CDK10 suppresses metastasis of lung adenocarcinoma through inhibition of the ETS2/c-Raf/p-MEK/p-ERK signaling loop
Xiujuan Zhang, Yu Zhao, Ruzetuoheti Yiminniyaze, and Ning Zhu contributed equally to this work and shared first authorship.
Abstract
The repertoire of aberrant signaling underlying the pathogenesis of lung adenocarcinoma remains largely uncharacterized, which precludes an efficient therapy for these patients, especially when distant metastasis occurs. Cyclin-dependent kinase 10 (CDK10) has been reported to modulate the progression of malignant tumors; however, contradictory effects have been found among different types of malignant tumors. In the present study, we found that CDK10 was downregulated in lung adenocarcinoma compared with the paired adjacent normal lung tissue, and lower expression level of CDK10 was associated with more frequent N2 staged lymph node and distant metastasis, higher TNM stage, and shorter overall survival. Further study indicated that CDK10 inhibited the migration and invasion abilities with no impact on the proliferation of lung adenocarcinoma cells. Mechanistically, CDK10 could bind to and promote the degradation of ETS2, a transcription factor for C-RAF and MMP2/9, thereby inactivating the downstream c-Raf/p-MEK/p-ERK pathway that drives epithelial–mesenchymal transition and impairing the expression of matrix metalloproteinases involved in cell invasion. In addition, the p-MEK/p-ERK pathway conducts a positive feedback regulation on the expression of ETS2. Knockdown of CDK10 in human lung adenocarcinoma cells significantly promoted the formation of metastatic foci in lungs in a xenograft mouse model. In conclusion, CDK10 suppresses metastasis of lung adenocarcinoma by disrupting the ETS2/c-Raf/p-MEK/p-ERK/ETS2 signaling and MMP2/9, providing a new therapeutic target for the treatment of lung adenocarcinoma with metastasis.
Abbreviations
-
- CDK
-
- cyclin-dependent kinase
-
- ECM
-
- extracellular matrix
-
- EGFR
-
- epidermal growth factor receptor
-
- EMT
-
- epithelial–mesenchymal transition
-
- EV
-
- empty vector
-
- HE
-
- hematoxylin–eosin
-
- KD
-
- knockdown
-
- LUAD
-
- lung adenocarcinoma
-
- MMP
-
- matrix metalloproteinase
-
- NGS
-
- next-generation sequencing
-
- OE
-
- overexpression
-
- qRT-PCR
-
- quantitative reverse transcription-polymerase chain reaction
-
- SD
-
- standard deviation
-
- shRNA
-
- short hairpin RNA
-
- siRNA
-
- small interference RNA
1 INTRODUCTION
The leading cause of cancer-related deaths worldwide is lung cancer, with a 5-year survival rate of only 19%.1, 2 Metastasis is the main cause of death in the majority of patients with lung adenocarcinoma (LUAD). The 2-year survival rates in lung cancer patients with stages IVA and IVB are only 23% and 10%, respectively, compared with more than 90% for stage I.3 However, more than half of the patients with lung cancer are at an advanced stage when diagnosed.2, 4 As recommended by the guideline of National Comprehensive Cancer Network, the therapeutic strategies for the metastatic LUAD include systemic chemotherapy, targeted therapy for the mutation of the driver gene, the antiangiogenic therapy, blocking of the immune checkpoints, and the local treatment for metastatic lesions, such as surgery for oligometastatic lesion and radiotherapy for bone and intracranial metastases.5 With the extensive use of immunotherapies in lung cancer in recent years, the prognosis of metastatic LUAD has improved significantly. Pembrolizumab, an inhibitor of programmed cell death 1 (PD-1), has been shown to increase the overall survival relative to chemotherapy in locally advanced/metastatic non-small-cell lung cancer patients with PD-L1 tumor proportion score ≥1%.6 However, there are still some obstacles in the treatment of LUAD, such as insensitivity to immunotherapy, severe adverse effects, or relapse after initial therapy.7 Given the poor prognosis of patients with advanced lung cancer, new therapies to suppress metastasis are required urgently. The mechanisms involved in the metastasis of lung malignancies are complex, and primarily include angiogenesis, dedifferentiation, increased plasticity of tumor cells, the epithelial–mesenchymal transition (EMT)/mesenchymal–epithelial transition, cancer stem cell hypothesis, and fitness genes that allow the tumor cells to survive stressors during metastasis.8-10 However, the therapeutics targeting metastasis of LUAD are still limited due to the poor understanding of molecular mechanisms of metastasis.
