Local Values and Data Empower Culturally Guided Ecosystem-Based Fisheries Management of the Wuikinuxv Bear–Salmon–Human System
Abstract
Despite numerous examples of ecosystem-based fisheries management (EBFM) addressing tradeoffs between ecological and commercial fishery interests, local social and cultural concerns are less frequently considered. We illustrate how Indigenous fishery harvest goals and data from locally driven wildlife research can inform EBFM, guided by cultural values of respect for and reciprocity with wildlife. Grizzly bears Ursus arctos horribilis hold particular importance for the Wuikinuxv First Nation in Rivers Inlet, British Columbia, where people and bears have coexisted as consumers of Sockeye Salmon Oncorhynchus nerka for millennia. The region’s valuable commercial fishery, active since the late 1800s, has been closed since the Sockeye Salmon population collapsed in the mid-1990s, but the Wuikinuxv maintain a modest food, social, and ceremonial (FSC) harvest. To address questions about balancing the needs of fishers and ecosystem recipients, we quantified tradeoffs between long-term maximum sustainable fishery yield and ecosystem benefits (for which bear density served as a proxy). We fitted age-structured state-space spawner–recruitment models and estimated relationships among spawner abundance, long-term fishery yields, and relative bear densities in time periods before and after the population collapse. We found that predicted maximum postcollapse bear density was 74% of maximum precollapse densities. We estimated a 94% decline in Sockeye Salmon equilibrium population size (from ˜3,115,000 to 200,000), resulting in a commensurate decline in maximum sustainable fishery yield. Despite this, we showed that Wuikinuxv FSC harvest goals are compatible with an EBFM target that seeks also to sustain relatively high bear densities, whereby relative fishery yield and bear density are reduced about 10% from their respective maxima, assuming that current Sockeye Salmon productivity and carrying capacity remain similar for the foreseeable future, although these estimates are highly uncertain. Collectively, our findings illustrate how EBFM can apply interdisciplinary approaches that draw not only on fisheries ecology but also local values to design sustainable harvest strategies for diverse beneficiaries.
Moving beyond a single-species focus, ecosystem-based fisheries management (EBFM) aims to sustain healthy ecosystems and resource use by balancing costs and benefits for ecological, economic, and social well-being. Whereas conventional fisheries management has often focused on maximizing the long-term commercial catch of single species, EBFM seeks to address cumulative impacts and tradeoffs among the different ecological and socioeconomic beneficiaries of fisheries (Pikitch 2004; Collie et al. 2016; Marshall et al. 2017). However, the representation of socioeconomic recipients in EBFM is typically limited to commercial interests (Marasco et al. 2007; Richerson et al. 2010), despite the presence of local subsistence and cultural harvesters who also rely on fisheries (Link 2010; Cinner et al. 2020).
The exclusion of local peoples from contemporary fisheries management often leaves communities disenfranchised and marginalized. Consequently, although ecosystem-based management strategies can incorporate ecosystem considerations into fisheries, implementation can be challenged by a lack of engagement or buy-in from local socioeconomic and cultural user groups (Read and Hartley 2006; Loring 2013; Bennett 2018). Accordingly, EBFM may have a greater chance of sustainable harvest for all beneficiaries if designed, incentivized, and governed by relevant place-based communities and the social capital embedded in them (Gutiérrez et al. 2011; Cox et al. 2015; Ban et al. 2017). As such, there is increasing recognition of the benefits of incorporating the values, knowledge systems, and decision-making agency held by local peoples to guide EBFM (Armitage 2005; Long et al. 2015; Pinkerton 2018; Okamoto et al. 2020; Cochrane 2021).
Pacific salmon Oncorhynchus spp. are vital to the cultures and economies of many local peoples. Indigenous people in the Pacific Northwest harvest salmon for food, social, and ceremonial (FSC) purposes as participants in complex, salmon-driven socioecological systems (Brown and Brown 2009; Campbell and Butler 2010; Housty et al. 2014). Common to Indigenous stories, songs, and dances from the region is respect for the plants and animals that also depend on salmon (Turner et al. 2013; Thornton et al. 2015; Artelle et al. 2018). Salmon have also supported commercial fisheries that are vital to settler and Indigenous economies of the Pacific Northwest. However, FSC and commercial harvests have waned due to declines in many salmon populations, which have been caused by overfishing (Slaney et al. 1996; Healey 2009; Price et al. 2017; but see Walters et al. 2018), habitat degradation (Waples et al. 2009), and changing marine conditions (Finney et al. 2000; Peterman and Dorner 2012).
The need for novel approaches to manage salmon for diverse beneficiaries is apparent in the Pacific Northwest. Ecosystem-based fisheries management requires not only quantitative methods but also political will to guide policies and implementation (Collie et al. 2016), including approaches that are guided by collaboration with Indigenous governments and local stakeholders. Canada’s Wild Salmon Policy (DFO 2005) acknowledges the need for ecosystem-based salmon management, including managing salmon for other, nonhuman consumers (Price et al. 2017; Walsh et al. 2017). Implementation for the Wild Salmon Policy is being supported through Canada’s revised Fisheries Act (2019), which includes provisions that Indigenous knowledge be considered in fisheries management decisions as part of a nation-to-nation process. A principal tenet of the Wild Salmon Policy is the guarantee of Indigenous fisheries harvest, second only to salmon conservation, followed by commercial harvest (DFO 2005). Concurrently, provincial and federal agencies in Canada are increasingly recognizing Indigenous rights and authority not only to access resources (e.g., R. v. Sparrow 1990; Tsilhqot’in Nation v. British Columbia 2014) but also to govern resources within their territories (e.g., Housty et al. 2014; Coastal First Nations et al. 2016; Ban and Frid 2018). We also note that from the perspective of many Indigenous Nations, authority to govern territories was never relinquished. Whereas federal agencies responsible for the management of salmon and other species are increasingly overextended (Marshall et al. 2017; Price et al. 2017), the potential for locally driven monitoring and governance of salmon by Indigenous governments—in this case supported by federal implementation of the Wild Salmon Policy (DFO 2005) and the Fisheries Act (2019)—creates a promising opportunity for management that can incorporate local values, governance, and ecosystem-based approaches (Atlas et al. 2021).
Grizzly bears Ursus arctos horribilis are well suited as a focal ecosystem indicator for the EBFM of salmon. Like local Indigenous harvesters, bears are terminal predators that consume salmon in their final spawning life history phase. Sufficient spawner abundances for bear populations may indicate adequate abundance for ocean-based consumers, such as seabirds, pinnipeds, and sharks, which access salmon before the fish become available to bears (Levi et al. 2012). Bears are a main vector of marine-derived nutrients from salmon carcasses into surrounding ecosystems (Hilderbrand et al. 1999; Quinn et al. 2009) that benefit many other species (Schindler et al. 2003; Hocking and Reimchen 2009; Hocking and Reynolds 2011; Wagner and Reynolds 2019). Accordingly, the productivity of grizzly bear populations can be considered a surrogate for salmon-influenced ecosystem function (Levi et al. 2012; Van Daele et al. 2013). Salmon-subsidized populations of bears are denser (Hilderbrand et al. 1999) and have a greater influence on their surrounding ecosystems (Harrer and Levi 2018; Shakeri et al. 2018). Grizzly bears also contribute to a growing coastal ecotourism economy for communities transitioning away from extractive industries (Clark et al. 2021). Finally, the charismatic appeal of a flagship species of conservation concern makes the grizzly bear a strong candidate species to motivate change in fisheries management.
