Choline Supplementation Ameliorates Behavioral Deficits and Alzheimer's Disease-Like Pathology in Transgenic APP/PS1 Mice
Abstract
Scope
Alzheimer's disease (AD) is a detrimental neurodegenerative disease and has no known effective treatment. The essential nutrient choline potentially plays an important role in cognition. Perinatal choline supplementation (CS) is critical for memory performance. Findings have shown that postnatal choline-containing compounds enhance memory functions in populations with memory impairments. However, whether CS can be targeted to decelerate the progression of AD remains unknown.
Methods and results
APP/PS1 mice and their wild-type littermates are fed either a control or CS diet from 2 to 11 months of age. As compared to WT mice, APP/PS1 mice on the control diet are characterized by the reduction in the number of cholinergic neurons in the basal forebrain, reduced cholinergic fiber staining intensity in the amygdala, and reduced hippocampal and cerebral cortical levels of choline and acetylcholine. CS partially prevents these changes and ameliorates cognitive deficits and anxiety. Furthermore, amyloid-β deposition and microgliosis are decreased in the APP/PS1 mice fed a CS diet. These effects may have been due to inhibition of NLRP3 inflammasome activation and restoration of synapse membrane formation.
Conclusion
These findings reveal a beneficial effect of CS on AD progression during adulthood and provide a likely therapeutic intervention for AD patients.
1 Introduction
Alzheimer's disease (AD) is the most common type of age-related dementia and has no effective treatment. This disorder, characterized by progressive memory decline and cognitive dysfunction, is often manifested histologically by parenchymal amyloid-β (Aβ) plaque deposits, neurofibrillary tangle formation, and neuroinflammation.1 Sporadic AD cases, which account for almost 95% of all AD cases, arise through complex interactions between various genetic and environmental factors.2 Epidemiological studies have repeatedly shown the protective and/or risk-reducing effects of nutrient intake on AD.3 However, the roles of specific nutrients in preventing or delaying the progression of AD remain poorly understood.
Choline is an essential nutrient and has a potential role in several pathways of AD, such as cholinergic dysfunction, reduced synaptic membrane formation and function, and phospholipid and methyl-group metabolism.4 In AD, the need for choline is increased because high brain levels of choline are required to correct the defects in the synapses.5 However, many people—particularly older people—fail to attain the recommended dietary choline intake. Research has documented lower choline and phospholipid levels in postmortem AD brains than in age-matched controls.6 We and others have reported that maternal dietary choline provides critical support for normal brain development through modulation of neurogenesis and angiogenesis.7, 8 Many studies show that perinatal choline supplementation enhances memory9 and prevents age-related memory decline.10-12 However, the effects of postnatal choline-containing compounds on human cognition are mixed, as no improvements are reported for mature, healthy humans and rodents,13, 14 but significant enhancements of a wide variety of memory and executive control functions, including working memory, verbal learning and memory, and maze pathway learning, are reported in populations with mild to moderate memory impairments.15, 16 These findings imply that supplementation of choline in adults is likely to improve cognitive function by delaying the progression of AD pathology.
Cholinergic degeneration plays a prominent role in the AD pathophysiology. The number of cholinergic neurons in the basal forebrain of subjects with advanced AD has consistently been found to be reduced. Cholinergic neurons provide the main source of acetylcholine (ACh), and a lack of ACh in the cerebral cortex and hippocampus directly correlates with cognitive decline. As a precursor to ACh, supplemental choline results in a dose-dependent increase in brain ACh concentrations.17 Recently, ACh was also suggested to contribute to inhibiting inflammasome activation by preventing mitochondrial DNA release.18 Inflammasomes are inducible protein complexes that play important roles in many inflammatory pathological processes. In AD, microglia sense Aβ and then promote pathological innate immune activation and inflammatory mediator release.19 NACHT-, LRR-, and pyrin (PYD)-domain-containing protein 3 (NLRP3) inflammasome activation has been detected in the brains of AD patients and APP/PS1 mice. NLRP3 inflammasome inhibition prevents microglial IL-1β secretion, AD pathology and cognitive impairment in APP/PS1 mice.20 Most recently, Venegas et al. demonstrated that inflammasome activation could seed Aβ deposition, suggesting that the inflammasomes are a new treatment target for AD.21
The present study was designed to determine whether choline supplementation during adulthood delays AD progression and, if so, by what underlying mechanism. We report here that choline-supplemented APP/PS1 mice exhibited enhanced spatial learning and memory and reduced anxiety levels. Furthermore, we showed that choline supplementation protected against the degeneration of cholinergic neurons and reduced Aβ deposition and microgliosis. Notably, these effects might involve the inhibition of NLRP3 inflammasome activation and restoration of synapse membrane formation. These findings shed light on the potential preventive and therapeutic application of choline for AD.