Cyclin-dependent kinase (CDK) 10 belongs to the CDK family, in which more than 20 members have been identified.11 The proteins of the CDK family contain an ATP-binding pocket, which catalyzes the phosphorylation of the serine/threonine residuals.12 After binding with their specific partner proteins, some CDKs phosphorylate the substrate proteins and enable the transition of the cell cycle. The hyperactivation of CDKs has been verified in some malignancies, providing a rationale for the repression of these kinases in cancer therapy. CDK4/6, the key checkpoint molecules driving the G2 to S phase transition of the cell cycle, have been validated as efficient therapeutic targets for breast cancer.13 Previous studies have revealed that cyclin M functions as a binding partner and an activator of CDK10 in the regulation of the G2/M phase of the cell cycle.14, 15 CDK10 could promote the division of osteosarcoma cells through facilitating entry into G2/M phases.16 In addition, CDK10 has also been demonstrated to engage the transcription factor ETS2 to promote its degradation and/or impair its enrichment on the promoter of target genes in mammalian cells, including breast cancer cells.14, 17 Moreover, some clinical studies have reported an elevated expression of CDK10 in prostate cancer and seminomas.18, 19 However, there is also accumulating evidence indicating an inhibitory effect on CDK10 on tumor progression.20, 21 Given that the role of CDK10 in lung cancer is still unknown, we aimed to explore the effects and molecular mechanisms of CDK10 on LUAD.
2 MATERIALS AND METHODS
2.1 Patients and clinical tissue specimens
The inclusion criteria were as follows: (1) patients who were diagnosed as LUAD on pathology and underwent epidermal growth factor receptor (EGFR) gene testing; (2) availability of enough tumor tissue and paired normal lung tissue acquired by surgery for further experiments in additional to routine clinical requirements; (3) patients who received comprehensive treatment according to clinical needs; (4) patients who agreed to participate in this study and were followed up regularly. LUAD patients who were unwilling to participate in the study, lost to follow up, or rejected systemic therapy were excluded from this research. In total, 71 LUAD patients were included. The clinical data of these patients were collected, and the LUAD tissues and paired adjacent normal lung tissues were used for immunohistochemical analysis. This study was approved by the Institution Review Board of Huashan Hospital, Fudan University, China (Permit No. KY2016-396), and each patient signed the informed consent form.
2.2 Cell lines and reagents
The human lung cancer cell lines NCI-H1975, HCC827, PC-9, and immortalized bronchial epithelial cell line BEAS-2B were obtained from the Cell Bank of Shanghai Institute for Biological Sciences, Chinese Academy of Sciences. NCI-H1299, NCI-H1650, and A549 were purchased from ATCC. No cross-contamination was observed in all of those cell lines, which was detected by polymerase chain reaction (PCR) of short tandem repeat DNA fingerprints. Cells were cultured in a complete growth medium with DMEM or RPMI 1640 medium containing 10% fetal bovine serum (FBS) and 100 U/mL penicillin–streptomycin. The cell dishes were placed in a humidified incubator with 5% CO2 maintained at 37°C, and passed on at 80%–90% confluence. Cells were split every 3–4 days or as needed. PCR was performed to screen for Mycoplasma contamination of cultured cells.