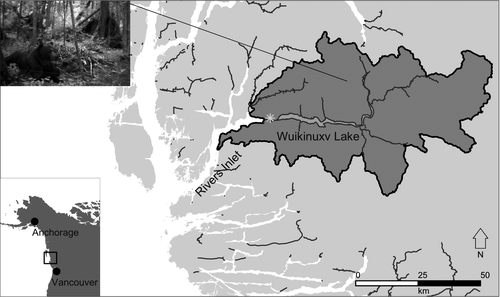
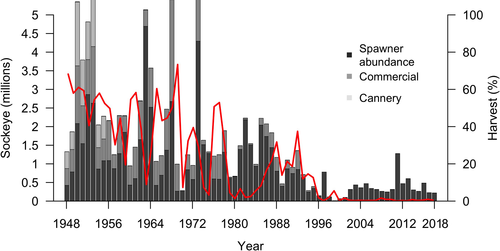
In the region of Wuikinuxv Territory (Owikeno or Rivers Inlet, British Columbia; Figure 1), people have harvested Pacific salmon alongside bears Ursus spp. for millennia. Bears are recognized by the Wuikinuxv Nation and its people not only as important ecologically but also as close cultural relatives that share similar resources on the landscape (Henson et al., in press; E. Windsor and F. Hanuse, Elders from Wuikinuxv Nation, personal communication). Rivers Inlet Sockeye Salmon O. nerka were historically abundant, with upwards of 3 million fish returning to the system each year (Groot and Margolis 1991; Cox-Rogers and Sturhahn 2005), thus supporting one of the largest commercial fisheries in British Columbia (Walters et al. 1993; McKinnell et al. 2001). In the mid-1990s, the Sockeye Salmon population collapsed, with fewer than 10,000 fish returning in 1999 (Figure 2; McKinnell et al. 2001). The decline and eventual collapse of the Sockeye Salmon population have been attributed to a legacy of overfishing and shifting environmental regimes (McKinnell et al. 2001; Shortreed and Morton 2003; Cox-Rogers and Sturhahn 2005; Ainsworth et al. 2011), leading to reduced food security for bears (Boulanger et al. 2004). Sharp increases in bear–human conflict ensued, with the destruction of at least 15 starving grizzly bears in Katit (Wuikinuxv village; Figure 1) in 1999 (Hanuse, personal communication). Although modest FSC harvest has continued, varying among years, the commercial fishery in the region has been closed since the collapse.
The Wuikinuxv Nation envisions an approach to a fishery that aligns with the Nation’s values to sustain its people and provide for bears and the ecosystem. Since the closure of the commercial fishery, the Wuikinuxv Nation has worked with federal managers to develop in-season monitoring that can inform FSC harvest efforts (e.g., CPUE, sonar observations of spawners), allowing the Nation to adjust fishing effort accordingly to ensure that bears and other wildlife have allocation of salmon. As the Sockeye Salmon population has begun to increase (e.g., spawner abundances upwards of 100,000), Wuikinuxv decision makers require more information about the recovery prospects of the population and the potential impacts that increased harvests might have for bears and, by extension, the ecosystem (Wuikinuxv Stewardship Committee, personal communication; T. Walkus [Xvu’sem’da’as], Wuikinuxv Hereditary Leader, personal communication). To support this vision, the Wuikinuxv began a bear monitoring program that focuses on population monitoring and foraging behavior of bears throughout Wuikinuxv territory (2013 to present; Adams et al. 2014, 2017).
Broadly, our goal is to illustrate how local cultural values can guide scientific management of natural resources, outlining the conceptual and practical steps in which these primary dimensions of resource management can interact in EBFM and beyond. Specifically, we examine potential tradeoffs between salmon harvest by both commercial and FSC fishers and benefits to grizzly bears—and, by extension, to salmon ecosystems—in order to understand the relationship between fisheries yield and benefits to bears across a range of spawner abundances, with the intent of informing future Wuikinuxv harvest management decisions. Our efforts were guided by the Wuikinuxv Nation’s principle of ńàńakila—to watch over someone and look ahead for them; to be a guardian or a protector—as it applies to neighboring grizzly bears. We fitted an age-structured state-space stock–recruitment model to data collected before and after the Sockeye Salmon population collapsed, and we estimated the relationship between spawning salmon abundance and relative long-term fishery yield and bear densities. We then characterized tradeoffs between fishery yield and benefits to bears to consider how food security for people and bears was related during the periods before and after the collapse.
METHODS
We used a multi-stage analysis to predict how bear population density would respond to variation in spawning salmon abundance as influenced by fisheries management in the Wuikinuxv Lake watershed and adjacent watersheds (3,580 km2; Figure 1), following the general method outlined by Levi et al. (2012).
First, we characterized the Wuikinuxv Sockeye Salmon spawner–recruit relationship based on available data (spawner abundance, catch, and age composition from 1948 to 2018), as well as for the precollapse (1948–1992) and postcollapse (1993–2018) time periods. We chose to focus on these two time periods because they generally coincide with a shift from relatively high to relatively low spawner abundance (Figure 2) and were coincident with a decline in marine survival (Rutherford and Wood 2000). We acknowledge that this demarcation of periods is somewhat subjective, but our intent was to illustrate EBFM tradeoffs across periods of pronounced system change as opposed to statistically identifying the most parsimonious explanation for when and how this system has changed, which we consider to be a worthwhile but separate area of future investigation. Second, we estimated relative dietary contributions of salmon to bears in the study area (2013–2018) by using stable carbon (δ13C) and nitrogen (δ15N) isotope ratio estimation from noninvasive hair samples (n = 79) collected from individual bears (n = 51). We used these data to estimate the relationship between salmon spawner abundance (i.e., escapement) and salmon consumption by bears. Third, we used the observed linear relationship between salmon consumption by bears and bear population density (Hilderbrand et al. 1999) to estimate bear density for a given spawner abundance relative to bear density in an unfished system. Fourth, holding all other salmon species (described below) constant at their median biomass densities (postcollapse), we quantified the predicted equilibrium tradeoffs between Sockeye Salmon fishery yields and bear densities across a range of spawner abundances and fishery targets before and after the collapse.
