Down-regulation of interferon tau gene transcription with a transcription factor, EOMES
Abstract
Interferon tau (IFNT), produced for a short interval during early pregnancy by the ruminant embryonic trophectoderm, is essential for the maintenance of early pregnancy, but the mechanism by which it is transcriptionally regulated has not been fully determined. To identify a transcription factor(s) involved in the down-regulation of IFNT genes, mRNAs for various known transcription factors were investigated by reverse-transcriptase and real-time PCR in conceptus tissues collected on Days 15, 17, and 21, or Days 17, 20, and 22 of ovine or bovine pregnancy, respectively. In particular, the T-box protein eomesodermin (EOMES) exhibited high mRNA expression in Day 17 or 22 ovine or bovine conceptuses. Interaction between EOMES and the identified transcription factors was studied using transient transfection, revealing that ovine/bovine IFNT-reporter transactivation was down-regulated by EOMES. Transcription factor interactions with EOMES were further studied through immunoprecipitation, demonstrating an association between EOMES and cAMP-response element binding protein (CREB)-binding protein (CREBBP). Uterine flushing media collected from cyclic or early pregnancy animals were added to bovine trophoblast CT-1 cells cultured on type-I collagen (monoculture) or bovine uterine epithelial cells (coculture) in an attempt to regulate EOMES expression. In the coculture, but not the monoculture, addition of uterine flushing from Day 17 pregnant animals resulted in increased EOMES expression in CT-1 cells. These results suggest that as conceptuses attach to the uterine epithelium, IFNT gene transcription is down-regulated by an increase in EOMES expression and EOMES–CREBBP binding in the attached trophoblast cells. Mol. Reprod. Dev. 80: 371–383, 2013. © 2013 Wiley Periodicals, Inc.
Abbreviations
-
- AP-1
-
- activator protein-1
-
- CDX2
-
- caudal-type homeobox transcription factor 2
-
- CREBBP
-
- cAMP-response element binding protein-binding protein
-
- DLX3
-
- distal-less 3
-
- EOMES
-
- eomesodermin
-
- IFNT
-
- interferon tau.
INTRODUCTION
Maternal recognition of pregnancy is the process in which communication is initiated between the conceptus and its mother, resulting in the altered maternal physiology required for a pregnancy to be established (Short, 1969). During the peri-implantation period, the embryonic trophectoderm of ruminant conceptuses secretes the cytokine interferon tau (IFNT) (Imakawa et al., 1987; Roberts et al., 1992), which inhibits the endometrial production of luteolytic pulses of prostaglandin F2α, resulting in the maintenance of corpus luteum function (Godkin et al., 1984; Spencer and Bazer, 1996; Salamonsen et al., 1998). Secretion of ovine IFNT starts on Days 8–9 of pregnancy (Day 0 being the day of estrus), peaks on Days 16–17, and then declines rapidly (Godkin et al., 1982; Ashworth and Bazer, 1989; Farin et al., 1989). Expression of trophoblast IFNT is regulated in a temporal and spatial manner, but the precise molecular mechanisms of this process have not been elucidated.
Ezashi et al. (1998) demonstrated that the transcription factor ETS2 binds to the promoter region from −79 to −70 bases (transcription-initiation site is +1) of the bovine IFNT gene, and that ETS2 transactivates a bovine IFNT-reporter construct in human choriocarcinoma JAR cells. In the upstream sequences of the ovine IFNT gene, there is a promoter region with an ETS2 binding site at the equivalent location of the bovine counterpart. Yamaguchi et al. (1999) then identified an enhancer region between −654 and −555 bases of the ovine IFNT gene, including activator protein-1 (AP-1) recognition sites to which nuclear proteins extracted from human choriocarcinoma JEG3 cells bind. Moreover, a transcription coactivator, cAMP-response element binding protein (CREB)-binding protein (CREBBP), which has histone acetyltransferase function, was shown to regulate ovine IFNT gene expression (Xu et al., 2003). It was also shown that caudal-type homeobox transcription factor 2 (CDX2) is expressed in the ovine and bovine trophoblasts during the conceptus elongation period (Degrelle et al., 2005; Imakawa et al., 2006). In fact, over-expression of trophectoderm lineage-specific Cdx2 along with Jun and ETS2 in JEG3 cells effectively increased the degree of ovine IFNT-reporter transactivation (Imakawa et al., 2006). Transcription factors GATA2 and GATA3 were also involved in trophoblast-specific regulation of the bovine IFNT gene (Bai et al., 2009). It was found that the homeodomain transcription factor distal-less 3 (DLX3) was also involved in trophoblast-specific expression of bovine IFNT (Ezashi et al., 2008). These results suggest that CDX2, and possibly GATA2/3 and DLX3, could be key elements that determine trophectoderm-specific activation of IFNT gene transcription. In spite of these efforts, the molecular events that down-regulate IFNT gene expression have not been clarified.
The unique expression of the IFNT gene could be mainly regulated by CDX2 (Imakawa et al., 2006; Sakurai et al., 2009, 2010), but evidence points to the existence of a negative regulator(s) of the IFNT gene. Previously, it was demonstrated that IFNT expression decreased as the trophoblast attached to the uterine epithelium (Guillomot et al., 1990; Imakawa et al., 2006). When in vitro-derived bovine blastocysts are cultured continuously, IFNT production remains strong even after the time its expression in vivo would have been attenuated (Hernandez-Ledezma et al., 1993). These findings indicate that the attachment of trophectoderm to the uterine epithelium could be an important determinant for the decrease in IFNT expression. The T-box protein eomesodermin (EOMES) is a prime candidate for IFNT gene down-regulation because it is expressed in the trophectoderm lineage and is thought to perform essential functions in trophectoderm development in mice (Hancock et al., 1999; Russ et al., 2000). A block in the early development of trophectoderm occurs in Eomes-mutant blastocysts, and, in fact, Eomes homozygous mutant mouse embryos die around the time of implantation (Russ et al., 2000; Strumpf et al., 2005). Furthermore, Degrelle et al. (2005) demonstrated that EOMES expression is located in embryonic and extra-embryonic tissues of bovine elongating conceptuses.
