Surface modification of PDMS substrates for tumour cell adhesion: Influence of roughness parameters
Abstract
Surface properties play a key role in how biomaterials interact with the environment. Surface topography has also been reported to be influential in some research, although its effect is still not well elucidated. In this study, nano-roughened polydimethylsiloxane (PDMS) substrates were developed through a chemically etched intermediate surface. Additionally, PDMS substrates containing 10–30 μm diameter micropillars were functionalized with multilayers of chitosan (CHI) and hyaluronic acid (HA) via layer-by-layer. Such substrates were submitted to cell adhesion assays with PC3 tumour cells. The characterization of these substrates was carried out using an atomic force microscopy (AFM), and some roughness parameters were estimated. Through a statistical description of the topography, we investigated the effects of these surface parameters on PC3 cell adhesion. AFM results indicated a significant modification in the PDMS surface topography and the cell adhesion assays suggest that smoother surfaces induce the PC3 cell adhesion, especially the ones with a high Hurst exponent value. In addition to the AFM analysis, the surface modification of the LbL-functionalized substrates was monitored by contact angle and UV-visible measurements. The improved wettability and the significant Alcian Blue absorbance of the functionalized substrates suggest that the HA/CHI film deposition was successfully accomplished. The LbL functionalization increased the cell capture potential of the PDMS substrates, in which lower diameter micropillars favour the cell adhesion mechanism. Although much work is still needed, the findings advance progress towards the fundamental understanding of the role of nanoscale fractal roughness in cell adhesion and can contribute to the development of new biomaterials with applications in biomedical systems, such as biosensors.
1 INTRODUCTION
Second leading cause of death worldwide, it is estimated that 18 million new cases of cancer appeared just in 2018 and 9.6 million people died because of the disease (GCO, 2018). The most diagnosticated types of cancer in that same year were as follows: lung (11.6%), breast (11.6%) and prostate cancer (7.1%) (Bray et al., 2018). Among men, prostate cancer (PC) is the leading type, knowing that it is expected that 1 in every 9 male individuals will endure the disease during their lifetime (Siegel et al., 2019). The development of the tumour is normally slow and the early detection of the disease can significantly increase the chances of cure and, therefore, the survival rate (Heidegger et al., 2019).
Conventional methods of prostate cancer diagnosis involve digital rectal examination and the prostate-specific antigen (PSA) test. However, several studies have been realized aiming to develop alternative methods of cancer early detection, normally by identification of biomarkers present in the patient's blood or urine (Álvarez-Maestro et al., 2020). Thus, the development of technologies able to selectively detect these types of markers is of tremendous importance in order to reduce the mortality of prostate cancer.
Additionally, the better understanding of cell–cell and cell–surface interactions can lead to great advances in the development of important tools in the field of biomedicine, such as biosensors, implants and drug delivery systems (Ermis et al., 2018). This is due to the fact that cells’ ability to interact with their surroundings is highly dependent of the chemical and structural properties involved in the process (Anselme & Bigerelle, 2014; Clark et al., 1990; Lu et al., 2008).
With the advance of nanofabrication technologies, several studies have been verifying the influence of surface structure on different factors affecting cells such as adhesion and proliferation (Yin et al., 2014), gene expression (Huang et al., 2016) and alignment and morphology (Ning et al., 2016). Also, surface roughness was found to have significant influence on cell adhesion and behaviour (Hu et al., 2014; Werner et al., 2017). Some studies even suggest a correlation between the roughness of the biological interaction sites and the cell interactions (Le Guehennec et al., 2008; Wu et al., 2015). However, in order to investigate the importance of each individual factor it is necessary to use a methodology which allows to isolate all of the other factors and focus on a specific one.
In this sense, layer-by-layer (LbL) becomes an interesting technique because of its capacity to functionalize surfaces with relatively simple adjustment of the desired properties by changing mild assembly conditions (Decher, 1997; Guo et al., 2017). Due to the innumerous possibilities of film production, the LbL technique has been widely used in biomedical applications, especially with the aim of promoting or inhibiting cell adhesion (Guo et al., 2017).
Polydimethylsiloxane (PDMS) is a widely used polymer in biomedicine due to its unique properties such as blood compatibility, high thermal and oxidative stability, low toxicity, physiological inertness. (Abbasi et al., 2001). However, its applicability faces limitations because of its intrinsic hydrophobic nature and its low surface energy (Chuah et al., 2015; Scharin-Mehlmann et al., 2018), which is why PDMS chemical or physical surface modification is highly desired in such applications (Abbasi et al., 2001).