Study Area within Wuikinuxv Territory
Wuikinuxv Lake (deep, cold, and oligotrophic; 96 km2) is located at the head of Rivers Inlet, British Columbia, draining into the inlet via the Waanukv/Wannock River (5 km), along which the village of Katit is situated (˜40 permanent residents; Figure 1). Nine salmon-bearing tributaries flow into the lake, all of which have Sockeye Salmon that spawn in them in addition to other salmon species. The FSC fishery provides Sockeye Salmon for Wuikinuxv people and their families from on and off the reserve and from neighboring communities. Wuikinuxv fishers’ efforts are generally concentrated in the Waanukv/Wannock River and not in the lake’s tributaries.
We defined the study area by using the lake’s watershed (based on the 1:50,000 third-order watershed delineation; Province of British Columbia 2018) plus all adjacent neighboring watersheds to align with average home range sizes and the mobility of grizzly bears within a season of salmon consumption (Figure 1; area = 3,580 km2, calculated in ArcMap version 10.2 [Environmental Systems Research Institute, Redlands, California]; Service et al. 2018).
Adult Sockeye Salmon migrate through the Waanukv/Wannock River and Wuikinuxv Lake to spawn in nine major tributaries (Figure 1), and juveniles are primarily lake rearing (Rutherford and Wood 2000). The tributaries and surrounding watersheds in the study area (including the Nx-ngvic/Nicknaqueet, Waanukv/Wannock, and Ćàq̌vala/Chuckwalla rivers) also support populations of Coho Salmon O. kisutch (no consistent enumeration), Pink Salmon O. gorbuscha (spawner abundance = 4,300–700,700), Chum Salmon O. keta (1,000–70,000), and Chinook Salmon O. tshawytscha (1,000–70,000). Median values of Pink, Chum, and Chinook salmon collectively comprise about 16% of all salmon biomass in the study area, with the remainder of total biomass contributions from Sockeye Salmon (ranges presented from 1993 to 2017; DFO 2016). We note, however, that historic estimates of abundance across species may be biased by changes in monitoring effort over time and are based on Sockeye Salmon abundance from the postcollapse period.
Estimating Spawner Abundance
Spawner abundance for Wuikinuxv Lake Sockeye Salmon has been annually estimated by the Department of Fisheries and Oceans Canada (DFO) since 1948 using stream walks and aerial surveys of clear tributaries and in recent years by the Wuikinuxv Nation with dual-frequency imaging sonar and adaptive resolution imaging sonar in the Waanukv/Wannock River (English et al. 2020). We used the relationship between sonar counts and estimates of spawner abundance on the spawning grounds from 2014 to 2018 to derive an expansion factor (mean = 6.29) for spawning ground surveys from 1948 to 2013 (see Supplementary Information [SI] available separately online). We excluded sonar and enumeration data for 2019 due to anomalous differences between count types (i.e., the 2019 expansion factor was ˜4 times larger than the average expansion factor; see SI), which are undergoing further scrutiny and consideration by DFO and the Wuikinuxv Nation in their application (Wuikinuxv Stewardship Director, personal communication). After applying the expansion factor, average annual reconstructed spawner abundance of Wuikinuxv Lake Sockeye Salmon from 1948 to 1992 was roughly 884,900 fish (ranging from 177,600 to 3,143,800 fish). Since the collapse in 1993, annual spawner abundance has averaged 252,200 (ranging from 7,500 to 855,100 fish).
We combined Sockeye Salmon spawner abundance with geo-referenced annual abundance estimates of the three remaining Pacific salmon species with consistent enumeration (Pink, Chum, and Chinook salmon) from the New Salmon Escapement Database (DFO 2016) to estimate total salmon biomass density assuming an average mass for each salmon species and sex (using a 1:1 sex ratio; Groot and Margolis 1991; Bryan et al. 2014). Salmon biomass density was calculated as total biomass divided by the spatial extent of the study area.
Spawner–Recruit Relationship for Wuikinuxv Lake Sockeye Salmon
Age composition
We reconstructed annual estimates of Sockeye Salmon population age composition using scale and otolith samples collected during enumeration efforts between 1960 and 2018 on the spawning grounds of Wuikinuxv Lake tributaries (Rutherford and Wood 2000; DFO, unpublished data). Age composition varied among years, with 5-year-old fish consistently the most abundant age-class, comprising on average 68% of spawners. Four-year-old fish comprised on average 31% of spawners, while 3-year-old and 6-year-old fish comprised 0.01% and 0.004% of spawners, respectively. For our spawner–recruit modeling, we applied the average age composition to years that lacked age composition data (1948–1959, 1968, 1969, 1976, 1977, and 1982). We note that a subset of the age composition estimates comes from the proportions per age-class reported by Rutherford and Wood (2000), which lacked counts associated with the estimates (1960–1967, 1970–1975, 1976–1981, 1983–1986).
Harvest reconstruction
We reconstructed Wuikinuxv Lake Sockeye Salmon harvest from databases maintained by the Wuikinuxv Nation and DFO. Commercial fishery harvests from 1948 through 1996 were reported by Rutherford and Wood (2000). We updated these harvest records to include Wuikinuxv FSC catch (1964–2018; D. Rolston, Wuikinuxv Fisheries Manager, personal communication). For years that lacked FSC catch data, we applied a mean FSC harvest rate from years with data to estimate FSC harvests (mean rate = 1% capped at 10,000 rather than a fixed harvest estimate to reflect Wuikinuxv FSC harvesting practices; Rolston, personal communication). Prior to the collapse in 1993, commercial harvest rates ranged from 2% to 80%, with an estimated average of 40%. After the collapse and closure of the commercial fishery in 1996, reported FSC harvest rates constituted 1% or less of the total run size (Figure 2).
Estimating the spawner–recruitment relationship
We used an age-structured state-space spawner–recruit model to estimate the population characteristics of Wuikinuxv Sockeye Salmon. This approach allows for temporal variability in productivity and age at maturity, process variation, and observation error (Fleischman et al. 2013; Connors et al. 2019; Staton et al. 2020), as well as more realistic incorporation of multiple sources of uncertainty than traditional spawner–recruit models that are fitted to salmon data. We fitted this model across the entire time series to quantify changes in productivity over time assuming a constant magnitude of density dependence (1948–2018; Figure 2) and then again for two truncated time series prior to (1948–1992) and following (1993–2018) the collapse (Figure 3). Equations (1)–(7) provided below closely follow the model description in Fleishman et al. (2013).
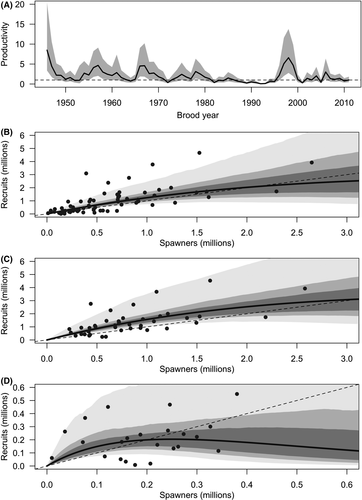
 + exp[residuals]) with 95% credible intervals from the Bayesian state-space age-structured model over the full time series (1948–2018). Relationships between recruitment and spawner abundance of Wuikinuxv Lake Sockeye Salmon for (B) 1948–2018, (C) precollapse years (1948–1992), and (D) postcollapse shift years (1993–2018) are also shown. The dashed black line is the 1:1 replacement line. Gray polygons are the 50th (dark gray), 75th (medium gray), and 95th (light gray) credible intervals predicted between spawner abundance and recruitment based on 1,000 random draws from the posterior distributions of the spawner–recruit relationship to illustrate uncertainty. Note the difference in magnitude between the y-axes in panels C and D.