2 Experimental Section
2.1 Animals
APP/PS1 transgenic mice (strain B6C3-Tg (APPswe, PSEN1dE9) 85Dbo/J) were purchased from Jackson Laboratory. Animals were housed according to a protocol described elsewhere.25 Eleven-month-old male APP/PS1 mice and their wild-type (WT) littermates were used for all behavioral experiments, immunohistochemical staining, choline/ACh assays, and Western blot analysis.
All animal experimental procedures were approved and received ethical approval from the laboratory animal care of Third Military Medical University (study number LLZM20160312).
2.2 Dosage Information
Two dietary groups were used according to a protocol described elsewhere: a control group (1.10 g choline chloride per kg) and a choline-supplemented group (4.95 g choline chloride kg).22 The control diet supplied adequate choline, and the choline-supplemented diet provided 4.5 times the concentration of choline consumed by the control group and was within the range of dietary variation observed in humans.23, 24 Unless otherwise noted, animals were maintained on a standard AIN76A rodent diet with the standard 1.10 g choline chloride per kg. From 2 months to 11 months of age, mice were randomly divided into two groups: a control AIN76A diet group and a choline-supplemented diet group.
For in vitro cell culture, media supplemented with 315 µm choline was used,7 a concentration that was 4.5 times higher than that used in the control group (70 µm) and has been demonstrated to protect neural cells.
2.3 Behavioral Tests
The mice used for all behavioral analyses were 11-month-old adult males. The following behavioral experiments were performed between 9:00 a.m. and 5:00 p.m. as described previously:25, 26 open-field locomotion, elevated plus maze, light-dark box test, Morris water maze, and nest building. For details, see Materials and Methods, Supporting Information.
2.4 Immunohistochemistry, Immunofluorescence, and Quantification
After the behavioral tests, the mouse brains were dissected and fixed. Forty-micron coronal serial sections were cut and incubated with primary antibodies and the corresponding secondary antibodies. For details, see the Materials and Methods, Supporting Information.
2.5 Choline/ACh assay
Brains were removed immediately on ice and bisected along the midsagittal plane, and from one randomly selected hemisphere, the frontal, parietal, temporal, and occipital cortices were collected by microdissection and pooled together, and the hippocampus was obtained. The tissues were snap frozen in liquid nitrogen and immediately stored at −80 °C. When the samples were ready for testing, they were thawed on ice. Choline and ACh were measured using a commercial kit (Abcam, Cambridge, UK).
2.6 Microglial Activation
To assess inflammasome activation, cells of the BV2 murine microglial line were primed with 1 µg mL−1 lipopolysaccharide (LPS, Escherichia coli O111:B4, Sigma) for 3 h and were activated with 5 µm Aβ for 24 h. Control (70 µm choline) and choline-supplemented (315 µm) media were used in different cultures. Cell culture supernatants and lysates were assessed by ELISA and Western blot.
2.7 ELISA
IL-1β in the cell medium was assessed by quantitative ELISA kits (R&D Systems, Minneapolis, MN) with reference to standard curves of purified recombinant proteins at various dilutions.
2.8 Western Blot Analysis
Western blot assays were performed as described previously.27 For details, see Materials and Methods, Supporting Information.
2.9 Statistical Analysis
To compare the group differences, Student's t-test, two-way ANOVA or repeated-measures ANOVA followed, when necessary, by least significant difference (LSD) post hoc analysis were used. Differences were considered significant at p < 0.05. Data are presented as the mean ± SEM. Statistical analysis was performed using SPSS v13.0 software (IBM, Chicago, IL).