To our knowledge, the expression and function of EOMES in the context of IFNT gene regulation during the peri-implantation period has not been studied. To find a transcription factor involved in the regulation of IFNT genes, reverse-transcriptase and real-time PCR were used to study various transcription factor mRNAs in trophoblasts collected on Days 15, 17, and 21 of pregnancy. Expression patterns of IFNT, CDX2, and EOMES mRNA observed in in vivo-derived trophoblasts were compared with in vitro cocultures of bovine trophoblast cells and endometrial epithelial cells. Experiments were extended to determine the 5′-upstream regions that regulate transcriptional activity of ovine IFNT genes through Eomes over-expression. Luciferase reporter constructs and immunoprecipitation were used to examine interactions between EOMES and other transcription factors on ovine IFNT-reporter transactivation. The results allow us to propose molecular mechanisms by which the IFNT gene is down-regulated through EOMES expression.
RESULTS
IFNT, EOMES, CDX2, CREBBP, ETS2, or JUN Transcripts in Ovine Conceptuses
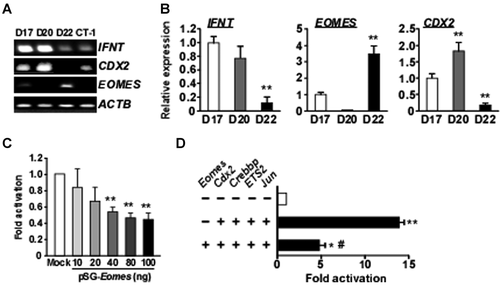
Transcripts for IFNT and the regulators of IFNT gene expression, including EOMES, CDX2, CREBBP, ETS2, and JUN, were assessed in ovine conceptuses by RT-PCR and real-time PCR, and are shown in Figure 1A,B, respectively. Expression of IFNT transcripts was relatively high in Days-15 and -17 conceptuses, but decreased on Day 21. mRNA expression of the T-box transcriptional factor EOMES in trophoblasts was lowest on Day 15, peaked on Day 17, and declined slightly to approximately 85% of maximum on Day 21. CDX2 mRNA was present at high levels in Days 15 and 17 trophoblasts, and decreased on Day 21. CREBBP mRNA gradually increased towards Day 21. While changes in IFNT, CDX2, CREBBP, and EOMES mRNA levels were seen, ETS2 and JUN mRNAs remained unchanged in Days-15, -17, and -21 ovine trophoblasts (Fig. 1A,B). Similar results were obtained from bovine conceptuses during the equivalent periods (Fig. 2A,B). In the bovine conceptus, EOMES mRNA level was lowest on Day 20 and highest on Day 22.
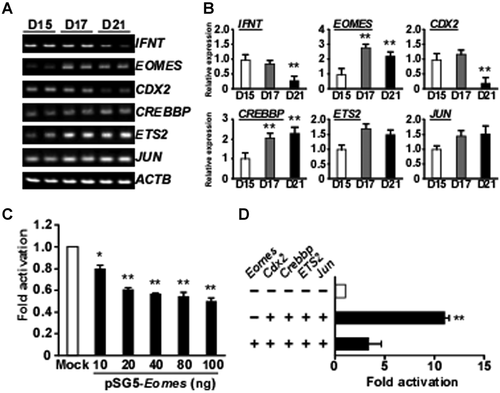
Negative Effects of EOMES on Ovine IFNT-Reporter Transactivation
To examine the effect of EOMES on ovine IFNT gene transcription, the ovine IFNT-reporter (−654 to +51 bp, wild type) construct with the pSG5 vector (Mock) or pSG5-Eomes was transfected into JEG3 cells (Fig. 1C). Luciferase activity decreased about 50% from the control (Mock) when pSG5-Eomes was present. The effect of EOMES was then examined in combination with Cdx2, Crebbp, ETS2, and Jun, which had previously been shown to be most effective in the transactivation of the ovine IFNT-reporter construct (Imakawa et al., 2006). EOMES reduced the effect of CREBBP, JUN, ETS2, and CDX2 on the up-regulation of ovine IFNT-reporter transactivation (Fig. 1D). Similar results were obtained from the bovine IFNT-reporter (−631 to +51 bp, wild type) construct (Fig. 2C,D). These findings indicate that EOMES could act negatively against IFNT transactivation in both species.
In deletion studies, EOMES exerted a negative effect on luciferase activity between −654 and −551 bases of the ovine IFNT construct (Fig. 3A). It has previously been demonstrated that there are JUN and CDX2 binding sites in this region of the ovine IFNT gene (Yamaguchi et al., 1999; Imakawa et al., 2006). Removal of this region or mutation of the AP-1 (JUN) binding site reduced transactivation in the bovine IFNT gene in a similar manner (unpublished observations). Luciferase activity was further decreased with deletions between −271 and −123 bases, but was unaffected by the presence of EOMES (Fig. 3A). In short, EOMES affected luciferase activity between −654 and −551 bases of the ovine IFNT gene, but exhibited no apparent effect when the serially deleted upstream region from −551 to −58 of the ovine IFNT gene was examined (Fig. 3A).
