Glycerol-Monolaurate-Mediated Attenuation of Metabolic Syndrome is Associated with the Modulation of Gut Microbiota in High-Fat-Diet-Fed Mice
Abstract
Scope
The gut microbiota plays an important role in the development of diet-induced obesity and metabolic syndrome. Glycerol monolaurate (GML), a widely consumed food emulsifier, is reported to promote metabolic disorder and gut microbiota dysbiosis in low-dose supplementation upon low-fat-diet feeding. However, little is known about whether GML produce the same effects in mice fed a high-fat diet (HFD).
Methods and results
C57BL/6 mice are fed a HFD with or without GML supplementation (150, 300, and 450 mg kg−1) for 10 weeks. The results demonstrated that higher GML treatment (450 mg kg−1) ameliorates HFD-induced metabolic disorders, supported by prevented visceral fat deposition, improved hyperlipidemia, modulated hepatic lipid metabolism, and reduced serum proinflammatory cytokine, TNF-α. Additionally, all doses of GML attenuated circulating lipopolysaccharide load and insulin resistance. Notably, GML ameliorates HFD-induced gut microbiota dysbiosis, with increases in Bacteroides uniformis, Akkermansia, Bifidobacterium, and Lactobacillus and decreases in Escherichia coli, Lactococcus, and Flexispira. Spearman's correlation analysis indicates that these enriched specific genera are significantly associated with the metabolic improvements of GML.
Conclusion
The findings identify the links between gut microbiota and GML-induced metabolic improvements, suggesting that the attenuation of HFD-induced metabolic disorders by higher GML supplementation may occur through targeting gut microbiota.
1 Introduction
The global prevalence of obesity and related metabolic comorbidities has increased considerably over the past decades.1 Metabolic syndrome, a cluster of metabolic disorders, contributes to the increasing prevalence of obesity and plays an important role in the development of type 2 diabetes, cardiovascular diseases, and other related diseases.2 The definition of metabolic syndrome includes glucose intolerance, insulin resistance, central obesity, dyslipidemia, and hypertension, as well as chronic inflammation and hepatic steatosis.3 Recent studies have demonstrated that the gut microbiota is closely associated with high-fat-diet (HFD)-induced obesity, insulin resistance, and other related symptoms of metabolic syndrome. Comparative analyses between lean and obese individuals have demonstrated significant differences in the community composition, functional genes, and metabolic activities of gut microbiota, implying that gut dysbiosis participates in the development of obesity and related metabolic disorders.4 The metabolites from intestinal bacteria play important roles in the influence of host physiology and pathology.5, 6 The release of lipopolysaccharide (LPS), which is the major component of the outer membrane of Gram-negative bacteria, is related to systematic inflammation, insulin resistance, and obesity.7 Accordingly, gut microbiota has become a new biomarker of diet-induced changes in host metabolic status. Modulating the composition of gut microbiota and their metabolic functions become key contributors to the inhibition of the development of obesity-related metabolic disorders.8
Glycerol monolaurate (GML), the monoglyceride of lauric acid, is widely used as an emulsifier and preservative in the food industry, including in meat products and bakery products, based on its emulsification properties and antibacterial and antiviral activities.9-12 GML naturally exists in coconut, palmetto, and breast milk and is recognized as a generally safe food additive by the USA Food and Drug Administration. Additionally, GML also plays an immunomodulatory role by suppressing inflammatory processes in T cells by altering lipid dynamics.13, 14
GML is a typical glycerol derivative of medium-chain fatty acids (C8-C12, MCFAs). Due to the short carbon chain length, MCFAs are directly absorbed via the portal system in the intestinal tract and transported to the liver, and most absorbed MCFAs are free fatty acids.15 Additionally, MCFAs can enter mitochondria independently without fatty acid binding-proteins for rapid oxidization.16 MCFAs and their glycerol derivatives, medium-chain triacylglycerols (MCTs), can be quickly metabolized. Based on their special metabolic characteristics, MCFAs and MCTs have been reported to increase energy expenditure, enhance lipid oxidation and downregulate lipogenic genes, leading to decreased body weight and fat accumulation as well as improved serum lipid profiles and insulin resistance in animal models and human studies.17-20 MCFAs and MCTs are known for their antimicrobial properties, which are still at play in the intestinal tract.21 Kono et al.22 demonstrated that MCTs could prevent intestinal permeability and endotoxemia through remodeling of gut microbiota. Nevertheless, it remains unclear whether the monoglycerides of MCFAs such as GML have similar metabolic effects.