3 Results
3.1 Choline Supplementation Increases the Abundance of Cholinergic Neurons in APP/PS1 Mice
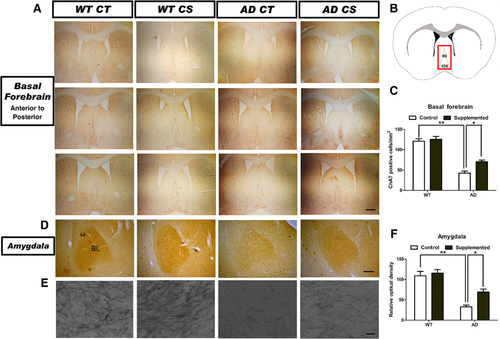
We performed immunostaining against choline acetyltransferase (ChAT) on brain slices collected from various brain regions. Choline supplementation could partially reverse the decreased number of ChAT-positive cholinergic neurons in the medial septal nuclei and vertical limbs of the diagonal band (MS/VDB) within the basal forebrains of 11-month-old APP/PS1 mice (Figure 1A,C). Optical density measurements of the ChAT-immunoreactive neurons in the basolateral amygdaloid nucleus (BL) showed significant differences between the APP/PS1 mice fed the control diet and the choline-supplemented diet (Figure 1D–F). Choline supplementation did not alter the number of ChAT-positive cholinergic neurons in the basal forebrain or amygdala of WT mice (Figure 1). These findings suggest that choline supplementation partially restores cholinergic function in AD mice.
3.2 Choline Supplementation Ameliorates Cognitive Deficits in APP/PS1 Mice
To determine whether choline supplementation can restore the impairment of spatial learning and memory in APP/PS1 mice, we conducted the Morris water maze test. In line with previous studies,28 AD mice had a longer escape latency than WT mice from days 2 to 5 (p < 0.05). The choline-supplemented AD mice required significantly less time (p < 0.05) to find the hidden platform than the AD mice fed the control diet on days 3, 4, and 5 (Figure 2A). Representative images of tracking data from probe trials are shown in Figure 2B. The choline-treated AD mice spent a significantly larger (p < 0.05) proportion of time in the goal quadrant than the control diet-treated AD mice (Figure 2B,C). Compared to that achieved with the control diet, the choline-supplemented diet increased (p < 0.01) the number of transits across the former platform location made by the AD mice (Figure 2B,D). However, choline supplementation had no effect on the WT mice in the Morris water maze test (Figure 2A–D).
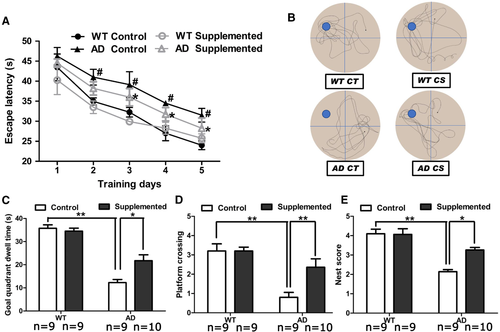
We further investigated nest-building behavior, which is dependent on the hippocampus.29 The abilities of AD mice to perform this task were significantly impaired (Figure 2E), and those fed the choline-supplemented diet substantially outperformed those fed the control diet, as indicated by their significantly increased nest scores (p < 0.05). These data indicate that choline enhances hippocampus-dependent cognitive function in AD mice.
3.3 Choline Supplementation Ameliorates Anxiety in APP/PS1 Mice
As we observed that choline supplementation markedly restored the abundance of ChAT-immunoreactive neurons in the amygdala, a brain region associated with anxiety, we examined the anxiety levels of mice receiving different treatments. In the open-field test, the AD mice exhibited less locomotor activity than the WT mice, and choline supplementation increased the total distance travelled by AD mice (p < 0.05) (Figure 3A,B). Moreover, choline supplementation significantly increased (p < 0.05) the time AD mice spent in the central area, suggesting that their anxiety level was reduced (Figure 3C).
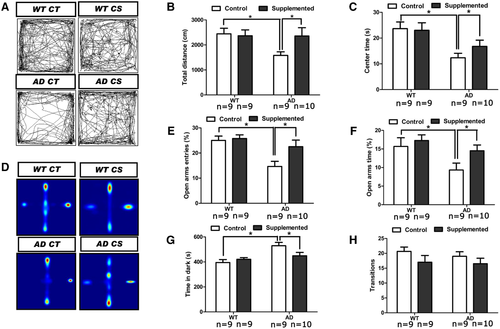
In the elevated plus maze assay, choline-supplemented AD mice entered the open arm more times and spent more time in the open arms (p < 0.05 for both) than the control diet-treated mice (Figure 3D–F). Furthermore, a light-dark box test revealed that choline supplementation decreased (p < 0.05) the total time AD mice spent in the dark chamber (Figure 3G), although no alteration (p > 0.05) was observed in the total number of transitions between the light and dark chambers (Figure 3H). Taken together, these results suggest that choline supplementation decreases anxiety-like behavior in AD mice.