In this work, PDMS substrates with different topographic profiles were produced through a chemically etched PS surface and characterized by their roughness according to three distinct parameters. This characterization was also performed on PDMS substrates containing different diameter micropillars, which were functionalized by LbL with multilayers of chitosan (CHI) and hyaluronic acid (HA). Additionally, prostatic tumour cells of the PC3 line were cultivated and exposed to the substrates in order to evaluate the influence of surface roughness and different topographies on cell adhesion mechanism. On the whole, the better understanding of properties that significantly impact cell–surface interactions can support the improvement or even development of important technologies in the field of biomedicine, where advances on biosensing, implant safety and drug delivery systems are paramount.
2 MATERIALS AND METHODS
2.1 Materials
Polyethylenimine (PEI, MM = 7.5 kDa), chitosan (CHI, deacetylation degree = 85%), hyaluronic acid (HA, obtained from Streptococcus equi sp) and Alcian Blue (MM = 1298.86 g/mol) were purchased from Sigma-Aldrich. Ethanol, acetone (both used for the chemical modification of PDMS surfaces), sodium hydroxide, sodium chloride, hydrochloric acid and sulphuric acid were all purchased from Synth. All reagents were analytical grade and used without further purification. Silicone elastomeric kit Sylgard 184® was purchased from Dow Corning.
2.2 Nano-roughened PDMS substrate preparation
Nano-roughened PDMS substrates were prepared by pouring uncured PDMS prepolymer over an etched 6-well plate of polystyrene (PS). Briefly, distinct levels of PS roughness were created by chemical etching with acetone in concentrations of 25, 50, 55 and 60% v/v in ethanol. After adding 0.5 ml of the etching solution, the openings of each well were covered with glass slides to prevent solvent evaporation during 90 s. When the etching time elapsed, each well was quickly rinsed with fresh etchant solution, thoroughly washed with deionized water and left to dry at room temperature.
Thereafter, the PDMS precursor and the curing agent (Sylgard 184®) were mixed at a mass ratio of 10:1, and 0.5 g of this mixture was casted onto each well. Before performing thermal curing at 80° for 1 h, this mixture was degassed in a vacuum desiccator. Then, the solidified PDMS with transferred nano-features was peeled off from the each well and sliced into 1 in x 1 in pieces.
2.3 PDMS substrate with ordered nanostructure preparation
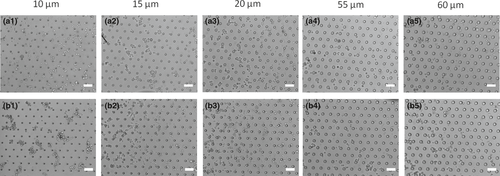
PDMS substrates containing 10–30 μm diameter micropillars of 55 μm in height were fabricated by photolithography and soft lithography (see Figure S1 for more details). The diameter values were chosen based on the PC3 cell average diameter (~30 μm), enabling to explore different diameter:cell ratios, as shown in Table S1. The micropillars were disposed hexagonally on the substrates surface, maintaining a space of 100 μm between them. SEM images were taken with a LEO Electron 440i Microscope (Cambridge, England) to ensure the adequate substrate fabrication under the required conditions.
2.4 Polyelectrolyte solutions
PEI and HA were dissolved in ultrapure water (resistivity of 18.2 MΩ.cm at 25°C) to prepare 0.1% (w/v) solutions. CHI was dissolved in acetic acid (100 mM) and also stirred for 24 h to prepare a 0.1% (w/v) solution. NaCl was added to adjust the ionic strength of the polyelectrolyte solutions to 100 mM. All of the solutions were stirred for 24 h. The pH of the PEI, CHI and HA solutions was adjusted to 4.0, 3.0 and 3.0, respectively.
2.5 Substrate coating through LbL
Before the coating process, all the substrates were treated with oxygen plasma to promote the formation of silanol groups on substrate surface. A PEI pre-coating was applied by dipping the substrates on PEI solution during 15 min. The pre-coated substrates were then immersed repeatedly and alternatively in CHI and HA solutions, 10 min during each step, separated by 3 rinse steps with Milli-Q water (pH = 3.0) for 2, 1 and 1 min, respectively. This cycle was repeated in order to obtain 3.5 bilayers. The whole process was executed in an automatic dipping equipment LbL Nanostructure Pro (ECSIA NanoScience).
2.6 Physicochemical characterization
2.6.1 Atomic force microscopy (AFM)
AFM analysis was carried out in a MultiMode™ SPM microscope (Digital Instruments) operated in the tapping mode at room temperature and humidity of approximately 5%. AFM images (5 × 5 µm2) of all samples were taken in triplicate. All data and images analyses were performed using Gwyddion open software.