Our previous study indicated that under a low fat diet (LFD, 10% fat, Cal%), low-dose GML consumption (150 mg kg−1) might promote metabolic syndrome, gut microbiota dysbiosis, and low-grade systematic inflammation.23 However, whether the same effects still exist upon HFD (45% fat, Cal%) feeding is still unknown. The present study elucidated and compared the effects of three different doses of GML supplementation on HFD-induced visceral fat deposition, hyperlipidemia, insulin resistance, and systemic inflammation. The modulation of gut microbiota composition mediated by GML supplementation was further analyzed and the circulating LPS load was also determined for investigating the impacts of GML on the gut microbiota in HFD-fed mice. Importantly, this research describes the links between gut microbiota and GML-mediated metabolic improvements in HFD-fed mice, which might help to uncover the mechanisms involved in the modulation of metabolic syndrome by GML consumption and provide a comprehensive evaluation of GML usage.
2 Experimental Section
2.1 Animals and Diets
All the experimental procedures were approved by the Animal Ethics Committee of Zhejiang University (Permission Number: ZJU-BEFS-2015013). Wild-type male C57BL/6 mice (6 weeks old, n = 75) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China) and maintained, three animals per cage in a controlled environment (12 h daylight cycle, lights off at 18:00, 22–24 °C) with food and water ad libitum. After 1 week of acclimation (week 0) on a normal chow diet, mice were randomly divided into five groups (n = 15) and fed specific diets for a period of 10 weeks. The groups included 1) Chow, mice fed a normal chow diet (provided kcal% with 10% fat, 20% proteins, and 70% carbohydrates; SLAC Laboratory Animal Co., Ltd, Shanghai, China); 2) HFD, mice fed a HFD (provided kcal% with 45% fat, 20% proteins, and 35% carbohydrates; Medicience Co., Ltd, Jiangsu, China); 3) HFD + 150 mg kg−1, mice fed an HFD supplemented with 150 mg kg−1 GML; 4) HFD + 300 mg kg−1, mice fed an HFD supplemented with 300 mg kg−1 GML; and 5) HFD + 450 mg kg−1, mice fed an HFD supplemented with 450 mg kg−1 GML. The components and energy densities of the diets are shown in Table S1, Supporting Information. Mice body weights and diet intakes were recorded weekly. Fresh fecal samples were collected once a week and immediately stored at −80 °C for subsequent analysis. After 10 weeks, mice were fasted for 12 h, blood samples were collected from the orbital vascular plexus, and then mice were euthanized. The livers and intestines as well as the epididymal, mesenteric, retroperitoneal, and perinephric fat pads were carefully collected, weighed, and then stored at −80 °C.
2.2 Glucose Homeostasis
At week 9, mice were fasted for 12 h (overnight) and an oral glucose tolerance test (OGTT) was performed after gavage with glucose (2 g kg−1 body weight). Blood samples were taken from the tail vein at different time points (before 0 min; and 30, 60, 120 min after), and blood glucose was measured by an Accu-Check glucometer (Roche, Mannheim, Germany).
2.3 Biochemical Analysis
Serum triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDLC), and low-density lipoprotein cholesterol (LDLC) were measured using commercially available kits from Nanjing Jiancheng Technology Co. (Nanjing, China), based on the manufacturer's instructions. Serum LPS (Clound-Clone Corp, Houston, USA) and tumor necrosis factor-alpha (TNF-α, R&D systems, Minneapolis, USA) were measured using commercial ELISA kit. Freshly harvested liver tissues were homogenized in PBS, and the total lipids were estimated by the previously described method.17 The same commercial enzymatic kits as serum analysis were used to determine the TG concentration in the liver. Hepatic malondialdehyde (MDA) and total antioxidant capacity, glutathione peroxidase (GPx), total superoxide dismutase (SOD), and catalase (CAT) assay kits were purchased from Nanjing Jiancheng Technology Co. (Nanjing, China).
2.4 Histological Analysis
Mice epididymal white adipose and jejunum were fixed in 10% neutral buffered formalin, then paraffin-embedded, sliced at 5 µm, and stained with hematoxylin and eosin (H&E) according to standard method. For oil red O staining, frozen sections from optimal cutting temperature embedded liver tissues were used and sectioned at 6 µm. The size of the white adipocytes, the morphological adaptation of the jejunum and the hepatic lipid accumulation were quantified by Image-Pro Plus 6.1 (Media Cybernetics, Inc., Rockville, USA).
2.5 RNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
Total cellular RNA from the liver was extracted using TRIzol reagent (Invitrogen, USA) according to the manufacturer's instructions. cDNA was synthesized using a HiScript Reverse Transcription kit (Vazyme, Jiangsu, China), and SYBR Green qRT-PCR was performed on a Roche LightCycler 480 system (Roche Applied Science, Indianapolis, USA) based on the manufacturer's instructions. The values were normalized to β2-microglobulin (B2M) and calculated based on the 2−△△Ct method. The detailed primer sequences used in qRT-PCR are listed in Table S2, Supporting Information.