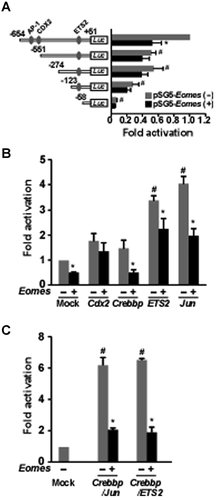
To further examine whether or not CDX2, CREBBP, ETS2, and/or JUN interaction with EOMES affected IFNT gene transcription, these transcription factors were evaluated in combination for effects in the ovine IFNT-reporter transactivation (Fig. 3B). Transfection with Cdx2, Crebbp, Jun, or ETS2 enhanced the luciferase activity of the wild-type ovine IFNT-reporter construct. When Eomes was co-transfected, however, the increase in luciferase activity brought forth by Crebbp, ETS2, or Jun was reduced (Fig. 3B). When Crebbp/Jun or Crebbp/ETS2 were co-transfected with Eomes, luciferase activity was also reduced (Fig. 3C).
Reduction of IFNT-Reporter Transactivation by EOMES Through Transcription Factor Interaction
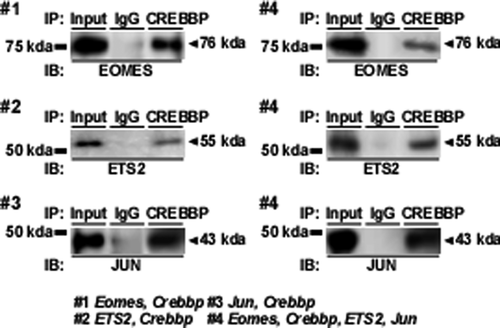
It was demonstrated that CREBBP/JUN and CREBBP/ETS2 complexes bind to the upstream region of the ovine IFNT gene (Xu et al., 2003). To investigate the protein-protein interactions among EOMES, CREBBP, JUN, and ETS2, an immunoprecipitation study was conducted with lysates from JEG3 cells that had been transfected with Crebbp/Eomes, Crebbp/ETS2, or Crebbp/Jun expression plasmids (Fig. 4). When the cell lysates were initially immunoprecipitated with mouse monoclonal antibody against CREBBP and then blotted with rabbit polyclonal antibody against EOMES, ETS2, or JUN, bands corresponding to EOMES, ETS2, or JUN were detected (Fig. 4; #1, #2, and #3). Immunoprecipitation was then conducted with lysates from JEG3 cells that had been transfected with Crebbp, Eomes, ETS2, and Jun expression plasmids. EOMES, ETS2, and JUN were detected in the lysates from JEG3 cells that had been transfected with Crebbp, Eomes, ETS2, and Jun plasmids (Fig. 4; #4).
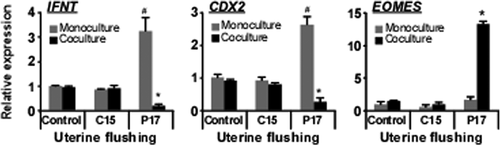
Coculture Experiments and Changes in IFNT, CDX2, and EOMES Transcripts
Next, we investigated how EOMES expression is regulated in trophoblast cells using the previously established coculture system of bovine trophoblast CT-1 cells and uterine endometrial cells (Sakurai et al., 2012). In monoculture, increased IFNT gene expression was observed after adding uterine flushing from Day-17 (P17) pregnant animals (Fig. 5). CDX2 transcripts were also increased after adding P17 uterine flushing (Fig. 5). Changes in EOMES mRNA, however, were not observed in CT-1 cells (Fig. 5). In coculture, decreased IFNT and CDX2 gene expression in CT-1 cells was detected when P17 uterine flushing was added (Fig. 5). Under these conditions, however, the same treatment resulted in four- to eight-fold increase in EOMES transcripts (Fig. 5). Regardless of culture conditions, no changes in IFNT, CDX2, or EOMES mRNA were seen when the uterine flushing from cyclic animals (C15) was used. Results from the coculture experiments with CT-1 and endometrial epithelial cells demonstrated that both the uterine flushing from pregnant animals and physical contact with endometrial cells was essential for the up-regulation of EOMES in CT-1 cells.
Changes in the Transcriptional Regulation of Bovine IFNT in Cocultured CT-1 Cells
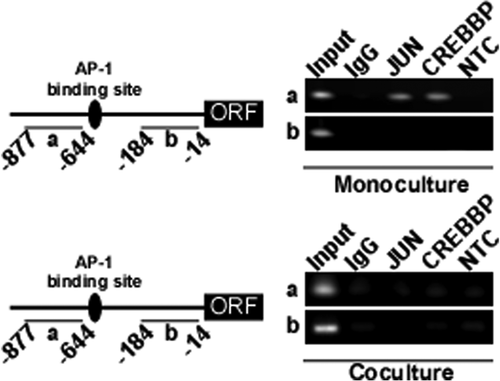
To determine how EOMES reduces the degree of IFNT-reporter transactivation, changes in the binding of CREBBP or JUN to the bovine IFNT loci was examined by chromatin immunoprecipitation from CT-1 cocultures with uterine endometrial cells (Fig. 6). JUN and CREBBP bound to upstream regions of IFNT gene in monocultured CT-1 cells, but not in cocultured cells. The data showed that the binding of JUN or CREBBP to the upstream region of the IFNT gene in CT-1 cells disappeared after the attachment of CT-1 to uterine endometrial epithelial cells.