+ exp[residuals]) with 95% credible intervals from the Bayesian state-space age-structured model over the full time series (1948–2018). Relationships between recruitment and spawner abundance of Wuikinuxv Lake Sockeye Salmon for (B) 1948–2018, (C) precollapse years (1948–1992), and (D) postcollapse shift years (1993–2018) are also shown. The dashed black line is the 1:1 replacement line. Gray polygons are the 50th (dark gray), 75th (medium gray), and 95th (light gray) credible intervals predicted between spawner abundance and recruitment based on 1,000 random draws from the posterior distributions of the spawner–recruit relationship to illustrate uncertainty. Note the difference in magnitude between the y-axes in panels C and D.Process model
 ) from 1948 to 2018 were treated as unobserved states and modeled as a function of spawner abundance (
) from 1948 to 2018 were treated as unobserved states and modeled as a function of spawner abundance (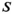 ), assuming a Ricker (1954) spawner–recruit relationship as is typically used in stationary modeling of Pacific salmon populations (Fleischman et al. 2013), with serially correlated lognormal process variation:
), assuming a Ricker (1954) spawner–recruit relationship as is typically used in stationary modeling of Pacific salmon populations (Fleischman et al. 2013), with serially correlated lognormal process variation:
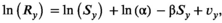 ((1))
((1))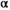 is productivity (intrinsic rate of growth or recruits per spawner at low population size),
is productivity (intrinsic rate of growth or recruits per spawner at low population size), 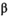 is the magnitude of within-brood year density-dependent effects, and
is the magnitude of within-brood year density-dependent effects, and 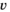 reflects interannual variation in recruitment, which was assumed to be correlated (
reflects interannual variation in recruitment, which was assumed to be correlated ( ) over time:
) over time:
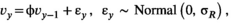 ((2))
((2))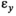 is independent, normally distributed process variation in survival, with an SD of
is independent, normally distributed process variation in survival, with an SD of 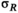 .
.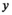 and at age
and at age 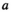 was the product of the total return in year
was the product of the total return in year 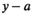 and the proportion of fish from brood year
and the proportion of fish from brood year 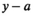 that returned at age
that returned at age 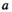 :
:
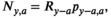 ((3))
((3))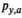 is the probability that a fish spawned in brood year
is the probability that a fish spawned in brood year 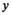 will mature at age
will mature at age 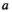 . We modeled brood year variation in maturity schedules as Dirichlet random vectors drawn from a common hyperdistribution characterized by a mean maturation-at-age probability vector (
. We modeled brood year variation in maturity schedules as Dirichlet random vectors drawn from a common hyperdistribution characterized by a mean maturation-at-age probability vector (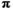 ) and an inverse dispersion parameter (
) and an inverse dispersion parameter (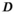 ):
):
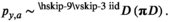 ((4))
((4))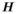 ) in a given year (
) in a given year (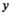 ) was then modeled as the product of the total run size and the harvest rate (
) was then modeled as the product of the total run size and the harvest rate (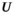 ) experienced:
) experienced:
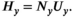 ((5))
((5))Data and observation model
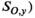 and
and 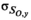 , with the CVs converted to lognormal variance (Forbes et al. 2011):
, with the CVs converted to lognormal variance (Forbes et al. 2011):
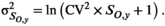 ((6))
((6))We had no direct quantitative estimates of observation error in harvest from the data reported by Rutherford and Wood (2000), but because commercial catch data in this area are considered to be of relatively high precision, we assumed a CV of 0.15 for years of reported commercial catch, which is similar to estimates applied in other systems (e.g., Fleischman et al. 2013). For the years in which cannery pack records contributed to commercial catch (1948–1956), we specified a large observation error (CV of 0.9) to account for the added uncertainty associated with deriving estimates of catch from cannery data. For years in which FSC harvest was the only reported harvest, we assumed a large observation error (CV of 0.90 for subsistence harvest as in Fleischman et al. 2013). We assumed that harvest observations were lognormally distributed with parameters ln (Ho,y) and σHo,y, with the CV converted to lognormal variance as per equation (6).
Age composition by return year was assumed to be observed with error. Sample sizes in multinomial distributions for fisheries composition data are typically down-weighted to an “effective” sample size (ESS) to account for correlations among fish within a given sample. For this reason, for years with age and count data (see above), we re-scaled annual age composition sample sizes to the maximum number of annual age samples across all years (n = 995) and then again to 200. For years in which age data were available but lacked corresponding sample sizes, we assumed an ESS of 100. For years in which age composition estimates were unavailable, we used the weighted average proportions from available years as the observed age-class proportions and we assumed an ESS of 25 to reflect the higher degree of uncertainty in age composition during those years (pre-1960 and post-1999). In sensitivity analyses, we explored the consequences of these ESS specifications by reducing the maximum ESS to 50 (Table S3 available in the SI).
Model fitting
We fitted the model (equations (1)-(6)) for each time series in a Bayesian estimation framework. We constrained the 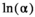 prior to follow a uniform distribution between 0 and 3 (Table 1). We used a vague
prior to follow a uniform distribution between 0 and 3 (Table 1). We used a vague 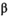 prior centered on the carrying capacity of the system (as estimated by Shortreed et al. 2001), with an SD of 0.3 (Table 1). Joint posterior probability distributions for all unknowns in the model were generated using a Markov chain–Monte Carlo approach via the JAGS sampler in R (Plummer 2017; R Development Core Team 2018). We ran six chains for 300,000 iterations after a burn-in of 50,000 and thinned every 15th iteration. Convergence was assessed by examining the potential scale reduction factor (
prior centered on the carrying capacity of the system (as estimated by Shortreed et al. 2001), with an SD of 0.3 (Table 1). Joint posterior probability distributions for all unknowns in the model were generated using a Markov chain–Monte Carlo approach via the JAGS sampler in R (Plummer 2017; R Development Core Team 2018). We ran six chains for 300,000 iterations after a burn-in of 50,000 and thinned every 15th iteration. Convergence was assessed by examining the potential scale reduction factor (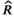 ) and was assumed to have occurred if
) and was assumed to have occurred if 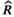 was less than 1.1 (Gelman and Rubin 1992).
was less than 1.1 (Gelman and Rubin 1992).