2.6 16S rRNA Sequencing and Analysis
Bacterial DNA was extracted from fecal samples which we collected at 10 week using a QIAamp DNA Stool Mini Kit (Qiagen, Germany) according to the manufacturer's instructions. The Illumina HiSeq platform was used for 16S rRNA gene high-throughput sequencing. The V4 region of the 16s rRNA gene was amplified by PCR with primers 515F and 806R for the microbial community structure analysis. The amplicons were purified using a Qiagen Gel Extraction kit (Qiagen, Germany), quantified with a Qubit 2.0 Fluorometer (Thermo Scientific) and Agilent Bioanalyzer 2100 system, and sequenced using an Illumina HiSeq 2500 platform to generate 250 bp paired-end reads. Subsequently, the paired-end reads were merged using FLASH V1.2.7, and quality-filtered according to QIIME V1.7.0 under specific filtering conditions to obtain high-quality clean tags.19 Chimera sequences were detected and removed according to the Gold reference database. Subsequently, sequences with 97% similarity were clustered into operational taxonomic units (OTUs) using UPARSE (V7.0.1001). For taxonomic classification, the Greengenes Database was used based on the RDP classifier (V2.2). α-diversity (Chao1, Observed species, Shannon index and Simpson index) and Venn diagrams were performed with QIIME (V1.7.0), and the results were displayed with R software (V2.15.3). To compare β-diversity, the weighted UniFrac distance and unweighted UniFrac distance were calculated and performed in a principal coordinate analysis (PCoA). The nonmetric multidimensional scaling (NMDS) was performed using OTUs for each sample based on Bray-Curtis. A heatmap which was based on the top 20% dominant genera and a principal component analysis (PCA) based on the top 10 large abundance genera were performed using R software (V2.15.3). The linear discriminant analysis (LDA) effect size (LEfSe) analysis was used for identifying the significant differences among groups (LDA > 4.0). Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) was applied to the prediction of the metagenome functional content from 16S rRNA gene sequencing data, and the resulting significant pathways were categorized from levels 1 to 3 into KEGG pathways and visualized by LEfSe analysis with an LDA score >2.0.24
2.7 Statistical Analysis
The results are expressed as mean ± SEM. Statistical analysis was performed using GraphPad Prism 6.0 (GraphPad Software Inc., San Diego, CA, USA) and SPSS 22.0 (SPSS Inc., Chicago, IL, USA). The Benjamini–Hochberg multiple testing procedure was conducted by R software (V2.15.3) for the false discovery rate (FDR); FDR-corrected p-values < 0.05 were considered significant. Differences among groups were determined by one-way analysis of variance (ANOVA) or two-way ANOVA with Tukey's post hoc multiple comparisons test or by the non-parametric factorial Kruskal–Wallis test followed by an unpaired Wilcoxon comparison test. The Spearman's correlations between bacterial abundance and metabolic syndrome-related indexes were determined and performed by a heatmap using R software (V2.15.3). A correlation network was also constructed based on the results of Spearman's correlations analysis for comprehensive presentation. All results were considered statistically significant at p ˂ 0.05, and are represented by superscript symbols (*vs HFD, #vs Chow, $vs H150).
3 Results
3.1 Effects of Different GML Supplementation on Features of Metabolic Syndrome in HFD-Fed Mice
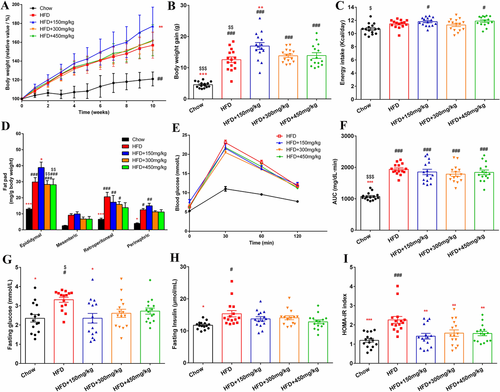
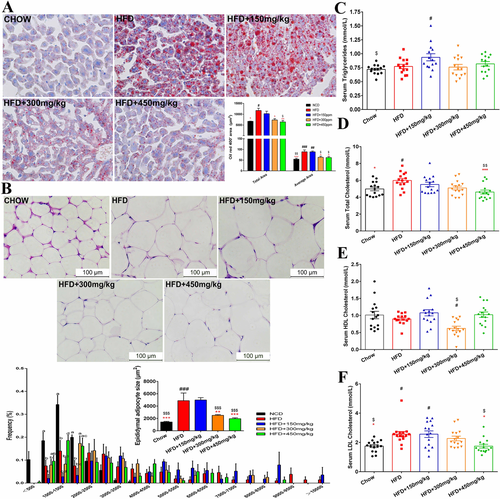