2.6.2 Contact angle
In order to assess the wettability of the surfaces, the sessile drop method was conducted by applying Milli-Q water droplets of 8 µl on the samples surface. The contact angle was measured in sextuplicate during the first 10 s using an Easy Drop DSA150 goniometer (Krüss).
2.6.3 Ultraviolet–visible (UV-vis) spectroscopy
To determine the availability of free carboxylic groups of HA on the films, PDMS substrates functionalized with HA/CHI films were dipped in Alcian Blue (0.001 M, pH 3.0) solution for 15 min. The samples were then rinsed in ultrapure water twice for 2 min each. Subsequently, dried substrates under room condition were analysed using a plate reader (Varioskan Lux, Thermo Scientific) in the absorbance mode. Alcian Blue peak was measured at 617 nm.
2.7 Determination of roughness parameters
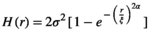 (1)
(1)2.8 Cell adhesion assays
PC3 cells cultures were maintained at 37°C with 5% CO2 in HAM-F12K media containing 10% foetal bovine serum and 1% streptomycin/penicillin. Before the adhesion assays, the two types of substrates produced in this work were treated for 15 min with UV-light. The substrates were also incubated with 400 µl of SFB for 12 h and washed extensively with sterile Milli-Q water before being used in the adhesion assay. Subsequently, suspensions containing 4.85 × 104 cells were pipetted directly onto both samples. The cell adhesion assays were carried out for 3 h, as previously described (Rocha Neto et al., 2019). After, only the nano-roughened PDMS substrates were gently rinsed in DPBS Flush buffer pH 7.4 to remove non-adherent cells. The adhered cells were fixed in 2% (w/v) paraformaldehyde for 15 min at room temperature and washed with DPBS. After, the nucleus of the adhered cells was stained with DAPI (1:1000) for 30 minutes and rinsed in DPBS Flush buffer. Cell adhesion on both samples was assessed by using an Axio Observer.Z1 Zeiss inverted optical microscope (Carl Zeiss AG), and the images were treated by using the ImageJ software.
3 RESULTS AND DISCUSSION
3.1 Substrate physicochemical characterization
Aiming to improve their cell adhesion capability, PDMS substrates with different levels of roughness were prepared. These nano-roughened surfaces were characterized by means of atomic force microscopy, and the obtained data were used to determine some roughness parameters.
3.1.1 Bare nano-roughened PDMS substrates
Topographical data obtained through AFM (Figure 1a) showed that the surfaces differ significantly depending on the etching process. The range of superficial heights displayed by samples PDMS50, PDMS55 and PDMS60 and their topographic pattern suggest that they possess higher values of roughness parameters than the PDMS0 and PDMS25 samples.
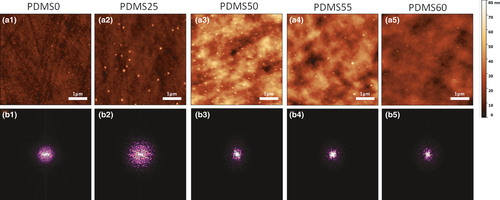
The histograms of superficial height plotted with AFM data all fit a Gaussian distribution model, which indicates that the topography is distributed randomly throughout the substrate (Figure S2A). The power spectra of the PDMS samples show that they are all circularly symmetric, meaning that the surfaces are isotropic (Figure 1b).
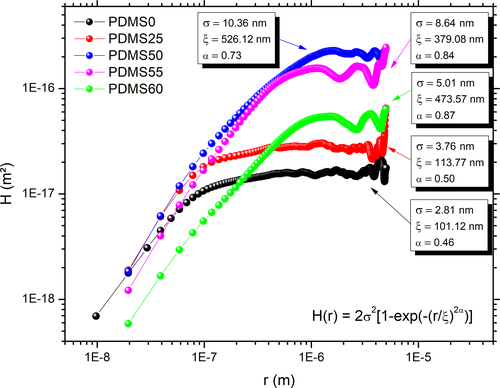
The data obtained through AFM analysis allowed the plotting of the height–height correlation curve and hence the calculation of surface roughness parameters: root mean square surface roughness (σ), lateral correlation length (ξ) and Hurst exponent (α), displayed in Figure 2. As expected from AFM images, the samples that underwent stronger chemical etching (PDMS50, 55 and 60) presented higher surface roughness parameters than the untreated one and the PDMS20 sample. However, the etching agents did not form jagged surfaces, as the untreated substrate is the one with the lower roughness exponent, which measures the short-range roughness (Krim & Indekeu, 1993), meaning it is the least smooth sample.