2.3 Immunohistochemistry
The procedures of immunohistochemistry were as described in a previous study.22 Generally, the tissues were fixed with 10% formalin, embedded in paraffin, and cut into a series of sections. Then, the sections were dewaxed, incubated with 3% H2O2 for 10 min in the dark, and blocked in 10% goat serum for 2 h. After incubation with primary CDK10 antibody (1:100, Cell Signaling Technology, #36106), ETS2 (1:100, Abcam, ab219948), anti-phospho-Ser217/221-MEK1/2 (1:100, Cell Signaling Technology, #9154), or phospho-Thr202/Tyr204-ERK1/2 (1:100, Cell Signaling Technology, #4370) at 4°C overnight, the sections were incubated with biotinylated secondary antibody (Cell Signaling Technology), stained with 3,3-diaminobenzidine and counterstained with hematoxylin. All slides were observed and photographed by an Olympus microscope (Nikon) under the same exposure conditions. The expression levels of CDK10, ETS2, p-MEK1/2, and p-ERK1/2 were scored by an experienced pathologist by an immunoreactive score, as described before.23
2.4 Gene knockdown (KD) and overexpression (OE) using lentivirus vectors
The short hairpin RNA (shRNA) oligonucleotides targeting CDK10 were designed and synthesized by Synbio Technologies, China. The sequences of CDK10 shRNA are shown in Supporting Information Table S3. Next, the paired shRNA oligos and control scrambled sequences were cloned into pGreen-Puro-CMV plasmid (Public Protein/Plasmid Library). The full-length CDK10 isoform a (NM_052988.5) was synthesized (GenScript) and cloned into the pCDH-CMV-MCS-EF1-Puro plasmid (Public Protein/Plasmid Library). The complementary DNA (cDNA) of ETS2 (NM_001256295.1) was cloned into the vector of EX-NEG-Lv183 (Gene Copoeia Company). These plasmids were cotransfected with psPAX2 and pMD2.G plasmids into HEK293T cells. The recombinant lentiviruses in the cell culture medium were collected and used to transfect the H1299, PC-9, and A549 cells. The KD and OE efficiencies of CDK10 in the transfected cells were verified by Western blot.
2.5 Quantitative reverse transcription-PCR (qRT-PCR) analysis
Total RNA was extracted using TRIzol reagent (Invitrogen) in accordance with the manufacturer's protocol. Then, 1 μg of RNA was reverse-transcribed into cDNA using a PrimeScript Reverse Transcriptase reagent kit (Takara). The qRT-PCR was conducted in C1000 Thermo Cycler (Bio-Rad), using the mixture of cDNA, SYBR Premix Ex Taq (Takara), and primers (Sangon Biotech). The primer sequences of the relative genes are shown in Supporting Information Table S2. The relative messenger RNA (mRNA) expression level was calculated as follows: ΔCt = Ctgene − CtInternal reference; ΔΔCt = ΔCtexperimental group − ΔCtcontrol group; the relative mRNA expression level = 2−ΔΔCt.
2.6 Immunoblotting
The protein sample was extracted from the cells by RIPA lysis buffer (Beyond, P0013B), and then, its concentration was examined using BCA kits (Thermo Fisher Scientific, #23225). After denaturation in a metal bath at 100°C for 15 min, the proteins were separated in 10% sodium dodecyl-sulfate polyacrylamide gel electrophoresis gels using electrophoresis tanks (Bio-Rad) and transferred to polyvinylidene difluoride transfer membranes. Five percent bovine serum albumin in Tris–buffered saline (TBST) was applied to block the blots for 1 h at room temperature. Primary antibodies were added and incubated overnight at 4°C. The following primary antibodies were used in this experiment: anti-CDK10 (Cell Signaling Technology, #36106), anti-E-cadherin (Cell Signaling Technology, #3195), anti-N-Cadherin (Cell Signaling Technology, #4061), anti-ETS2 (Abcam, ab219948), anti-phospho-Thr202/Tyr204-ERK1/2 (Cell Signaling Technology, #8544), anti-ERK1/2 (Cell Signaling Technology, #9101), anti-phospho-Ser217/221-MEK1/2 (Cell Signaling Technology, #9154), anti-MEK1/2 (Cell Signaling Technology, #4694), anti-MMP2 (Abcam, ab92536), anti-MMP9 (Abcam, ab76003), anti-C-Raf (Erpantech, AB-06-3811), anti-GAPDH (Cell Signaling Technology, #97166), anti-β-Tubulin (Cell Signaling Technology, #2146), and anti-Vimentin (Cell Signaling Technology, #5741). After washing out of the primary antibody by TBST solution, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody for 1 h, followed by a reaction with ECL chemiluminescence reagent (Amersham Life Science). Finally, the blots were visualized and scanned in a chemiluminescence imaging system (Tanon).