| Parameter | Prior | Posterior | ||
|---|---|---|---|---|
| 50th percentile | 2.5th percentile | 97.5th percentile | ||
| Precollapse (SEQ = 3,114,921; SMSY = 1,413,154) | ||||
|
˜Uniform(0,3) | 0.662 | 0.163 | 1.365 |
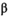
|
˜Normal(4.18 × 10−6, 0.3a) | 2.13 × 10−7 | −1.50 × 10−8 | 5.99 × 10−7 |
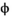
|
˜Uniform(−1, 1) | 0.593 | 0.216 | 0.905 |
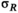
|
˜Uniform(0, 100) | 0.498 | 0.197 | 0.914 |
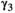
|
˜Dirichlet(0.25, 0.25, 0.25, 0.25) | 0.016 | 0.009 | 0.027 |
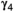
|
0.312 | 0.274 | 0.353 | |
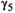
|
0.662 | 0.617 | 0.701 | |
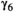
|
0.01 | 0.005 | 0.019 | |
| Postcollapse (SEQ = 199,556; SMSY = 88,666) | ||||
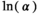
|
˜Uniform(0, 3) | 0.796 | 0.051 | 2.300 |
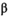
|
˜Normal(4.18 × 10−6, 0.3a) | 3.99 × 10−6 | 4.27 × 10−7 | 1.05 × 10−5 |
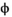
|
˜Uniform(−1, 1) | 0.449 | −0.024 | 0.854 |
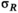
|
˜Uniform(0, 100) | 0.994 | 0.408 | 1.711 |
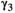
|
˜Dirichlet(0.25, 0.25, 0.25, 0.25) | 0.027 | 0.016 | 0.046 |
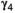
|
0.322 | 0.276 | 0.369 | |
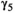
|
0.641 | 0.589 | 0.687 | |
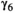
|
0.09 | 0.004 | 0.019 | |
- a In SD units.
Estimating Relative Fishery Yield
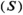 , following Levi et al. (2012):
, following Levi et al. (2012):
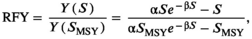 ((7))
((7)) and density dependence
and density dependence 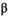 (equation 1); and
(equation 1); and 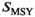 is the spawner abundance predicted to maximize long-term yield from the system under equilibrium conditions (Hilborn 1985). We estimated RFY under the pre- and postcollapse scenarios (Tables 1, S2).
is the spawner abundance predicted to maximize long-term yield from the system under equilibrium conditions (Hilborn 1985). We estimated RFY under the pre- and postcollapse scenarios (Tables 1, S2).Estimating Salmon Consumption by Bears and Relative Bear Density
We used hair samples from individual grizzly bears collected in the study area to estimate individual salmon consumption and subsequent population density (Figure 1; survey methods detailed by Adams et al. 2017). Considering the spatial distribution of salmon-bearing watersheds in the area and the mobility of grizzly bears, we used the watershed region of Wuikinuxv Lake and all adjacent neighboring watersheds to comprise the study area (Figure 1; Service et al. 2018). We collected hair samples from 23 noninvasive hair snag stations that sampled habitable regions from shore to alpine environments throughout the study area in May and June of 2013–2018 (>575 km2; Adams et al. 2017). Because collection occurred during the shedding phase of the annual molt (May–June), we assumed that the stable isotope ratios in samples represented diet assimilated in the previous year during the hair growth stage (˜June–October; Hilderbrand et al. 1996; Deacy et al. 2018).
Noninvasive hair collection from coastal grizzly bears was approved by the Animal Care Committee at the University of Victoria (Permit 2012-018), following applicable requirements concerning animal care and wildlife research by the Canadian Council on Animal Care (Sikes and Gannon 2011; Field et al. 2019). We also obtained a permit (Number 106703) from British Columbia Parks to sample in conservancies.
Estimating dietary contributions
We estimated the relative annual proportion of salmon in the grizzly bear diet from stable isotope analysis (SIA) using the same approach described by Adams et al. (2017). We performed SIA on hair samples from 79 detections of grizzly bears (n = 51 individuals, some sampled multiple times across years). We prepared and processed hair samples and isotope ratio estimation using established SIA procedures (full details in SI). We estimated annual dietary contributions from predetermined food groups (see below; Table S4) by using Bayesian mixing models via MixSIAR in R (Stock and Semmens 2016). These models use the δ15N and δ13C isotope ratios in consumer and food resource samples, trophic discrimination values, and associated uncertainties to predict the relative proportion of a diet comprised of given food types (Moore and Semmens 2008; Ben-David and Flaherty 2012; Hopkins and Kurle 2016). We modeled annual proportional contributions by the following food groups: terrestrial meat (i.e., ungulates), Pacific salmon (the five main species, collectively considered as one salmon group), intertidal food sources, and plants (Table S4). We used the posterior median contribution from salmon to the yearly diet (hereafter, “salmon consumption”) in subsequent analyses (Adams et al. 2017).
Estimating relative bear density
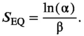 ((8))
((8))We assumed that long-term spawner abundance at equilibrium would not be affected by the potential for depensatory effects of bear predation, based on the high quality of spawning habitat and the productive nature of Sockeye Salmon, even at low population densities (Quinn et al. 2014).
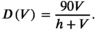 ((9))
((9))We tested the relationship for the saturation curve (equation 9) with multi-year population salmon consumption means and salmon biomass density from 18 grizzly bear populations in British Columbia, as reported by Levi et al. (2012; details in SI, Figure S2).
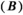 was estimated by linking equation (9) with a linear relationship between the proportion of meat in the annual diet and bear population density, assuming a zero intercept (Hilderbrand et al. 1999; Levi et al. 2012). We assumed that the vast majority of meat in the study area was comprised of salmon (Adams et al. 2017). The bear density for a given spawner abundance was therefore
was estimated by linking equation (9) with a linear relationship between the proportion of meat in the annual diet and bear population density, assuming a zero intercept (Hilderbrand et al. 1999; Levi et al. 2012). We assumed that the vast majority of meat in the study area was comprised of salmon (Adams et al. 2017). The bear density for a given spawner abundance was therefore
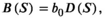 ((10))
((10))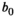 when considering relative density. Salmon in the bears’ diet saturates with salmon abundance (equation 9),
when considering relative density. Salmon in the bears’ diet saturates with salmon abundance (equation 9),
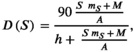 ((11))
((11))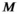 is an estimate of the median biomass of other enumerated species present in the study area from 1993 to 2018 (283,108 kg),
is an estimate of the median biomass of other enumerated species present in the study area from 1993 to 2018 (283,108 kg), 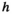 is the half-saturation parameter estimated in equation (9) (93.06 kg/km2; SI), and
is the half-saturation parameter estimated in equation (9) (93.06 kg/km2; SI), and 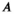 is the area of the study area. The relative bear density (RBD) can be written by combining equations (10) and (11) as
is the area of the study area. The relative bear density (RBD) can be written by combining equations (10) and (11) as
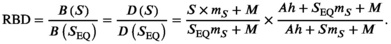 ((12))
((12))We compared maximum bear density (over a range of 0 to 1) from the pre- and postcollapse periods, where maximum bear density would be achieved in the absence of a fishery (i.e., SEQ; equation 12), assuming precollapse conditions as the denominator.