DISCUSSION
IFNT gene expression in the ruminant trophectoderm is characterized by a drastic increase followed by a rapid decrease during early pregnancy. Although molecular mechanisms by which IFNT gene transcription is up-regulated have been studied extensively, mechanisms associated with the down-regulation of the IFNT gene have not been well-characterized. We demonstrated in this report that EOMES was up-regulated in vivo as well as in vitro following attachment of trophoblast cells to uterine epithelial cells, and EOMES was a transcription factor associated with the down-regulation of IFNT gene transactivation. We propose that the mechanism of IFNT down-regulation proceeds as follows (Fig. 7): Due to a decrease in the expression of CDX2 just after attachment of conceptus to the uterine epithelium, chromatin conformation of IFNT gene loci is changed from euchromatin to heterochromatin. At the same time, the expression of EOMES is increased. EOMES can then associate with the JUN/CREBBP/ETS2 complex, negatively affecting its ability to maintain high IFNT transcription. While not directly binding, EOMES could thus contribute to the down-regulation of IFNT expression.
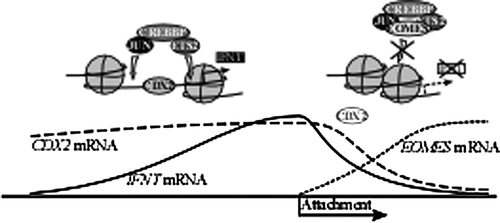
Transcription factors ETS2 and JUN, which are known to regulate IFNT gene expression (Ezashi et al., 1998; Yamaguchi et al., 1999), were consistently expressed in Day-15 to -21 conceptuses (Fig. 1). During the same period, a decrease in IFNT and CDX2 mRNA levels and an increase in CREBBP and EOMES mRNA levels were found in the ovine conceptuses, corresponding with the period during which the attachment process to the uterine epithelium occurs (between Days 15 and 17). Because the decrease in IFNT production coincides with conceptus attachment to the uterine epithelium (Guillomot et al., 1990; Imakawa et al., 2006; Sakurai et al., 2010), previously established cocultures of endometrial epithelial cells and trophoblast CT-1 cells (Sakurai et al., 2012) were used to model the in vitro attachment between trophoblast and endometrial cells. An increase in EOMES transcripts in CT-1 cells was detected only in the coculture upon the addition of P17 uterine flushing (Fig. 5). In the coculture, low expression levels of IFNT mRNA were found only with high EOMES expression. Conversely, high IFNT and CDX2 expression occurred only when EOMES expression was low. Similar results were found in in vivo-derived conceptuses (Figs. 1 and 2). EOMES expression increases only in the presence of pregnant uterine flushing, suggesting that it is regulated by factors from the conceptus, uterus, and/or their interactions. Further experimentation is required to identify molecules present in the Day 17 uterine flushing and to characterize cascades required for conceptus attachment to the uterine epithelium.
It was demonstrated that the transcription factors CDX2, ETS2, JUN, CREBBP, and possibly DLX3 are involved in IFNT gene transcription (Ezashi et al., 1998, 2008; Imakawa et al., 2006; Sakurai et al., 2009, 2010), and that JUN, ETS2, and CREBBP likely form a transcription factor complex when the IFNT gene is actively transcribed (Xu et al., 2003). Among these factors, CDX2 was the most effective in increasing ovine IFNT-reporter transactivation (Imakawa et al., 2006). Yet, the inhibitory effect of EOMES on ovine IFNT-reporter transactivation was observed even when Cdx2 and Crebbp/ETS2/Jun were cotransfected with Eomes in JEG3 cells (Figs. 1-3), indicating that the expected increase in luciferase activity brought forth by Crebbp and either Jun or ETS2 was down-regulated by Eomes transfection. In over-expression and subsequent CREBBP immunoprecipitation studies in JEG3 cells, EOMES, ETS2, and JUN were detected (Fig. 4), further indicating that EOMES bound with the JUN/CREBBP/ETS2 complex. On the other hand, in coculture conditions in which high EOMES was found (Fig. 5) along with moderate levels of JUN and CREBBP, the putative activators were not found on their respective binding sites within the INFT promoter (Fig. 6). It would have been ideal if EOMES over-expression in bovine trophoblast CT-1 cells were performed; in recent trials, however, CT-1 cells could not be transfected with expression plasmids, regardless of which transfection methodologies were employed (e.g., lipofectamine, electroporation, and/or Moloney murine leukemia virus (MMLV)-based introduction). This has limited our ability to demonstrate the effect of EOMES on endogenous IFNT gene transcription. Nevertheless, these results suggest that although EOMES did not bind directly to the upstream region of the ovine IFNT gene, it may reduce the effect and/or DNA binding of the JUN/CREBBP/ETS2 complex, resulting in the down-regulation of ovine IFNT-reporter transactivation.
Exhaustive experiments have been conducted to elucidate the role of Cdx2 in murine embryonic development; however, a loss or down-regulation of Cdx2 following lineage specification has not been well characterized. It has been determined that Cdx2 expression in mouse embryos is initiated through changes in cell polarity, resulting from trophoblast cells differentiating external to the embryo proper (Jedrusik et al., 2008; Ralston and Rossant, 2008). CDX2 expression in bovine embryos is initiated in the same manner as in the mouse blastocyst (Yang et al., 2011). It was found that CDX2 expression in bovine trophoblast cells is reduced at the time or immediately following conceptus attachment to the uterine epithelium (Imakawa et al., 2006; Sakurai et al., 2010). This could also be due to changes in cell polarity (Yamakoshi et al., 2012). In addition, the expression of the transcription factor GATA3, which is reported to be involved in Cdx2 expression in the trophoblast lineage (Ralston et al., 2010), is reduced after conceptus attachment to the uterine epithelium (Bai et al., 2009). Following lineage specification and initiation of implantation in murine embryos, the expression of CDX2 and EOMES is localized only in the extraembryonic ectoderm, but not in spongiotrophoblast or trophoblast giant cells (Simmons and Cross, 2005). These observations and results from our studies suggest that down-regulation of CDX2 soon after conceptus attachment to the uterine epithelium may be unique to ruminants.