2.7 Transwell migration and invasion assay
In the cell migration assay, cells in the culture medium without FBS were seeded on the upper chamber (Corning, 8 μm), and 1 mL of complete cell culture medium containing 10% FBS was added into the lower chamber in a 24-well plate as well. After incubation for 14–16 h, the cells attached to the upper chamber were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. The cells on the upper side of the membrane were gently removed by cotton swab, while the cells on the lower side of the membrane were photographed and counted in five random fields. In the cell invasion assay, each chamber was pretreated with 40 μg matrigel (Corning) in line with the manufacturer's protocol, and the remaining processes were similar to those in the transwell migration assay. All experiments were repeated for at least three times.
2.8 Immunoprecipitation
pCDH-CDK10-Myc (6 μg) and an empty vector (6 μg) were transfected into H1299 cells separately. Twelve hours later, MG132 (10 μM; MedChemExpress) was added to inhibit the degradation of proteins. After incubation for another 24 h, the protein samples were prepared in 0.5% NP-40 lysis buffer containing protease and phosphatase inhibitors and were further lyzed by an ultrasonic cell crusher (Scientz). The lysate (1 mg/sample) was incubated with 25 μL of anti-Myc-tag magnetic beads for 3 h at 4°C on a rotary shaker. Then, the beads were washed with TBST, boiled for 10 min, and subjected to Western blot assay.
2.9 Immunofluorescence imaging
H1299 cells in 50%–70% confluence were fixed with 4% paraformaldehyde, blocked with 5% BSA, incubated with primary antibodies against CDK10 (1:100, Thermo Fisher Scientific, PA5-96192) and ETS2 (1:50, SantaCruz, sc-365666) overnight at 4°C, and then incubated with Alexa Fluor 488-conjugated and Dylight 550-conjugated secondary antibodies for 1 h. Then, 4ʹ,6-diamidino-2-phenylindole dye was applied to stain the nucleus, and cells were observed and photographed under a fluorescence microscope.
2.10 RNA interference
Small interference RNAs (siRNAs) targeting ETS2 and control scrambled siRNAs were designed and synthesized by Genepharma. Cells were transfected with ETS2 siRNAs and control siRNAs using Lipofectamine 3000 (Invitrogen). The sequences of siRNAs targeting ETS2 and negative control are shown in Supporting Information Table S3. The qRT-PCR and Western blot were conducted to verify the KD efficiency of ETS2.
2.11 In vivo model of LUAD metastasis
The lentiviral vector expressing firefly-luciferase (Genechem) was transfected into CDK10-KD and control H1299 cells. Twelve female BALB/c nude mice aged 4 weeks were randomly divided into two groups, injected with shCDK10 H1299 cells and control cells (5 × 105 cells in 200 μL PBS/mouse) via the caudal vein, respectively. Six weeks later, metastatic lesions in distant lungs were detected by bioluminescence imaging after intraperitoneal injection with d-luciferin (150 mg d-luciferin/kg body weight). The mice were killed, and the lung was isolated, fixed with 10% formalin, embedded in paraffin, and cut into a series of continuous sections at an interval of 50 μm. Each slide was then processed with hematoxylin–eosin (HE) staining and the metastatic lesions were counted. All of the mice were raised in a pathogen-free animal laboratory animal housing with free access to food and water, in accordance with the National Institutes of Health guide. All animal experiments were approved by the Fudan University Veterinary Medicine Animal Care and Use Committee.
