWLEDGMENTS
The authors would like to thank Joani Zary (East Carolina University) for technical assistance, Drs. E. Mitch Eddy (National Institute of Environmental Health Sciences) and Ann Sperry (East Carolina University) for experimental advice, and Dr. Carol Otey (University of North Carolina at Chapel Hill) for critical reading of the manuscript.