In addition to transcription factor regulation of IFNT gene expression, recent evidence supports a role for the epigenetic regulation of IFNT expression (Sakurai et al., 2009, 2010). In MDBK cells that normally do not produce IFNT, Trichostatin A treatment by itself was unable to induce IFNT transcription, but resulted in substantial histone acetylation and subsequent up-regulation of IFNT transcription in conjunction with CDX2 over-expression (Sakurai et al., 2009). It was later demonstrated that CDX2 was involved in changes in H3K18 acetylation on the trophectodermal IFNT gene via recruitment of CREBBP (Sakurai et al., 2010). CREBBP possesses strong histone acetyltransferase activity, which controls remodeling of local chromatin structures and increases DNA accessibility to other regulators (Bannister and Kouzarides, 1996; Kawasaki et al., 1998; Agalioti et al., 2000). It is possible that a complex of EOMES/JUN/CREBBP/ETS2 rather than JUN/CREBBP/ETS2 might be associated with a loss of histone acetyltransferase activity, resulting in a switch from the euchromatin-influenced state, which is involved in active IFNT gene expression, to the inactive heterochromatin state, even when CDX2 is present on the IFNT upstream region.
In conclusion, the transcription factor EOMES was observed to be inhibitory to IFNT gene transcription in this study. This inhibition resulted from EOMES binding to CREBBP, which possibly reduces the effect and/or DNA binding of the JUN/CREBBP/ETS2 complex to the IFNT gene. This could be one of the molecular mechanisms by which IFNT is rapidly down-regulated following its massive expression in utero.
MATERIALS AND METHODS
Animal Experimentation and Uterine Flushing Preparation
Whiteface crossbred ewes were maintained at the farm of the University of Tennessee, Knoxville, TN, USA, and the protocol for sheep experimentation had previously been reviewed and approved by the animal care committee at the University of Tennessee. Animal care, estrous synchronization procedures, and tissue collections were done as previously described (Sakurai et al., 2010). Hysterectomy was performed on designated days. After hysterectomy, uteri from Day-15, -17, or -21 pregnant animals (P15, P17, or P21, respectively, n = 3 each day) were flushed with 20 ml of sterile phosphate-buffered saline (PBS, pH 7.2), and conceptuses were removed from the medium. In addition to flushings from pregnant animals, the uterine flushings from Day 15 cyclic animals (n = 3) were collected. Recovered PBS (18–19 ml) was centrifuged at 4,000g for 5 min to remove cell debris, supernatants were filtered through 0.22 µm membrane, and then samples were stored at −80°C until use. After thawing, the samples (2 ml from 18 ml uterine flushings) were concentrated and desalinated through the use of Microcon filter device (Ultracel YM-3, Millipore, Billerica, MA). Protein concentrations were determined with the Bradford reagent (BioRad Laboratories, Hercules, CA), and the concentrations were adjusted to 1 µg/µl with distilled water.
Experimental procedures for Japanese black cattle were approved by the Committee for Experimental Animals at Zen-noh Embryo Transfer Center and the University of Tokyo. At the Embryo Transfer Center, conceptuses were collected non-surgically from superovulated Holstein heifers by uterine flushing with lactated Ringer's solution on Days 17, 20, or 22 (n = 3 each), as described previously (Ideta et al., 2009). Briefly, all animals were crossbred beef heifers (n = 9), and were fed the same feed; water was supplied ad libitum. These heifers between 13 and 15 months old were synchronized by insertion of a progesterone-releasing intravaginal device (PRID, ASKA Pharmaceutical Co., Ltd., Tokyo, Japan) placed into the vagina on randomly selected days of the estrous cycle for 9 days. Two days before removal of the PRID, prostaglandin F2α (PGF2α, 2 ml, Resipron-C containing 0.25 mg/ml Cloprostenol, ASKA Pharmaceutical) was administered to the heifers by intramuscular (i.m.) injection. The heifers were also given 500 IU equine chorionic gonadotropin by i.m. injection (Serotropin, ASKA Pharmaceutical) at the time of PRID removal. The superovulatory treatments began on Day 9 of the estrous cycle, and consisted of eight decreasing doses of follicle-stimulating hormone by i.m. injection (total of 28 Armour units, Antrin R-10, Kanagawa, Japan) for 4 days. All heifers were observed for standing estrus within 48 hr after the first PGF2α treatment, and were randomly assigned to three groups.
Cell Propagation
Human choriocarcinoma JEG3 cells (HTB36, American Type Culture Collection) were grown in Dullbecco's modified Eagle's medium (DMEM, Wako, Tokyo, Japan) supplemented with 10% (v/v) fetal bovine serum (FBS; JRH Biosciences, Lenexa, KS) and antibiotic/antimycotic solution (Invitrogen, Carlsbad, CA) at 37°C in air with 5% CO2.
Bovine trophoblast CT-1 cells, a generous gift from Dr. Alan Ealy, University of Florida, were derived from 10- to 11-day in vitro-produced blastocysts, and propagated as described previously (Talbot et al., 2000). In this study, CT-1 cells were cultured on plastic plates coated with Matrigel at 37°C in air with 5% CO2 in DMEM containing 10% (v/v) FBS (JRH Biosciences) supplemented with 4.5 g/L D-glucose (Invitrogen, Carlsbad, CA), nonessential amino acids (Invitrogen), 2 mM glutamine (Invitrogen), 2 mM sodium pyruvate (Invitrogen), 55 µM β-mercaptoethanol (Invitrogen), and antibiotic/antimycotic solution (Invitrogen). Cells have since been propagated through more than 50 passages on Matrigel-coated plates, and continue to grow as a monolayer in distinct colonies while retaining characteristic features of trophectoderm cells.