All the samples displayed statistically equal contact angles (Figure S3), meaning that the roughing of the surfaces through chemical etching was not capable of changing the wettability properties of PDMS. Similar results were found by Xue and coworkers, who varied acetone concentration during the etching process and were not able to alter the wettability of the material (Xue et al., 2018). Such results can be due to the intrinsic high hydrophobicity of the material (Ai et al., 2002).
3.1.2 Nanostructured PDMS chips
Considering that topography can strongly affect the cell adhesion mechanism on surfaces (Guo et al., 2017), we explored the influence of different diameter micropillars in PDMS substrates on the tumour cell capture. These samples were functionalized with 3.5 multilayers of hyaluronic acid (HA) and chitosan (CHI) by layer-by-layer method under specific assembly conditions as a strategy to induce cell adhesion, as recently reported by Rocha Neto and coworkers (Rocha Neto et al., 2019, 2020). To illustrate adequate film assembly, this section monitors the films physicochemical properties assessed by atomic force microscopy, UV-visible absorption and water contact angle analyses.
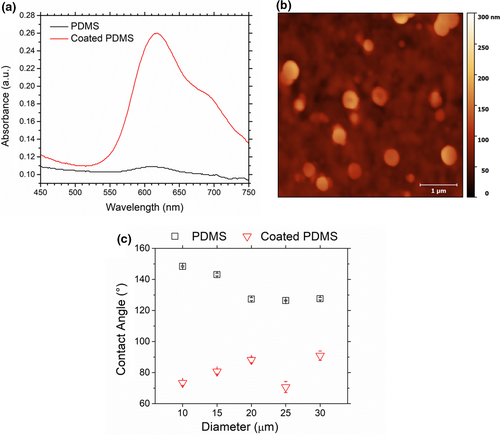
The deposition of 3.5 bilayers of HA/CHI on PDMS substrate promoted a significant increase in the Alcian Blue (AB) absorbance of the substrate, as shown in Figure 3a. This result is a relevant evidence of the successful functionalization since the adsorption of AB suggests the presence of free carboxyl groups of HA on the substrate surface. Indeed, the AB dye has been widely employed to track presence of HA and, consequently, to provide information about the film assembly (Hernández-Montelongo et al., 2016; Vasconcellos et al., 2010). AFM image in Figure 3b indicates the presence of pronounced polymeric islets, which is characteristic of the assembly of weak polyelectrolyte-based films (Picart et al., 2002). In addition to the film roughness, it was possible to determine its thickness by using the AFM analysis. The HA/CHI film presented an average thickness of 29.2 ± 2.7 nm (see Figure S4 for more information), which is also in agreement with previous work that covered PDMS substrates with biocompatible ultrathin films via layer-by-layer (Ai et al., 2002). The contact angle measurements for all PDMS substrates containing different diameter micropillars are displayed in Figure 3c. The HA/CHI film deposition onto the PDMS substrates decreased the water contact angles for all samples, indicating an increase in material's wettability, which can be attributed to the intrinsic high hydrophilicity of the HA molecule (Fallacara et al., 2018). The same trend was reported by Hernandez-Montelongo and coworkers that developed HA/CHI films with a strong hydrophilic behaviour (Hernandez-Montelongo et al., 2016), reinforcing that the PDMS surface modification was successfully accomplished.
3.2 Cell adhesion assays
3.2.1 Nano-roughened PDMS substrates
Substrates with higher roughness (PDMS 50, 55 and 60) displayed more capability to adhere cells, as it can be seen from Figure 4. This behaviour was expected as the same samples adsorbed more foetal bovine serum at the incubation step of the process. Such increase in adhesion/adsorption is due to the fact that cells/molecules have to rearrange geometrically to adapt to rough PDMS surfaces (Rechendorff et al., 2006; Vlachopoulou et al., 2009).
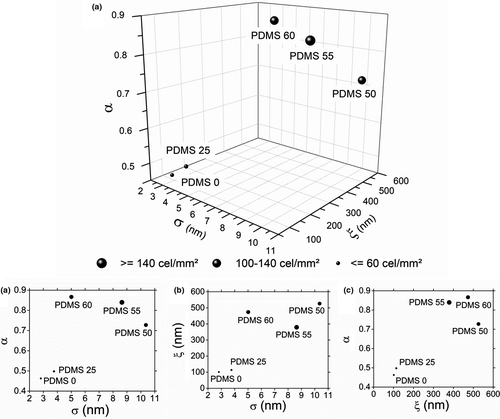
Observing Figure 4a it is possible to see a grouping of points in the upper-right position of the graphs, showing that the PDMS samples with higher roughness parameters also were the ones that adhered the most cells/mm2. Because of Figure 4d, where the points on the X-axis were more spaced than when comparing with the other parameters, the Hurst exponent was thought to be a significant predictor of PC3 cell adhesion. Bright field microscopy images of adhered cells for all samples are shown in Figure S2B. These results corroborate with the ones found by a study developed by Rocha Neto and coworkers, where smoother surfaces also resulted in higher PC3 cell adhesion (Rocha Neto et al., 2020).