3.4 Choline Supplementation Reduces Aβ Deposition and Microgliosis in both the Cortices and Hippocampi of APP/PS1 Mice
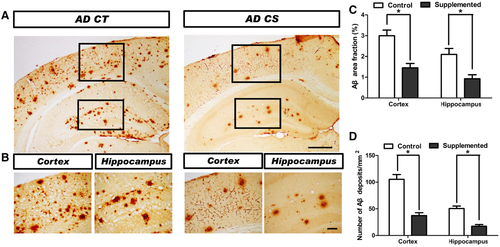
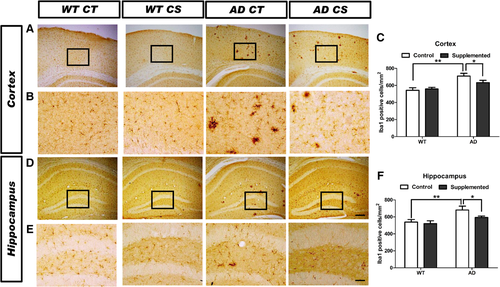
To investigate whether chronic choline supplementation affects Aβ deposition in vivo, we analyzed the brains of mice at 11 months of age. Choline-supplemented AD mice had obviously fewer Aβ deposits within the cortex and hippocampus than the control diet-treated AD mice (Figure 4A,B), as revealed by the reduced (p < 0.05) area fraction (Figure 4C) and decreased (p < 0.05) density of Aβ-immunopositive deposits (Figure 4D). We analyzed the effect of choline supplementation on microglia in the somatosensory cortex and hippocampus using the microglia marker Iba1 (Figure 5A,B,D,E). The number of Iba1+ cells in AD mice was markedly enhanced (p < 0.01) in both the cortex (Figure 5C) and the hippocampus (Figure 5F), and choline supplementation ameliorated microgliosis in both of these brain areas in AD mice (p < 0.05, Figure 5C,F). Together, these data suggest that choline supplementation protects against AD pathogenesis.
3.5 Choline Supplementation Inhibits NLRP3 Inflammasome Activation
To investigate the possible role of choline supplementation in NLRP3 inflammasome activation, we first evaluated NLRP3 expression, caspase-1 (CASP1) activation, and IL-1β production in the cortex and hippocampus by Western blot analysis. We observed considerable NLRP3 expression in the cortices of control diet-treated AD mice, and choline supplementation decreased (p < 0.05) NLRP3 expression (Figure 6A,B). When we assessed the indications of functional inflammasome CASP1 cleavage as well as IL-1β maturation, we confirmed the specific effect of choline supplementation on NLRP3 inflammasome activation. Both the cleaved CASP1 product CASP1 p20 and mature IL-1β (IL-1β p17) were increased in the brains of APP/PS1 mice compared with those in the brains of WT mice. However, choline supplementation decreased CASP1 cleavage and IL-1β maturation in the APP/PS1 mice (Figure 6A–C).

Additionally, we cultured microglia of the BV2 cell line, and analysis of IL-1β by ELISA revealed that the increase induced by combined LPS and Aβ treatment was attenuated by choline supplementation (p < 0.05, Figure 6D). Additionally, choline inhibited NLRP3 inflammasome activation as well as pro-CASP1 and pro-IL-1β expression in microglia (p < 0.05, Figure 6E,F). Collectively, these findings establish that choline supplementation inhibits NLRP3 inflammasome activation in the brains of AD mice.