Compared with the HFD treatment, 150 mg kg−1 GML supplementation led to a significant increase in body weight, and this effect was seen as early as 2 weeks after-GML treatment (p = 0.039, Figure 1A,B). No significant difference in daily energy intake was observed among the HFD-fed groups (Figure 1C). Additionally, 150 mg kg−1 GML supplementation also produced significant increases in the relative weight of epididymal white adipose tissues (p = 0.014, Figure 1D). GML supplementation at 300 and 450 mg kg−1 did not affect the body weight of the HFD-fed mice; however, the relative epididymal fat pad weight to body weight ratio showed an obvious decrease in mice from these groups compared to the 150 mg kg−1 GML-treated mice (p = 0.002 and p = 0.004, respectively). Notably, the relative weight of the retroperitoneal and perinephric adipose tissues in 450 mg kg−1 GML-treated group had no significant difference with the Chow diet control group. Similarly, the H&E staining results revealed that 150 mg kg−1 GML supplementation did not attenuate HFD-induced adipose hypertrophy. However, the size of adipocytes was significantly decreased when HFD-fed mice were treated with 300 or 450 mg kg−1 GML (p = 0.005 and p < 0.001, respectively, Figure 2B,C). Higher GML supplementation (300 and 450 mg kg−1) also efficiently protected against HFD-induced hepatic steatosis, as demonstrated by oil red O staining (p = 0.012 and p = 0.001, respectively, Figure 2A). Importantly, the 450 mg kg−1 GML treatment showed the potential to improve HFD-induced serum hyperlipidemia with lower concentrations of TC and LDLC (p < 0.001 and p = 0.022, respectively), while 150 mg kg−1 GML supplementation slightly increased the serum levels of TG compared to the HFD control group (p ˃ 0.05) (Figure 2D,E,G). GML treatment did not improve HFD-induced glucose intolerance with no significant change in the AUC of the OGTT (Figure 1E,F). However, all the GML-treated groups significantly improved the high HOMA-IR induced by the HFD (p = 0.003, p = 0.010, and p = 0.007, respectively), especially in 150 mg kg−1 GML-treated group where the treatment significantly reduced the level of serum fasting glucose (p = 0.048) (Figure 1G–I). Taken together, these results demonstrated the modulation of visceral fat deposition, hyperlipidemia and insulin resistance were depended on the supplemented dose of GML in HFD-fed mice.
3.2 Effects of Different GML Supplementation on Hepatic Lipid Metabolism
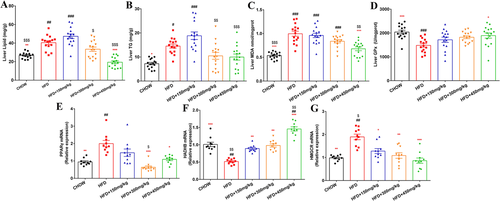
The results demonstrated by oil red O staining indicated that the 300 and 450 mg kg−1 GML supplementation modulated HFD-induced hepatic fat deposition (Figure 2A). Concomitantly, 450 mg kg−1 GML supplementation induced significant reductions in the hepatic lipid level compared to HFD control group (p < 0.001, Figure 3A). Both 300 and 450 mg kg−1 GML treatment significantly reduced hepatic TG level compared to the 150 mg kg−1 GML supplemented group (p = 0.002 and p = 0.001, respectively, Figure 3B). Oxidative stress is also associated with obesity-related diseases. 450 mg kg−1 GML treatment remarkably modulated lipid peroxidation induced by HFD, as shown by a lower MDA content and increased GPx activity (p < 0.001 and p = 0.014, respectively, Figure 3C,D). Furthermore, we observed downregulation of the adipogenesis gene peroxisome proliferator-activated receptor γ (PPARγ) and upregulation of the fatty acid oxidation-related gene HADHB in the livers of GML-treated HFD fed mice, while both the 300 and 450 mg kg−1 GML groups showed significant changes (p < 0.001 and p = 0.012, respectively, in Figure 3E, and p = 0.003 and p < 0.001, respectively, in Figure 3F). Additionally, the reduction in serum TC after 450 mg kg−1 GML supplementation was supported by the significantly downregulated expression level of 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR), which encoded the rate-limiting enzyme of cholesterol synthesis pathway (p < 0.001, Figure 3G).