Bovine endometrial epithelial cells were cultured in DMEM/F12 containing 10% (v/v) FBS, 40 units/mL of penicillin, and 40 µg/mL of streptomycin at 37°C in air with 5% CO2, and propagated as described previously (Skarzynski et al., 2000; Sakurai et al., 2012). The cells were used within three passages to avoid changes in their epithelial cell characteristics.
RNA Extraction and RNA Analysis
Total RNA was extracted from Day-15, -17, and -21 ovine conceptuses; Day-17, -20, and -22 bovine conceptuses; and monocultured or cocultured CT-1 cells as described below with ISOGEN (Nippon Gene, Tokyo, Japan), according to the manufacturer's recommendations. For PCR analyses, isolated RNA (total 1 µg) was reverse-transcribed to cDNA using ReverTra Ace qPCR RT Kit (Toyobo, Tokyo, Japan) including 1× RT buffer, Enzyme Mix, and primer Mix in a 10 µl reaction volume, and the resulting cDNA (RT template) was stored at 4°C until use. The cDNA reaction mixture was diluted 1:10 using DNase- and RNase-free, molecular-biology grade water and 2 µl were taken for each amplification reaction. PCR was carried out with 0.5 units of ExTaq HS polymerase (Takara Biomedicals, Tokyo, Japan), 1× ExTaq HS buffer, 0.2 µM of the oligonucleotide primers (Table 1), and 0.2 mM of dNTP in a final volume of 20 µl. The thermal profile for PCR was at 94°C for 10 min, followed by 30 cycles of 94°C for 30 sec, 60°C for 30 sec, and 72°C for 30 sec. The products were analyzed by 1.5% agarose gel electrophoresis, and the bands were then visualized with ethidium bromide staining, followed by photography with ATTO ImageSaver (AE-6905C) under UV light. The expression levels of beta-actin (ACTB) were used to confirm equal loading (Sakurai et al., 2010).
| Name (Genbank accession) | Sequence of forward and reverse primers | Length |
|---|---|---|
| IFNT (AF238613) | F: 5′-CATCTTCCCCATGGCCTTCG | 206 bp |
| R: 5′-TCATCTCAAAGTGAGTTCAG | ||
| EOMES (XM_001251929) | F: 5′-ACTGGTTCCCACTGGATGAG | 225 bp |
| R: 5′-CACAGCAATGAACTGCGTTT | ||
| CDX2 (XM_871005) | F: 5′-GCCACCATGTACGTGAGCTAC | 140 bp |
| R: 5′-ACATGGTATCCGCCGTAGTC | ||
| ETS2 (NM_001080214) | F: 5′-GTGGGCCTATCCAGCTGTGG | 227 bp |
| R: 5′-TTCCCTGACGTCTTGTGGAT | ||
| JUN (NM_010591) | F: 5′-GAGTCTCAGGAGCGGATCAA | 225 bp |
| R: 5′-TGAGTTGGCACCCACTGTTA | ||
| CREBBP (XM_581740) | F: 5′-CAAGGAGCTGCCCTACTTTG | 226 bp |
| R: 5′-TTTTTCTGGGCGTTCTTGCT | ||
| ACTB (BC102948) | F: 5′-CTCTTCCAGCCTTCCTTCCT | 177 bp |
| R: 5′-GGGCAGTGATCTCTTTCTGC |
- F, forward primer; R, reverse primer.
Reverse-transcribed cDNA (2 µl) synthesized from ovine and bovine conceptus and cultured CT-1 cells RNAs (n = 3 from each day) were analyzed by quantitative real-time PCR with a 7900HT Fast Real-Time PCR System (Applied Biosystems, Tokyo, Japan). For each reverse-transcribed template, the PCR reaction was carried out with an ExTaq HS (Takara Biomedicals), SYBR Green I (Cambrex Bio Science, Rockland, ME), ROX Reference Dye (Invitrogen), and oligonucleotide primers for each target (Table 1). Amplification efficiencies of each target and the reference gene beta-actin (ACTB) were examined through their calibration curves and found to be comparable (Bustin et al., 2009). The PCR amplification consisted of 40 cycles at 95°C for 10 sec, annealing at 60°C for 20 sec, and extension at 72°C for 40 sec. The threshold cycle (Ct) value for each target was determined by Sequence Detection System software v1.2 (Applied Biosystems). Expression levels of each mRNA were normalized by calculating the Ct values based on subtracting the Ct value of targets mRNA from the Ct value of the internal control, ACTB mRNA. Each amplification ended with a melting curve analysis to confirm the specificity of amplification and absence of primer dimers (Sakurai et al., 2009).
Reporter Gene Constructs and Expression Plasmids
Preparation of 5′-deletion constructs representing various lengths of ovine and bovine IFNT promoter-enhancer regions (ovine TP-010, GenBank accession number: M88773 and bovine IFNTc1, GenBank accession number: AF238613, respectively) was performed as described previously (Yamaguchi et al., 1999; Xu et al., 2003). The nucleotide structure of a murine Jun expression vector, pRVSV Jun, has been described previously (Yamaguchi et al., 1999). A pRVSV plasmid, which contains only the Rous Sarcoma Virus (RSV) long terminal repeat (LTR) and a poly-(A) signal sequence, was used as the control for the determination of basal transcriptional activity. The human ETS2 expression vector was a pSG5-based construct containing the SV40 promoter and SV40 enhancer (Wakiya et al., 1996). The mouse Cdx2 and Eomes expression plasmids in pRC-cytomegalovirus (CMV), kindly provided by Drs. EunRan Suh and Steve Reiner, respectively (University of Pennsylvania School of Medicine), were subcloned into the pSG5 plasmid (Agilent Technologies Japan, Tokyo, Japan), and nucleotide structures were confirmed by DNA sequencing. The mammalian expression vector encoding the full-length mouse Crebbp cDNA (pRc/RSV-Crebbp), driven by RSV LTR, was a generous gift from Dr. D. Thanos, Columbia University (Merika et al., 1998). A pRc/RSV plasmid (The Institute of Physical & Chemical Research [RIKEN] Gene Bank, Tsukuba, Japan) that contains only RSV LTR and a poly-(A) signal was used as a control to determine the basal transcriptional activity. It should be noted that sequence similarity between transcription factor expression plasmids used in this study and those of the bovine were more than 80%.