3.6 Choline Supplementation Increases Synaptic Membrane Formation
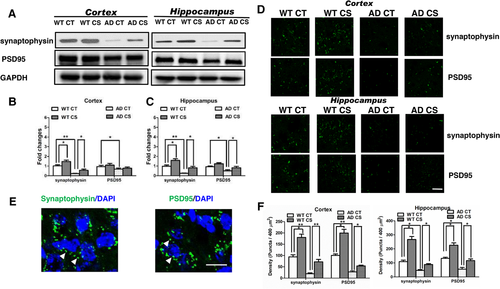
As loss of synapses is a hallmark of AD, we assayed the levels of presynaptic and postsynaptic membrane proteins to determine the potential effect of choline supplementation on synapse formation. Similar to previous reports,30 we observed decreased levels of the presynaptic marker synaptophysin in APP/PS1 mice. Notably, Western blot analysis revealed that choline supplementation increased the levels of synaptophysin in both WT and AD mice. Moreover, the levels of the postsynaptic protein postsynaptic density-95 (PSD95) were reduced in AD mice compared to those in age-matched WT mice. Choline supplementation restored PSD95 levels in the hippocampi of the APP/PS1 mice (p < 0.05, Figure 7A–C). Furthermore, immunofluorescence data showed that choline supplementation increased synaptophysin and PSD95 in both WT and AD mice (Figure 7D–F). Together, these results support the possibility that choline supplementation can correct AD-related deficiencies in synaptic membranes.
4 Discussion
Prenatal and early postnatal high choline intake has been shown to be neuroprotective. Here, we further confirmed that choline supplementation could alleviate cognitive deficits, anxiety and AD-like pathology in an adult AD mouse model, and we elucidated some new findings regarding its underlying mechanisms.
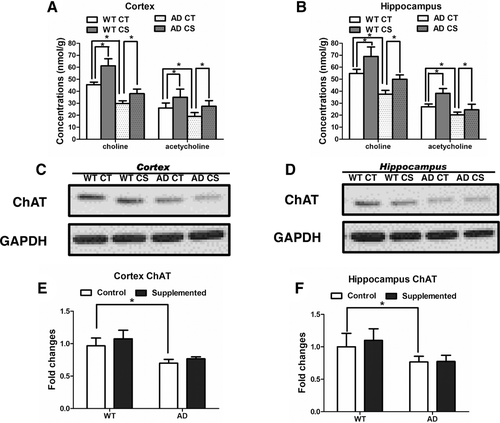
First, choline supplementation ameliorates the cognitive and noncognitive behavioral effects of AD. AD is considered a progressive cognitive disorder; however, noncognitive psychiatric symptoms, such as depression, anxiety, apathy and aggression, are very common and accompany all stages of the disease.31 The pathological basis of noncognitive behaviors is complicated and still controversial. Dekker et al. considered that neurotransmission is vital in neuropsychiatric behavioral manifestations of AD.32 Degeneration of the cholinergic system is a critical part of the AD pathophysiology and is thought to be due to the reduced expression and activity of ChAT as well as deficits of cholinergic neurons.33 The cholinergic system regulates various aspects of brain function by modulating neural activity via ACh and its receptors. Currently, three-quarters of the drugs approved by the Federal Drug Administration (FDA) for the treatment of AD are acetylcholinesterase inhibitors, which can increase both the level and duration of action of the neurotransmitter ACh. Additionally, supplementation with ChAT has been proposed as an alternative therapy for AD.34 We measured free choline and ACh in the cerebral cortex and hippocampus following choline administration and found that both were markedly increased in the WT and APP/PS1 mice (Figure 8A,B), which is consistent with a previous report.17 We also performed ChAT immunoblots of the cortex and hippocampus, and our results were consistent with the published literature. ChAT protein levels were significantly decreased in the hippocampi of 9- to 12-month-old APP/PS1 male mice.9 Neither the APP/PS1 mice nor the WT mice exhibited a significant increase in ChAT protein expression after choline supplementation (Figure 8C–F). We speculate that the change in ACh detected in the cortex and hippocampus could have resulted from increased free choline levels. The increased ACh in the cortex and hippocampus after choline supplementation had no effects on the spatial memory and anxiety of WT mice, implying that choline supplementation may affect the cholinergic neurons specifically under the AD condition. Most cholinergic neurons are located in subcortical regions and have axons that innervate many brain regions, distinct subtypes of cholinergic neurons serve different regulatory functions in the brain.35 A previous study reported no obvious changes in the number of cholinergic neurons or the optical density of ChAT in the nucleus basalis of APP/PS1 mice compared to those in nontransgenic mice.36 We observed that the abundance of cholinergic neurons in AD mice was decreased in the MS/VDB, which is the major cholinergic brain area within the basal forebrain and sends cholinergic projections to many cortical and subcortical regions as well as to the hippocampus.35 The inconsistency in these results could be explained by the facts that we conducted our counts in different regions of the basal forebrain and used a different animal gender or by the possibility that the control diet used in this study exacerbated AD-like phenotypes compared to those generated by the mouse chow likely used in other studies.