3.3 GML Modulates Metabolic Endotoxemia, Systematic Inflammation, and HFD-Induced Intestinal Morphology
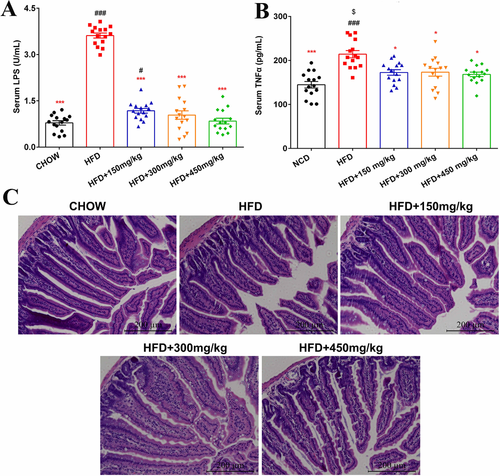
Ten weeks of HFD feeding produced a significant increase in serum LPS levels, which were significantly reduced by all doses of GML supplementation (p < 0.001, p < 0.001, and p < 0.001, respectively, Figure 4A). Among the HFD-fed groups, 450 mg kg−1 GML-treated group showed the lowest LPS load, which approached the level in the Chow diet-fed mice. Notably, all doses of GML supplementation significantly reduced the high level of serum pro-inflammatory cytokine, TNF-α, which was induced by HFD (p = 0.032, p = 0.039, and p = 0.025, respectively, Figure 4B). The jejunum H&E stained sections from different groups are shown in Figure 4C. Compared with the Chow-fed mice, the HFD-fed mice exhibited decreased villus height and increased crypt depth, which were related to a decrease in motility and slower intestinal transit. Remarkably, the 300 mg kg−1 and 450 mg kg−1 GML treatments significantly ameliorated the negative effects caused by HFD (Table S3, Supporting Information). These changes suggested that higher GML supplementation contributed to enhanced digestive and absorptive capacity and intestinal health.
3.4 GML Ameliorates HFD-Induced Gut Microbiota Dysbiosis
To profile the effects of diet and GML on gut microbiota composition, we performed 16S rRNA sequencing by Ilumina HiSeq platform. A total of 1626 bacterial OTUs were obtained, and 71, 17, and 250 OTUs were found only in the 150, 300, and 450 mg kg−1 GML-treated HFD-fed mice, respectively, whereas 36 OTUs were specifically identified in the HFD group (Figure 5A). The effects of GML supplementation on the diversity of gut microbiota were presented in Figures S2 and S3, Supporting Information, GML-treated groups showed no significant difference in the α-diversity of gut microbiota compared to the HFD control. The results of PERMANOVA test indicated a statistically significant separation among groups (p = 0.001), the β-diversity of gut microbiota from GML-treated groups clearly separated from the Chow and HFD groups by PCoA based on the unweighted UniFrac. And the NMDS plot based on Bray–Curtis confirmed these effects.
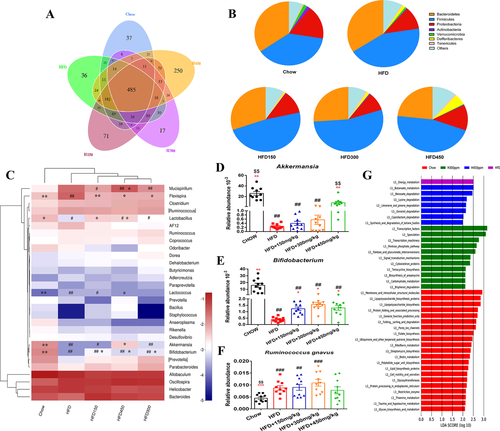
Taxon-based analysis revealed structural changes after GML treatment. At the phylum level, the abundance of Firmicutes was significantly increased by HFD feeding compared with Chow diet (p = 0.049, Figure 5B). GML supplementation did not reverse the HFD-induced increase in Firmicutes and showed a trend of decreased abundance of Bacteroidetes. Interestingly, although the abundance of LPS-suppressing phylum Verrucomicrobia was decreased after 150 or 300 mg kg−1 GML treatment, 450 mg kg−1 GML treatment resulted in a slight increase in this phylum. The PCA analysis based on the top 10 large abundance genera was performed in Figure S4, Supporting Information; 300 and 450 mg kg−1 GML-treated groups displayed separations with the HFD group. HFD significantly reduced the abundance of Bifidobacterium and Lactobacillus (p = 0.002 and p = 0.016, respectively), while GML supplementation increased the abundance of these genera (Figure 5C,E). Notably, the abundance of Akkermansia significantly increased in the 450 mg kg−1 GML supplemented group compared to the HFD control group (p = 0.024, Figure 5D). Moreover, the significantly increased abundance of Lactococcus and Flexispira induced by HFD were reversed by GML treatment. Notably, the abundance of Flexispira was significantly decreased in all the GML-treated groups compared to the HFD-fed group (p = 0.009, p = 0.011, and p = 0.014, respectively). Based on the analysis at the species level, the abundance of Ruminococcus gnavus, which was significantly increased by HFD (p < 0.001), showed no significant difference between 450 mg kg−1 GML-treated HFD-fed mice and Chow control group (Figure 5F). 450 mg kg−1 GML treatment reversed the HFD-induced significant decrease in the abundance of Bacteroides uniformis (p = 0.001, Figure S1A, Supporting Information). Importantly, in comparison with HFD control group, 300 and 450 mg kg−1 GML treatments significantly reduced the abundance of Escherichia coli (p = 0.021 and p = 0.014, respectively, Figure S1B, Supporting Information). The LEfSe analysis was also performed to reveal the differentially enriched taxa at the OUT level in each group (Figure S5, Supporting Information). Collectively, these results indicated that GML supplementation modulated the composition of gut microbiota in HFD-fed mice, and a higher GML concentration (450 mg kg−1) was more effective.