In order to assess the statistical significance of α and the other parameters, an experimental processing was realized and a linear regression model was adequate (p-value <.1) to fit the data (Table S2). Also, the Hurst exponent was confirmed to be the most significant factor, with a p-value of .09, having the most impact on cell adhesion.
3.2.2 Nanostructured PDMS substrate
The cell adhesion results for PMDS substrates before and after LbL functionalization are shown in Figure 5. The prominent circular morphology of PC3 cells observed on uncoated substrates (Figure 5a) suggests that there was no cell adhesion in these samples, which can be attributed to both the high hydrophobicity and the low surface energy of the PDMS. On the other hand, the typical spread morphology of adhered cells assumed by PC3 cells when in contact with functionalized PDMS (Figure 5b) suggests that these samples are promising platforms to promote the tumour cell capture. Furthermore, our results suggest that decreasing the diameter of the micropillars increases the cell adhesion performance, as shown in Figure 5b2,b3. The improved cell-substrate adhesion properties of the functionalized PDMS may be associated with their greater wettabilility as indicated by water contact angle measurements. Shi and coworkers described the same trend, in which PDMS substrates had their wettability increased when treated with poly (dopamine), increasing the spreading of MG-63 cells on their surface (Shi et al., 2015). The presence of HA molecules on the surface of functionalized samples not only improves their wettability as discussed before, but also provides the specific binding mediator for establishing the interaction with CD44 receptors. CD44 is a well-known surface receptor for HA, and it has been used as a biomarker for prostate cancer progression since it is broadly distributed on the cell surface and commonly overexpressed in many tumour cells (Liu et al., 2011; Patrawala et al., 2006). Thus, these results highlight the use of the specific CD44-HA interaction as a promising strategy to induce the tumour cell adhesion on surfaces, reinforcing the findings recently reported in the literature (Rocha Neto et al., 2020). Furthermore, these findings also highlight lower diameter micropillars as the best condition to produce improved devices for cell adhesion, opening up new possibilities to the development of smartly designed biosensors.
4 CONCLUSIONS
The experiments carried out throughout the execution of this study were able to produce PDMS substrates of different roughness and topography profiles, as it was shown by AFM analysis. Nano-roughened PDMS surfaces developed by chemical etching displayed distinct behaviour regarding PC3 cell adhesion. Bigger concentrations of the etching agent resulted in bigger values of roughness exponent (α), which was the most statistically significant factor influencing cell adhesion. The less jagged the PDMS surfaces (presenting higher roughness exponents: α ~ 1) the higher was the cell density adhered to the substrates. The functionalization of PDMS substrates with HA/CHI multilayers by the layer-by-layer method increased their wettability, roughness and, consequently, their cell capture potential. In addition to the changes in the mentioned surface properties, the improved cell-substrate adhesion properties can also be attributed to the presence of HA chains on PMDS substrates, as indicated by their significant Alcian Blue absorbance. HA molecules interact with CD44 extracellular receptors, acting as a direct mediator of the cell adhesion mechanism. Exploring the specific CD44-HA interaction, our results serve as a proof-of-concept that PDMS substrates functionalized with HA/CHI films have a promising potential to develop smartly biosensors for cell detection. Furthermore, with the understanding about the influence of roughness parameters on the cell adhesion it will be possible to modify the surface properties of materials unfavourable for cell adhesion as the PDMS for the development of cell immobilization devices.
5 ACKNOWLEDGMENT
The authors acknowledge the financial supports given by National Council for Scientific and Technological Development - CPNq (147536/2016-2, 147443/2017-2 and 114307/2020-2) and São Paulo Research Foundation - FAPESP (2016/19976-6) in this particular research field. The authors also acknowledge the Brazilian Nanotechnology National Laboratory (LNNano/CNPEM), Institute of Science and Technology on Photonics Applied to Cell Biology and Department of Materials and Bioprocess Engineering for their consistent support and guidance throughout this work.
CONFLICT OF INTEREST
The authors declare no conflict of interest.