Our study is the first to explore the effects of dietary choline on AD-like phenotypes using a chronic paradigm in a mouse model. Some previous studies reported no effect of choline supplementation on memory function in aged human patients.37, 38 In contrast to these studies, we have tested the effects of choline in the long term. This study and others suggest that choline supplementation participates in synapse formation. A possible explanation for the null results of previous studies on humans is that choline affects synapse function, which takes time to recover. Future studies need to clarify whether prolonged choline administration could attenuate AD symptoms. Another possibility underlying the null results is that in clinical trials with patients, the sample sizes were not large enough to identify subjects who responded to choline. Finally, lecithin and choline chloride, both substances containing the chemical structure of choline, did not successfully improve memory performance. However, CDP-choline and choline alfoscerate, both alternative compounds that contain choline, improved cognition in human patients with dementia.16, 39 The discrepancy between the effects of these different substances may relate to the complexity of the digestive system.14 Because substances that can convert to choline undergo different processes, they may be more or less effective at producing choline depending on where and how they are broken down or connected to other chemical elements. Nonetheless, it remains unclear which choline-containing substances improve memory and why.
Second, choline supplementation inhibits NLRP3 inflammasome activation. Accumulating data emerging from genetics, clinical studies, and animal experimentation point to the integral role inflammation may play in the pathogenesis of AD.19 This role is further supported by epidemiological evidence that known risk factors for AD have an inflammatory component, including stroke, head trauma, diabetes, midlife obesity, and infection.40 Moreover, the level of inflammation correlates with the severity of AD. Some findings indicate that brain inflammation connects the cognitive and noncognitive symptoms of AD. Inflammation drives neuronal dysfunction in different brain structures, thereby triggering clinical symptoms related to distinct brain regions.41
Furthermore, recent studies show that the NLRP3 inflammasome and its downstream targets are key components in the development of AD.20, 21 The activated NLRP3 inflammasome contributes to AD pathogenesis in at least three ways: generating toxic IL-1β, impeding Aβ clearance, and prompting Aβ aggregation and spreading. Furthermore, the role of NLRP3 in AD has been verified in clinical studies. Components of the NLRP3 inflammasome were upregulated in the monocytes of AD patients compared with those in the monocytes of age-matched healthy controls.42 Thus, targeting inflammasome components, particularly in the early stages of disease, could slow the formation of Aβ plaques and thus delay neurological sequelae in patients. In the present study, we indeed observed that the inflammasome complex components NLRP3 and pro-caspase-1 were downregulated by choline supplementation. A reduced amount of active caspase-1 consequently reduced the cleavage of pro-IL-1β, decreasing the release of mature IL-1β in the choline-supplemented AD mice. The inhibition of inflammasome activation likely partly explains the reduced Aβ deposition, microgliosis and proinflammatory cytokine production observed in the brains of APP/PS1 mice after 9 months of choline supplementation.
The mechanisms underlying the ability of choline supplementation to inhibit inflammasome activation are not clear. Several pharmacological investigations demonstrated that choline had anti-inflammatory activity in peripheral tissues. Such anti-inflammatory activity of choline was identified as being due to stimulation of the alpha 7 nicotinic acetylcholine receptor (α7 nAChR).43 As the primary resident immune cells of the brain, microglial cells express the α7 nAChR, and its activation attenuates the proinflammatory response and prevents the production of reactive oxygen species (ROS) in microglia stimulated by fibrillar Aβ.44 Interestingly, both choline and ACh are α7 nAChR agonists; in particular, choline is a natural specific α7 nAChR agonist.45 Previous studies indicate that α7 nAChR activation triggers two critical signalling pathways (NF-κB activation and JAK/STAT signal transduction) required for cellular inflammation.44 More recently, α7 nAChR signalling was shown to inhibit NLRP3 inflammasome activation by preventing mitochondrial DNA release.18 These results raise the intriguing possibility that choline may control brain inflammation via α7 nAChRs in AD. Free choline can be transported to the brain due to the availability of the choline supply under physiological conditions. In our study, whether both increased choline and ACh mediate the activation of α7 nAChR to suppress the proinflammatory response remains unclear.