PICRUSt analysis was performed to understand the potential effects of GML supplementation on predicted functional pathways in gut microbiota. The changes in KEGG metabolic pathways (level 3) among the five experimental diets are shown in Figure 5G. The energy metabolism pathway was the top discriminative microbial pathway in HFD control group. 300 mg kg−1 GML-treated group revealed an enrichment of genes belonging to pathways related to carbohydrate metabolism, including the pentose phosphate pathway in addition to pentose and glucoronate interconversions. Lipid and amino acid metabolism-related pathways, such as lysine degradation and synthesis and degradation of ketone bodies, were enriched in 450 mg kg−1 GML-treated HFD-fed mice. These data suggested that GML supplementation mediated functional alterations of gut microbiota, and these changes might be associated with GML-induced significant modification of gut microbial composition.
3.5 Correlation of Gut Microbiota Composition with Metabolic Syndrome Related Parameters
Spearman's correlation analysis was performed to investigate the associations between the changes in gut microbiota at the genus level induced by GML supplementation and specific metabolic syndrome-related parameters in all the mice groups. Fifteen genera were correlated with decreased body weight, weight gain, HOMA-IR index, and serum LPS concentration in addition to improved hyperlipidemia, suggesting that these genera were negatively correlated with metabolic syndrome phenotypes (Figure 6A). Conversely, 12 genera were positively associated with body weight and weight gain, serum LPS level and hyperlipidemia, implying a positive correlation with metabolic syndrome symptoms. Notably, these increased genera in the GML-treated groups included, Bifidobacterium and Lactobacillus, which were significantly, negatively correlated with increases in body weight and energy intake. The abundance of Bifidobacterium also showed a significant negative correlation with serum LPS concentration. The abundance of Akkermansia was significantly, negatively associated not only with the increased body weight but also with insulin resistance (indicated by the serum glucose level and HOMA-IR index). Lactococcus and Flexispira, which were decreased after GML supplementation, were significantly positively correlated with serum insulin and LDLC levels, and Lactococcus also displayed significantly positive correlations with the AUC of the OGTT and serum LPS concentration.
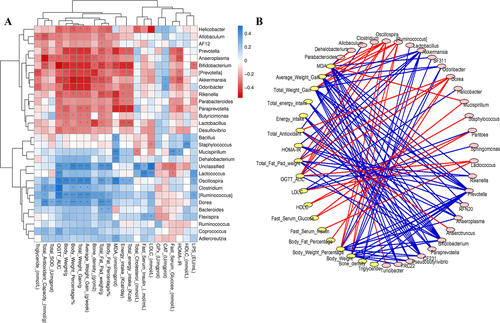
The network analysis in Figure 6B also exhibited the correlation of gut microbiota and host metabolic responses. Oscillospira, [Ruminococcus], Akkermansia, Dorea, Prevotella, Anaeroplasma, and Bifidobacterium were most correlated with metabolic parameters. Among them, Bifidobacterium was increased by all the GML treatments and Akkermansia was increased after 450 mg kg−1 GML supplementation compared to the HFD control. For metabolic responses, the body weight related parameters, hepatic MDA concentration and glucose homeostasis (the AUC of the OGTT and the HOMA-IR) were most involved in the correlation with gut microbiota. These results indicated that the GML-induced changes in gut microbiota composition were closely correlated with its metabolic effects in HFD-fed mice.
4 Discussion
Our previous study showed that relatively low-dose dietary GML in LFD-fed mice increased body weight, induced low-grade systematic inflammation, and gut microbiota dysbiosis.23 However, whether the same effects of GML on metabolic syndrome and gut microbiota still exist in HFD-fed mice had not been investigated. Our present results demonstrate that 450 mg kg−1 GML supplementation mediated metabolic improvements and modulation of gut microbiota. The same low-dose dietary GML supplementation (150 mg kg−1) that was used in our previous study also led to a significant increase in body weight upon HFD feeding. Interestingly, higher dose GML treatment (450 mg kg−1) improved HFD-induced hyperlipidemia, prevented visceral obesity, modulated hepatic lipid metabolism, and reduced systematic low-grade inflammation except body weight which was not modified. The glucose tolerance was not affected by GML supplementation, which might be related to the improper concentration of gavaged glucose in the OGTT. However, the HOMA-IR index, an insulin sensitivity indicator, was significantly lower in all the GML-treated HFD-fed mice. Notably, GML treatment also reduced the circulating LPS levels and improved the intestinal epithelium structure. Moreover, the observed metabolic improvements induced by GML were associated with the modulation of gut microbiota composition.