Transient Transfection and Luciferase Assay
JEG3 cells were placed on 24-well plastic microplates, and grown to 50–60% confluence before transfection (Imakawa et al., 2006). Plasmid DNA was transfected into JEG3 cells with TransFast (cationic lipids, Promega, Madison, WI) according to the manufacturer's instructions. In brief, 0.8 µg of each DNA construct and 3 µl of TransFast were mixed in 200 µl DMEM. Amounts of total plasmids for each transfection were adjusted with the inclusion of pSG5 only (empty vector, Mock). After 10–15 min, plated cells were overlaid with the plasmid mixture and incubated at 37°C for 48 hr. To normalize transfection efficiency, pRL-TK vector (containing the herpes simplex virus thymidine kinase promoter and the Renilla luciferase gene, Promega) was transfected along with each pGL3 vector into JEG3 cells (Imakawa et al., 2006). The ratio of pGL3 to pRL-TK vector was 160:1 (800:5 ng).
After 48 hr of transfection, the cells were washed with PBS and lysed with 100 µl Passive Lysis Buffer (Promega) at room temperature for 20 min. Luciferase activity was determined by the Dual-Luciferase Reporter Assay System (Promega) on a Microlumat LB 96 P luminometer (Perkin–Elmer, Foster City, CA). Luciferase activity resulting from various ovine IFNT-reporter constructs was normalized through the determination of transcriptional efficiency with Renilla luciferase activity (Imakawa et al., 2006; Sakurai et al., 2009). Luciferase activity was expressed as fold-activation relative to an appropriate control within the experiment.
Co-Immunoprecipitation Analysis
JEG3 cells were plated in 10-cm plastic plates and grown to 70–80% confluence before transfection. Plasmid DNAs, Eomes/Crebbp, ETS2/Crebbp, Jun/Crebbp, or Eomes/Crebbp/ETS2/Jun, were transfected into JEG3 cells using HilyMax reagents (Dojin Chemicals) according to the procedures recommended by the manufacturer, as described previously (Imakawa et al., 2006), and cultured for 48 hr. JEG3 cells were suspended in 1 ml Nonidet P-40 (NP-40) buffer (NP-40:10 mM Tris–HCl, pH 7.4, 1% NP-40, 150 mM NaCl, 0.5 mM EDTA, and 0.5 mM NaF) containing protease inhibitors (2 mM PMSF, 25 µg/ml Aprotinin, 5 µg/ml Leupeptin, and 1 µg/ml Pepstatin A), and lysed on ice for 1 hr. The protein concentrations of these samples were determined with the Bradford reagent (BioRad Laboratories) and the concentration adjusted to 1 mg/ml. One ml (1 mg/ml) of each sample was passed through chromatin immunoprecipitation (ChIP)-Grade Protein G Magnetic Beads (Cell Signaling, Danvers, MA) at 4°C for 1 hr. To these samples, 2 µg of mouse monoclonal antibody against the carboxyl terminus of human CREBBP (sc-7300, Santa Cruz Biotechnology, Santa Cruz, CA) were added at 4°C for 2 hr. Normal mouse IgG (Sigma–Aldrich) was used as a negative control. After incubation with each antibody, these samples were incubated with 30 µl of ChIP-Grade Protein G Magnetic Beads for 1 hr. Protein–antibody-magnetic bead complexes were washed three times with NP-40 buffer, and then protein was diluted with Sample buffer. Diluted samples were subjected to Immunoblot analysis.
Immunoblot Analysis
Immunoprecipitates with anti-CREBBP antibody was separated on 10% reducing SDS–PAGE gels, and transferred onto PVDF membrane (Millipore, Bedford, MA). After blocking with 2.5% (w/v) skim milk and 2.5% (w/v) bovine serum albumin (BSA, Sigma) at 4°C overnight, the membranes were incubated with rabbit anti-mouse EOMES antibody (1:1,000, ab23345, Abcam, Tokyo, Japan), rabbit anti-human ETS2 (1:1,000, sc-351, Santa Cruz Biotechnology), or rabbit anti-mouse JUN (1:1,000, #9162, Cell Signaling) antibody at room temperature for 1 hr.
To determine EOMES and IFNT protein expression in mono- or cocultured CT-1 cells, cell lysates were prepared in RIPA Lysis buffer (50 mM Tris–HCl, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% SDS, 1 mM Na3VO4, 50 mM NaF). After incubation, the CT-1 cells were lysed with RIPA lysis buffer, and protein concentrations were determined with the Bio-Rad protein assay dye reagent concentrate (Bio-Rad laboratories, Hercules, CA). Cell lysates (10 µg) were loaded into each lane and separated through 10% SDS–polyacrylamide gel electrophoresis (SDS–PAGE), and were then transferred onto PVDF membrane (Millipore). After blocking them with 2.5% (w/v) skim milk and 2.5% (w/v) BSA overnight, PVDF membranes were treated with rabbit monoclonal anti-IFNT antibody (Operon, Tokyo, Japan), rabbit polyclonal anti-TBR2/EOMES antibody (Abcam, Tokyo, Japan), or rabbit anti-human nucleoporin P62 (internal control) (NUP62, BD Biosciences, Pharmingen, NJ) diluted 1:1,000 with 2.5% (w/v) skim milk and 2.5% (w/v) BSA. After washing, horseradish peroxidase-conjugated anti-rabbit IgG (1:10,000 for each antibody) was applied as the secondary antibody and the membranes were further incubated at room temperature for 1 hr. For visualization, ECL Plus Western Blotting Detection Reagents (GE Healthcare, Buckinghamshire, UK) were used, and signals were detected on X-ray film (Kodak, New York, NY).