The resulting RBD and RFY were both dimensionless values that could be directly compared. Finally, we solved for an EBFM spawner abundance that aligned with Wuikinuxv values of respect for and reciprocity with bears as well as fishers, defined as RBD = RFY. This level of spawner abundance imposes equal costs to bears and people, defined by the same scaled reduction from their respective maxima.
RESULTS
We estimated the shape of the Wuikinuxv Sockeye Salmon spawner–recruit relationship for the full time series as well as prior to and following the collapse (Figure 3; Tables 1, S1). From 1948 to 1992 (precollapse), the predicted equilibrium population size was roughly 3,115,000 fish (median 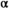 = 1.94 recruits/spawner, 95% CI = 1.18–3.92; median
= 1.94 recruits/spawner, 95% CI = 1.18–3.92; median 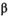 = 2.13 × 10−7, 95% CI = 1.50 × 10−8 to 5.99 × 10−7). From 1993 to 2018 (postcollapse), the predicted equilibrium population size decreased by 94% to roughly 200,000 fish (median
= 2.13 × 10−7, 95% CI = 1.50 × 10−8 to 5.99 × 10−7). From 1993 to 2018 (postcollapse), the predicted equilibrium population size decreased by 94% to roughly 200,000 fish (median 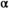 = 2.17 recruits/spawner, 95% CI = 1.05–9.72; median
= 2.17 recruits/spawner, 95% CI = 1.05–9.72; median 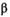 = 3.99 × 10−6, 95% CI = 4.27 × 10−7 to 1.05 × 10−6). Productivity varied over the full time series (Figure 3A; median
= 3.99 × 10−6, 95% CI = 4.27 × 10−7 to 1.05 × 10−6). Productivity varied over the full time series (Figure 3A; median 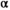 = 1.51 recruits/spawner, 95% CI = 1.04–3.11). With the reduction in carrying capacity following the collapse, the SMSY declined by 93% from roughly 1,413,000 to 89,000 fish (based on median
= 1.51 recruits/spawner, 95% CI = 1.04–3.11). With the reduction in carrying capacity following the collapse, the SMSY declined by 93% from roughly 1,413,000 to 89,000 fish (based on median 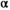 and
and 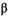 values).
values).
For those years in which we had both salmon abundance data and bear dietary data (2012–2018), we estimated how bear diet and relative population density responded to increasing salmon abundance in the study area. Male bears consumed more salmon in their annual diet than did females (proportion of salmon in annual diet: mean ± SD = 0.86 ± 0.13 for males, 0.54 ± 0.20 for females). After the Sockeye Salmon population collapse (mean salmon biomass density sampled in 1998–1999 = 77 kg/km2), the weighted mean proportion of salmon in the annual diet of bears between sexes and among sampled years was 23% (sampled in 1998–1999; Levi et al. 2012). In recent years with greater spawner abundance (mean salmon biomass density from 2013 to 2017 = 257 kg/km2), the weighted mean of annual salmon consumption was 63.6%. Consistent with these observations, predictions of salmon consumption by bears (equation 9) increased as spawner abundance increased (Figure S2). The patterns in this relationship help to explain corresponding increases in predicted bear densities with increasing spawner abundances (equation 12). We estimated that maximum bear density at the postcollapse equilibrium population size of Sockeye Salmon was 74% of bear densities predicted at the precollapse equilibrium size.
We quantified tradeoffs between RBD and RFY across a range of spawner abundances. Predicted RBD increased and saturated as spawner abundance increased (Figure 4). In contrast, expected fishery yield increased until the spawner abundance that produces SMSY and declined thereafter. We estimated the spawner abundance that corresponded to equal relative reductions in bear densities and fishery yield, which we defined as an EBFM goal. Postcollapse RBD and RFY were predicted to be reduced by about 10% from their relative maxima at an EBFM spawner abundance of roughly 120,000 (50% CI = ˜82,000–159,000; Figure 4B; Table S5), which corresponds to a surplus yield of roughly 45,000 (Figure 5). We found that under precollapse conditions, RBD and RFY were equally reduced by approximately 3% from their maxima at a spawner abundance of roughly 1,694,000 (50% CI = ˜1,154,000–2,533,000; Figure 4A; Table S5), which corresponds to a surplus yield of roughly 578,400. Changes in carrying capacity have exacerbated fishery–ecosystem tradeoffs in this system, such that the postcollapse tradeoff was 233% greater than the precollapse reduction in maximum RFY and RBD to meet EBFM goals. However, it is important to note that while the relative tradeoff was greater postcollapse, the absolute tradeoff was in fact almost an order of magnitude smaller (precollapse tradeoff with a yield difference of ˜37,000 fish versus postcollapse tradeoff with a yield difference of ˜4,400) because yield declines with declining carrying capacity.
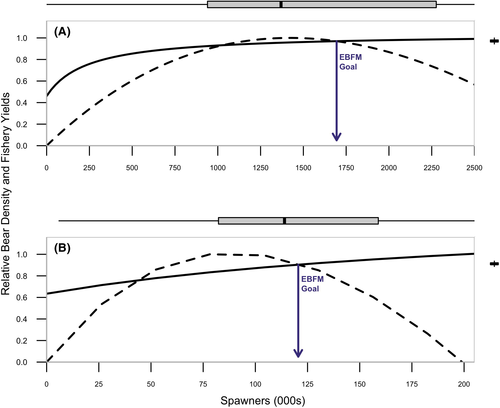
 = 1.94, 95% CI = 1.18–3.92) and (B) postcollapse (1993–2018) spawner–recruit model (median
= 1.94, 95% CI = 1.18–3.92) and (B) postcollapse (1993–2018) spawner–recruit model (median  = 2.17, 95% CI = 1.05–9.72). Under precollapse productivity, ecosystem-based fisheries management (EBFM) is achieved when RBD and RFY are equal—at a spawner abundance goal of about 1,694,000 (purple arrow; 50% CI = 115,424–2,533,468). Under postcollapse conditions, this target would be achieved at a spawner abundance of about 120,000 (purple arrow; 50% CI = 82,372–158,783). Note that biomass from other salmon species in the system was held constant per time period under both calculations of RBD, so as to compare the relative effect of the Sockeye Salmon biomass on RBD (i.e., the additional contributions of Sockeye Salmon over other available salmon biomass, represented as the y-intercept in each panel; because other salmon biomass varies between the two periods, RBD in the absence of Sockeye Salmon [the y-intercept] also varies between the two periods). Credible intervals predicted for the spawner abundance and relative tradeoff of the EBFM, based on 1,000 random draws from the posterior distributions of α and β and subsequent calculations of SMSY (spawner abundance that produces maximum sustainable yield) and SEQ (spawner abundance in the absence of a fishery), are shown as box plots (median = line within box; 50th percentiles = ends of box; whiskers = ±1 interquartile range) on the top and right side of each panel.