In this study, three different concentrations of GML were tested individually for their effects on HFD-induced metabolic syndrome. GML treatment did not reduce body weight or total weight gain in HFD-fed mice, and even 150 mg kg−1 GML significantly increased body weight compared with the HFD control group. However, HFD-induced visceral adiposity was significantly improved by higher dose GML supplementation (300 and 450 mg kg−1). Visceral lipid deposition contributes to systematic inflammation, insulin resistance, and further adiposity. 300 and 450 mg kg−1 GML treatments significantly reduced hepatic fat accumulation and the size of epididymal adipocytes and subsequently resulted in improved insulin sensitivity and systematic inflammation. 450 mg kg−1 GML supplementation also significantly improved serum hyperlipidemia. Hepatic lipid metabolism disorders contribute to the development of hepatic steatosis, which might lead to systematic inflammation and steatosis hepatitis.25, 26 Our results suggested that the modulation of GML on hepatic lipid metabolism in HFD-fed mice was associated with the supplementary dose. 450 mg kg−1 GML-treated mice showed a significant decrease in hepatic lipid content and a tendency of reducing hepatic TG level (p = 0.07) compared with HFD-fed mice. Interestingly, compared with 150 mg kg−1 GML-treated HFD-fed mice, both 300 and 450 mg kg−1 GML significantly lower the content of hepatic lipid and TG. Notably, an obvious downregulation of PPARγ expression was observed in 300 and 450 mg kg−1 GML treatments. PPARγ is a nuclear hormone receptor and is mainly present in adipose tissue and liver.27 Hepatic PPARγ regulates triglyceride homeostasis and promotes the development of fatty liver by upregulating the expression of lipogenic genes.28 The downregulation of PPARγ after GML treatment was consistent with lower hepatic lipid and TG content, as well as reduced hepatic fat deposition, which confirmed the role of PPARγ in hepatic lipogenesis. Similarly, the MCTs diet has been reported to reduce fat mass and suppress adipocyte hypertrophy by regulating the expression of PPARγ in adipose tissue.29, 30 Additionally, GML supplementation upregulated HADHB gene, which is involved in fatty acid β-oxidation. HADHB, as a subunit of the mitochondrial trifunctional protein, catalyzes two step reactions in mitochondrial fatty acid β-oxidation and its deficiency may lead to abnormal fatty acid metabolism.31, 32 Shinohara et al.33 showed that consumption of a diet rich in MCTs improved adiposity by enhanced fat oxidation. The research of Papamandjaris et al.34 indicated that MCTs increased the endogenous oxidation of long chain fatty acids, which consequently promoted lipolysis in healthy female subjects. Furthermore, GML treatment significantly reduced the expression of HMGCR gene, which encodes HMG-CoA reductase, the rate-limiting enzyme in cholesterol synthesis. This effect was supported by the significantly reduced serum TC level in 450 mg kg−1 GML treatment. MCTs and MCFAs have also been found to have positive effects on the reduction in serum TC levels by regulating hepatic cholesterol secretion and reducing intestinal reabsorption of sterols in intestine.18, 35 Altogether, our results indicated that the modulation of hepatic steatosis by higher GML doses (300 and 450 mg kg−1) could be attributed to the limitation of lipogenesis and improvement of lipid β-oxidation.
There is growing evidence that HFD-induced metabolic disorder is closely associated with gut microbiota dysbiosis. The beneficial effects of GML supplementation on metabolic phenotypes were concomitant with the modulation of gut microbiota composition. We observed an increase in the abundance of Firmicutes in all HFD-fed mice and a decrease in the abundance of Bacteroidetes after GML treatment. Turnbaugh et al.36, 37 reported decreased levels of Bacteroidetes and increased levels of Firmicutes in obese humans and animals, which indicated that these major phyla might play important roles in the development of obesity. However, whether the change in the Firmicutes to Bacteroidetes (F/B) ratio is associated with obesity is still controversial. The results of Zhang et al.38 indicated that the F/B ratio showed no correlation with body weight or adiposity percentage. They found that both berberine and metformin inhibited dietary-induced obesity and improved insulin resistance in HFD-fed rats with an increased F/B ratio. Intriguingly, compared with the Chow and HFD control treatments, 450 mg kg−1 GML supplementation reversed the decreased abundance of LPS-suppressing phylum Verrucomicrobia in 150 and 300 mg kg−1 GML treatments. 450 mg kg−1 GML supplementation also significantly increased the LPS-suppressing genus Akkermansia compared with the HFD control group. These results were consistent with our observations of the circulating LPS concentration; GML supplementation significantly reduced the level of serum LPS and 450 mg kg−1 GML supplementation resulted in the lowest LPS level in all HFD-treated groups. The increased concentration of circulating LPS has been reported to be a consequence of HFD-induced changes in the composition of gut microbiota.4 The synthetic dietary emulsifiers CMC and P80, whose one direct important target are gut microbiota, significantly elevated the LPS levels in HFD-fed mice.39, 40 Conversely, GML, a typical and natural dietary emulsifier, significantly reversed the HFD-induced increased LPS load, suggesting ameliorated systematic inflammation and improved intestinal epithelium structure, which were supported by decreased serum TNF-α levels and improved jejunum morphology. As Kono et al.41 demonstrated, rats fed MCTs showed a significant improvement in intestinal permeability and prevented LPS-mediated endotoxemia by remodeling gut microbiota based on its antimicrobial properties.