Coculture CT-1 Cells and Endometrium Cells
Coculture assay was performed as described previously (Sakurai et al., 2012). In short, CT-1 monocultures on collagen type I-coated 6-well plates were incubated with 10 µg proteins from uterine flushing (C15 or P17) for 48 hr. In cocultures, endometrial cells were initially incubated on 6-well plates coated with collagen type I to reach confluence. CT-1 cells and 10 µg protein from uterine flushing (C15 or P17) were then plated over the endometrial cells for a 48 hr incubation period. After incubation, cells were washed twice with PBS and incubated in PBS for 5 min, and then CT-1 cells were separated from substratum of collagen type I-coated dish (monocultured) or adhering bovine endometrial epithelial cells (cocultured) with gentle pipetting of PBS. To collect cocultured CT-1 cells without contamination of uterine cells, the cells in PBS were passed through a 70-µm cell strainer (BD Biosciences), and the cells that remained on the membrane were purified further using a Percoll gradient. Percoll was diluted with DMEM to obtain dilutions of 12.5%, 25%, and 50%. The gradient column was prepared in a 15-ml Falcon tube by gently depositing a 1 ml layer of each solution, starting from a 50% dilution at the bottom. One ml of the CT-1 cell suspension in PBS was deposited at the top layer, and the gradient was centrifuged at 800g for 25 min. CT-1 cells were located in the Percoll layers of 12.5% and 25%, from which CT-1 cells were collected. Trypan blue exclusion tests confirmed cell viability over 95%. Mono- or cocultured CT-1 cells were then subjected to RNA isolation or transcription factor binding analysis through the use of chromatin immunoprecipitation (ChIP).
Chromatin Immunoprecipitation Assay
ChIP was performed as described previously (Sakurai et al., 2012). Briefly, CT-1 cells were cultured with or without endometrial epithelial cells (coculture or monoculture, respectively), and then cultured for 48 hr. Potential protein–DNA complexes in cultured CT-1 cells were cross-linked through the addition of 1% formaldehyde (Wako, Osaka, Japan) for 15 min. DNA from cells was sonicated to less than 500 base pairs. The supernatant of sonicated cells was diluted 10-fold, and 1% of the diluted lysates was used for total genomic DNA as input DNA control. Immunoprecipitation was performed overnight at 4°C with rabbit monoclonal anti-JUN antibody (10 µg; ab32447, Abcam) or rabbit polyclonal anti-human CREBBP antibody (ab2832, Abcam). Normal rabbit IgG (Santa Cruz Biotechnology) was used in place of specific antibody as a negative control. For isolation of DNA, 30 µl protein-G magnetic beads (Cell Signaling Technology) were added, and incubated for 30 min. Beads were then washed with High-salt or Low-salt buffer. Histone complexes were eluted from the antibody by freshly prepared elution buffer (1% SDS, 0.1 M NaHCO3, 10 mM DTT). Protein–DNA cross-links, including the input DNA control samples, were incubated with 5 M NaCl and proteinase K (Invitrogen) at 65°C for 4 h. DNA fragments were extracted with a column (QIAquick PCR purification kit; Qiagen) following the protocol provided by the manufacturer, and resuspended in 60 µl elution buffer, from which 2 µl were used for PCR (35 cycles) with primers for the upstream region of the bovine IFNT (IFN tau-c1, Genbank accession number; AF238613) gene. The sequences of primers for the region from −877 to −644 bp are 5′-GTGAAGAGTTGACTCATTGG (sense) and 5′-TACACCTGTGGGCTTAGTTG (anti-sense), and for the region from −184 to −14 bp are 5′-TTGACAAACCCAAATTTTATTGG (sense) and 5′-GCAAGGCTTTTAAATAGGGAAGA (anti-sense). The PCR products were separated on a 1.5% agarose gel containing ethidium bromide, and were visualized under UV light.
Statistical Analysis
The real-time PCR data for gene expressions or luciferase assay data were analyzed by one-way ANOVA followed by Tukey's test for multiple comparisons against the control for each experimental group with the StatView statistical analysis software (version 5; SAS Institute Inc.). Differences of P < 0.05 were considered to be significant. In these analyses, treatment and day were considered independent variables, with replicate as the dependent variable.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Lutz W. Weber, Oak Ridge Institute of Science and Education, Oak Ridge, TN, USA, for his critical reading of the manuscript and Ms. Mary Roberts, Department of Animal Science, University of Tennessee, for her excellent technical assistance. The authors thank Drs. E. Suh and S. Reiner (University of Pennsylvania School of Medicine) for kindly providing mouse Cdx2 and Eomes expression plasmids. The authors also thank Dr. D. Thanos (Columbia University) for generously providing the Crebbp expression plasmid. This work was supported by a Grant-in-Aid for Scientific Research (23780279) to T.S. from the Japan Society for the Promotion of Science (JSPS). H.B. is a Research Fellow of JSPS. The original portions of this study were supported by the program for Promotion of Basic Research Activities for Innovative Bioscience (BRAIN).