= 2.17, 95% CI = 1.05–9.72). Under precollapse productivity, ecosystem-based fisheries management (EBFM) is achieved when RBD and RFY are equal—at a spawner abundance goal of about 1,694,000 (purple arrow; 50% CI = 115,424–2,533,468). Under postcollapse conditions, this target would be achieved at a spawner abundance of about 120,000 (purple arrow; 50% CI = 82,372–158,783). Note that biomass from other salmon species in the system was held constant per time period under both calculations of RBD, so as to compare the relative effect of the Sockeye Salmon biomass on RBD (i.e., the additional contributions of Sockeye Salmon over other available salmon biomass, represented as the y-intercept in each panel; because other salmon biomass varies between the two periods, RBD in the absence of Sockeye Salmon [the y-intercept] also varies between the two periods). Credible intervals predicted for the spawner abundance and relative tradeoff of the EBFM, based on 1,000 random draws from the posterior distributions of α and β and subsequent calculations of SMSY (spawner abundance that produces maximum sustainable yield) and SEQ (spawner abundance in the absence of a fishery), are shown as box plots (median = line within box; 50th percentiles = ends of box; whiskers = ±1 interquartile range) on the top and right side of each panel.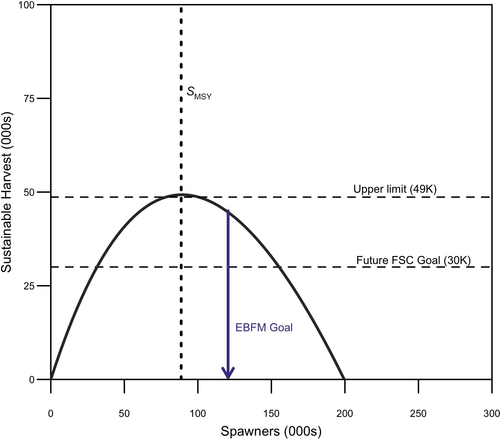
Assuming that the postcollapse conditions remain the same for the foreseeable future, we estimated that maximum sustainable surplus production from the system (i.e., harvest that can occur without causing the population to decline) is approximately 49,000 fish. At the EBFM spawner abundance goal, surplus production is approximately 45,000 (Figure 5). Notably, this EBFM goal and corresponding estimates of production are compatible with the Wuikinuxv Nation’s stated harvest goals for future FSC harvests (30,000 fish; Figure 5), although we note the uncertainty associated with these estimates (Table S5).
DISCUSSION
We quantified the predicted tradeoffs between long-term fishery yield and ecosystem benefits in a salmon system that has undergone profound change. Cultural values of respect for and reciprocity with neighboring wildlife guided how we evaluated the tradeoffs in this EBFM approach. We demonstrated that potential fishery yield from the system has declined by an order of magnitude after the collapse. While our intent was not to recommend specific allocations for bears, we emerge with a spawner abundance goal within a tradeoff framework in which human fishers and bears (and, by extension, the ecosystem) assume an equal relative cost. We found that managing for this EBFM goal requires forgoing relatively modest amounts of harvest but that the magnitude of the tradeoff is highly dependent on the state of the system (i.e., pre- or postcollapse). The relative tradeoff was larger postcollapse, but the absolute tradeoff in terms of numbers of fish was small, though highly uncertain; fishers need not forgo substantial harvest to provide an allocation to bears that supports relatively high density (relative to maximum). As a result, despite large reductions in potential future fishery yield after the collapse, the FSC harvest goals of the Wuikinuxv Nation are compatible with an EBFM goal that can simultaneously sustain relatively high bear densities if the productivity and carrying capacity of the system remain similar for the foreseeable future.
Our analyses suggest that the Sockeye Salmon carrying capacity of Wuikinuxv Lake changed substantially over time, as illustrated by our comparison between the spawner–recruit models in the two time periods and across the full time series. We note that our delineation of the two time periods was done a priori—based on the beginning of the marked declines in spawner abundance and marine survival (Rutherford and Wood 2000). Our assumption of an apparent collapse of spawner abundances in this system (i.e., our use of two time periods) allowed us to illustrate a framework for how Indigenous harvest goals and data from locally driven fisheries and wildlife programs could inform EBFM. However, the hypothesized timing of the collapse was not explicitly tested and therefore warrants further consideration in future studies. Model-based approaches to identifying the timing of the collapse or regime shift could be used—for example, a hidden Markov model can be implemented, as has been done for productivity in other systems (Cunningham et al. 2018). However, incorporating temporal variation in productivity and density dependence in an age-structured state-space spawner–recruitment framework like the one used here is a nontrivial undertaking that requires future research. Although we did not seek to identify the cause of the collapse or subsequent reductions in spawner abundance, we note the extent to which the carrying capacity has affected equilibrium population size. Productivity was variable across the entire time series (Figure 3A) and was modestly higher in the postcollapse period (Table 1), whereas carrying capacity decreased by an order of magnitude from the precollapse period to the postcollapse period (Table 1). We cannot rule out that a change in productivity over time confounded our estimates of capacity over the two time periods, but our findings suggest stronger within-population per-capita density effects on survival after the collapse, which may indicate a reduction in habitat capacity in Wuikinuxv Lake. It is possible that the extensive timber harvest, road building, and subsequent siltation and watershed instability in major tributaries to Wuikinuxv Lake from the 1960s to the mid-2000s, glacial retreat, or a combination of these factors have interacted in nonlinear ways and have led to reductions in rearing habitat capacity, which have pushed the system into an alternative and reduced state (e.g., Platts et al. 1989; Croke and Hairsine 2006). However, these are simply hypotheses that warrant further investigation. In addition, large reductions in spawner abundance as a result of the large commercial fishery prior to the 1960s may have reduced nutrient loading into the lake. Further limnological research on the turbidity and productivity of Wuikinuxv Lake, along with analyses or relationships with other stressors and time-varying capacity, could help to provide clarity on the current and future carrying capacity of the system (Atlas et al. 2020).
Our approach required the use of informed assumptions, both theoretically and empirically. Despite recent sonar observations, uncertainty in estimates of spawner abundance in this system is quite high (C. Carr-Harris, DFO, personal communication). We also acknowledge that assuming a linear relationship between the sonar and the count data is limiting. The accuracy of FSC catch reporting is also uncertain (Rolston, personal communication). However, we found that our inference was robust to these assumptions about the magnitude of uncertainty in observations (see SI for sensitivity analyses with alternative priors and observation errors). Our estimates of RBD were limited by our assumption of a linear relationship of salmon in the diet and productivity (i.e., density) of 13 grizzly bear populations as reported by Hilderbrand et al. (1999), without accounting for uncertainty. We used this across-population pattern of a meat diet to density and applied it within one population exposed to differing levels of the predominant meat source in the region—salmon (Adams et al. 2017). Hilderbrand et al. (1999) suggested that relative dietary meat, an important determinant of fitness (Belant et al. 2006; Bryan et al. 2014), is linearly related to bear density and thus is roughly equivalent to RBD. Salmon are of particular importance to density patterns in grizzly bear populations, and our work here corroborates other patterns of salmon consumption in grizzly bear populations as reported by Hilderbrand et al. (1999; Figure S2), which underlies our assumption in this linear relationship. Finally, to assess the long-term consequences of Sockeye Salmon contributions to the bears’ diet, we held the biomass of other adjacent salmon species at their median values. Future efforts could consider accounting for annual variability in spawner abundance of these species, some of which is substantial (e.g., Pink Salmon returns in the nearby Ćàq̌vala/Chuckwalla River can fluctuate by an order of magnitude and can be in excess of Sockeye Salmon returns).