We also observed a significant increase in the abundance of Bifidobacterium after GML supplementation compared to HFD-fed mice. Consistently, previous studies have reported that Bifidobacterium, a carbohydrate-degrading genus, significantly decreased after HFD feeding and was associated with alleviated metabolic syndrome.42 In addition, GML treatment enhanced the abundance of Lactobacillus, which was also decreased in HFD-fed mice and associated with lipid metabolism.43 In the correlation analysis of gut microbiota and GML-induced metabolic effects, the abundance of Bifidobacterium and Lactobacillus was significantly, negatively associated with metabolic syndrome phenotypes. The studies of Griffiths et al.44 and Hevia et al.45 indicated that the increased abundance of Bifidobacterium and Lactobacillus by prebiotics was associated with reduced intestinal endotoxin, which was supported by decreased serum LPS levels in GML-treated groups. Moreover, the proliferation of these bacteria could improve gut barrier function and modulate hepatic inflammation, and whether GML has the same effects needs further investigation. Another lactic acid bacterium, Lactococcus, which has been reported to be increased in obese animals,46 was significantly reduced by 450 mg kg−1 GML supplementation compared to the HFD control group. Our observations were same with the results of Zhu et al.47 Lactococcus was strongly positively correlated with insulin resistance, serum dyslipidemia, and high LPS load, suggesting its important role in the development of obesity. Interestingly, all GML-treated groups showed significant reduced Flexispira compared with the HFD-fed mice. As Zhang et al.48 reported, the decreased abundance of Flexispira was related to the improvement in the health status of the host intestinal tract. The analysis at the species level revealed that 450 mg kg−1 GML-treated group had similar abundance of R. gnavus, B. uniformis, and E. coli as the Chow group. Among these, R. gnavus was previously reported to be associated with metabolic disorder and inflammatory bowel diseases,49, 50 and compared with the Chow controls, all the HFD-fed groups except for 450 mg kg−1 GML-treated group showed significant increased abundance of this bacterium. The results of Cano et al.51 demonstrated that B. uniformis could alleviate HFD-induced obesity, and this bacterium was significantly reduced in both HFD control and lower-dose GML (150 and 300 mg kg−1) treated groups. Conversely, compared with the Chow-fed mice, 450 mg kg−1 GML supplementation showed no obvious differences in the abundance of B. uniformis. Our previous study found that 150 mg kg−1 GML treatment in LFD resulted in an increased abundance of the Gram-negative pathogens E. coli, which was related to higher levels of serum LPS and proinflammatory cytokines.23 Consistently, 150 mg kg−1 GML supplementation did not reverse the increased E. coli induced by HFD in our present study. However, 300 and 450 mg kg−1 GML treatment significantly reduced E. coli compared with the HFD controls, which might be associated with the lower concentration of serum LPS. These results indicated that GML may ameliorate HFD-induced metabolic syndrome by modulating gut microbial composition in multiple ways.
In conclusion, our findings suggest that higher-dose GML supplementation (450 mg kg−1) improved metabolic syndrome in HFD-fed mice by modulating lipid metabolism and gut microbial composition. The metabolic improvements of dietary GML are associated with the increased abundance of some specific bacterial groups, such as Akkermansia, Bifidobacterium, Lactobacillus, and B. uniformis. These findings offer new perspectives into the potential to use dietary GML as a functional ingredient for the attenuation of HFD-induced metabolic syndrome. However, the optimum dose of GML supplementation requires further analysis.
Acknowledgements
This work was supported by Key Project of Natural Science Foundation of Zhejiang Province (Grant No. LD19C200001), General Project of Natural Science Foundation of Zhejiang Province (Grant Nos. LQ19C200005 and LY18C200006), Basic Research Project of Education Department of Zhejiang Province (Grant No. Y201737161), Technology and Achievement Transformation Project of Hangzhou, China (Grant No. 20161631E01), and Zhejiang University New Rural Development Research Institute Agricultural Technology Promotion Fund (Grant No. 2017006). M.Z., H.C., Z.J., and F.F. conceived the project. M.Z. performed the experiments, interpreted the results, generated the figures, and wrote the manuscript. H.C., Z.J., Y.L., and H.Z. performed the experiments and interpreted the results. H.Z. and F.F. supervised the experiments, interpreted the results, and revised the manuscript. All authors approved the final manuscript.
Conflict of Interest
The authors declare no conflict of interest.