Another possibility is that choline supplementation rescues “lipid raft ageing” in the brain membranes of AD mice. Choline is a critical component of both phosphatidylcholine and sphingomyelin in lipid rafts and has been suggested to reduce the disruption of neurons by alcohol exposure via lipid raft-dependent signalling cascades and protein trafficking.46 Neuroinflammation in AD is mediated through a number of pattern-recognition receptors, such as CD36 and Toll-like receptor 4.47 Whether choline influences NLRP3 inflammasome activation through lipid rafts to suppress proinflammatory signalling in microglial cells awaits further study.
Third, choline supplementation increases synaptic membrane formation. Synapses are considered the earliest site of AD pathology, and synaptic loss is the best pathological correlate of cognitive impairment in subjects with AD. Reducing synapse loss and membrane-related pathology may preserve or improve neurotransmission and thereby positively affect memory and other cognitive functions. In adulthood, neuronal activity controls synapse formation. Because choline constitutes synapse membrane formation, choline availability can affect synaptogenesis.48 Previous data showed that dietary enrichment with Fortasyn Connect, a specific nutrient combination including choline, significantly increased the expression of the presynaptic marker synapsin-1 and the postsynaptic marker PSD-95 in aged rats, suggesting enhanced synthesis of synaptic membranes.49 A recent report showed that compounds affecting NLRP3 inflammasome activation prevented the synaptic plasticity deficit in an animal model of AD amyloidosis.50 Additionally, inflammasomes trigger IL-1 release, which can inhibit hippocampal long-term potentiation. In this study, both increased synaptic membrane formation and NLRP3 inflammasome inhibition were observed, indicating that choline could be a potent agent for ameliorating synapse loss and synaptic dysfunction in AD. We also investigated whether neuronal cell death was modulated by choline supplementation. We found that choline supplementation decreased the number of apoptotic neurons in AD mice compared to that in AD mice fed the control diet, whereas no significant change was detected between the control and choline-supplemented WT mice (Figure S1, Supporting Information), suggesting that choline supplementation may affect the survival of pre-existing neurons specifically under the pathological conditions of AD and not under normal physiological conditions.
Studies on dietary choline as a methyl-group donor provide evidence of nutrient-responsive epigenetic mechanisms. Choline and methyl metabolism play an important role in regulating liver cancer carcinogenesis. In particular, maternal dietary choline deficiency changes DNA and histone methylation in the fetal brain, and gene expression, neurogenesis and angiogenesis are known to be influenced by choline during foetal brain development.22, 51 Therefore, it is tempting to speculate that choline supplementation may be a key resource for protecting against AD via methylation.
The adequate intake (AI) of choline recommended by the United States Institute of Medicine is 550 mg d−1 for men and 425 mg d−1 for women. However, many people fail to meet the AI for choline, and single-nucleotide polymorphisms (SNPs) in several genes related to choline metabolism alter the dietary requirement for this nutrient, such as SNPs in phosphatidylethanolamine N-methyltransferase (PEMT) and methylene tetrahydrofolate reductase (MTHFR). These SNPs, which are differentially distributed among various racial and ethnic populations, increase the risk of choline deficiency.52 Thus, oral choline could meet the additional choline requirement for people with a choline deficiency or for AD patients.
In summary, our data provide new explanations for the multifaceted beneficial effects of choline on AD patients. These findings suggest that choline supplementation in patients with early-stage AD may pave the way for therapeutic interventions against AD.
Acknowledgements
Y.W. obtained financial support, designed this study, conducted the behavior, immunohistochemistry and immunofluorescence tests, choline/ACh assay, ELISA, and wrote the manuscript. X.G. performed the animal experiment, behavior, and immunohistochemistry tests. X.C. conducted immunohistochemistry and western blot. Y.C. conducted the behavior tests. Y.M. performed immunohistochemistry. J.M., Q.Z., and L.D. performed the cell culture and western blot. X.F. and Y.B. designed this study and reviewed the manuscript. This work was supported by the grants from the National Natural Science Foundation of China (No. 81573153) and Chongqing Science and Technology (No. cstc2016jcyjA0350 and cstc2018jcyjAX0499).
Conflict of Interest
The authors declare no conflict of interest.