We quantified how bears respond to salmon abundance under alternative salmon production scenarios. Although the Wuikinuxv Lake Sockeye Salmon population may not recover to support previous densities of bears, the dietary contributions we observed and RBDs predicted under current conditions suggest that grizzly bears have adequate access to salmon. These results indicate that under current conditions, the benefits to surrounding ecosystems from salmon-fed bears (e.g., fertilization, seed dispersal; Quinn et al. 2009, 2018; Darimont et al. 2010; Shakeri et al. 2018) can be sustained and the risk of potential conflicts with Katit residents (which are more likely when salmon returns are poor; Artelle et al. 2016) can be managed.
The opportunity for and apparent benefits of values-driven EBFM are illustrated in the Wuikinuxv Sockeye Salmon system. Here, values of local food sovereignty and respect for ecological well-being support a balance among cultural, economic, and ecological goals (Marshall et al. 2017). We found that the current and future FSC harvest goals of the Wuikinuxv Nation are compatible with an EBFM approach, which in our case accounts for benefits to bears and, by extension, ecosystems. The Wuikinuxv local fishery in the Waanukv/Wannock River is terminal, occurring directly before the spawning area, and is informed by CPUE indices and sonar observations conducted by the Wuikinuxv Nation. In contrast, relatively large mixed-stock commercial harvests that intercept Wuikinuxv Sockeye Salmon, like those that occurred precollapse (average commercial catch of ˜600,000 prior to 1992, with the majority of the stock intercepted in Rivers Inlet and additional minimal catch occurring to the north; Rutherford and Wood 2000), are likely not possible postcollapse given the profound changes to the carrying capacity of the system (Figures 2, 5). Put plainly, our analysis indicates that the needs of local Wuikinuxv FSC fishers and the surrounding ecosystem are likely to be supported by the Sockeye Salmon stock, while there simply may not be enough to support commercial interests. The consideration of these consequences is reflected in the precautionary approach taken by Wuikinuxv decision makers in the management of the local fishery, which also guided the design and analysis of this work.
The increasing relevance of locally derived data and decentralized Indigenous governance (and the value systems they draw upon) create conditions under which our approach is particularly well suited (Artelle et al. 2018; Atlas et al. 2021). Specifically, data collected by the Wuikinuxv Nation and other coastal First Nations fill important gaps created by decreased capacity of centralized agencies (e.g., DFO or the Province of British Columbia) to monitor and manage fish and wildlife populations over large regions (Price et al. 2008, 2017; Loring 2013). Locally driven and executed research, including the data on bears and salmon from which we draw, can continue to support place-based and localized decision making (e.g., Adams et al. 2014; Service et al. 2014, 2018). Federal and provincial policy supports such opportunities for transfer of authority in the form of the Wild Salmon Policy (DFO 2005) and the Reconciliation Protocol (Coastal First Nations et al. 2016), respectively. Sustained local monitoring and management efforts are particularly important given the nonstationarity in salmon population dynamics, which poses a major challenge for stock assessment and harvest management across the Pacific Northwest (Holt 2010; Collie et al. 2012; Peterman and Dorner 2012). More broadly, Indigenous governments are turning their collective efforts toward holistic and increasingly de-centralized regional monitoring and management to guide their integrated stewardship of marine and terrestrial species (e.g., the Coastal Guardian Watchmen Network, the Central Coast Indigenous Resource Alliance, Nation-based integrated resource stewardship departments, or otherwise; Ban et al. 2017, 2018).
Future directions for EBFM in this and other systems include applying additional analytical and collaborative approaches so as to attend to remaining uncertainties. Increased analytical investments are required to more comprehensively and justly account for the inherent complexity of socioecological systems that are reliant on fisheries (Bennett 2018; Okamoto et al. 2020). For example, we note that the approach we have taken to quantify fishery yields and evaluate tradeoffs with bear densities under an EBFM framework assumes long-term equilibrium conditions. This is a limiting assumption because it neither fully considers the current state of the system nor accounts for the imperfect nature of observing a system and managing the fisheries that seek to harvest from it (Cenek and Franklin 2017). A closed-loop simulation of stock dynamics and an ecosystem-based management system could be developed to rigorously evaluate the predicted ability of alternative management actions (e.g., fishery management reference points and FSC and commercial harvest goals) to meet ecosystem, Indigenous harvest, and commercial fishery objectives under plausible future states of nature. This would allow the dynamics of the system to be simulated forward in time over a meaningful period (e.g., 20–50 years), where the biological state of the system is conditioned on the most recent data. Such an approach is most decision relevant if it is undertaken in a collaborative manner with Indigenous titleholders in conjunction with local stakeholders. This team could define the social, economic, and ecological objectives against which alternative management actions are evaluated to support a more comprehensive suite of dimensions for decision making. This type of approach is commonly referred to as “management strategy evaluation” in fisheries literature (e.g., Punt et al. 2016) and is increasingly being applied in salmon systems (e.g., Cunningham et al. 2018; Connors et al. 2020). The age-structured state-space spawner–recruit model we developed is well suited to form the basis of the biological model used in such an exercise. As such, these directions have potential to contribute to EBFM efforts focused on delivering ecologically, economically, and culturally sustainable harvest to diverse fishery beneficiaries. Such efforts can be guided and realized through cultural values and leadership of local governance systems in the Pacific Northwest and beyond.
ACKNOWLEDGMENTS
We are grateful to Tom Quinn, Charmaine Carr-Harris, Christina Service, Dave Rolston, and Lindsay Davidson for their initial feedback on the manuscript, as well as two anonymous reviewers for their constructive feedback. We thank the DFO, Raincoast Conservation Foundation, and Indigenous stewardship staff who made their data or expertise available to us, and we appreciate the Wuikinuxv Stewardship Committee for their ongoing input and support of this work. Fieldwork was supported by the Coastal Guardian Watchmen Network, Raincoast Conservation Foundation, the Hakai Institute, and the Grizzly Bear Foundation. M.S.A. was supported by Mitacs and Wilburforce fellowships. C.T.D. was supported by Natural Sciences and Engineering Research Council Discovery Grant 435683 and the Wilburforce and SkyeMikko foundations. There is no conflict of interest declared in this article.