2.12 Statistical analysis
ImageJ software was used for grayscale scanning of Western blots, and the GraphPad Prism 8.0 software was used for statistical analysis. The data of continuous variables were expressed as mean ± standard deviation (SD). The t test was used to compare the data between two groups, and the analysis of variance was used to compare the data between multiple groups if these data were homogeneous and distributed normally. If not, the continuous variables were analyzed by rank-sum test. Log-rank test was used to analyze the differences between Kaplan–Meier survival curves. If p was <0.05, the difference was considered as significant.
3 RESULTS
3.1 CDK10 downregulation correlates with metastasis and poor overall survival in LUAD patients
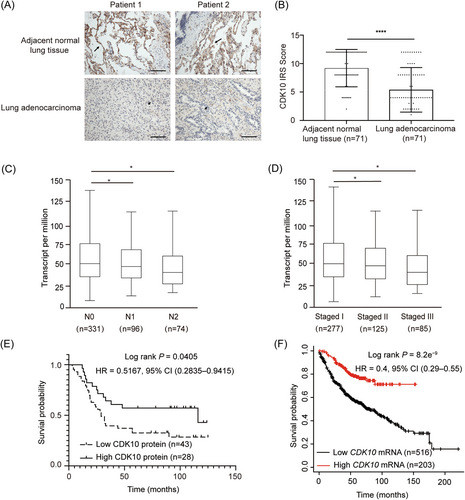
In this study, the paired tumor tissues, adjacent normal lung tissue specimens, and metastatic lesions in lymph nodes from five T1aN1M0 and five T1aN0M0 stage LUAD patients were collected for the next-generation sequencing (NGS). Analysis of the NGS data resulted in the identification of CDK10, which harbored a missense mutation in tumors from 3 of 5 enrolled patients but not in the paired normal tissues (Supporting Information Table S1). The correlation of CDK10 expression with the clinical characteristics of 71 patients with LUAD was next analyzed. Immunochemical staining indicated that the levels of CDK10 protein in tumor tissues were downregulated compared with those in the paired normal lung tissues (Figure 1A,B). As shown in Table 1, CDK10 was lower in patients with stages N2, M1, and TNM stage II/IV than in patients with stages N0, M0, and TNM stage I, respectively. Besides, CDK10 was also lower in LUAD of pathologic grade III versus grades I and II. Consistently, data on UALCAN.24 showed a reduced mRNA level of CDK10 in LUAD patients with N1 and N2 stages compared with those with N0 stage (Figure 1C). Patients with TNM stages II and III also had lower mRNA levels of CDK10 than those with TNM stage I (Figure 1D). Kaplan–Meier survival curve analysis indicated a shorter overall survival in patients with lower protein expression of CDK10 (hazard ratio [HR] = 0.5167; 95% confidence interval [CI], 0.2835–0.9415; Log-rank P = 0.0405; Figure 1E). We further examined the association of CDK10 mRNA levels with the prognosis of LUAD patients in the public Kaplan–Meier Plotter database,25 and verified shorter overall survival in patients with lower CDK10 mRNA expression (HR = 0.40; 95% CI, 0.29–0.55; Log-rank P = 8.2e−9; Figure 1F). Thus, CDK10 was downregulated in LUAD, and the downregulation of CDK10 level was significantly correlated with increased metastatic capacity of tumors and poor prognosis of LUAD patients.
| Classification | Total | CDK10 IRS (mean ± SD) | P |
|---|---|---|---|
| Gender | |||
| Male | 39 | 4.62 ± 0.57 | Ref |
| Female | 32 | 5.68 ± 0.70 | ns |
| Age (years) | |||
| ≤60 | 42 | 5.19 ± 3.62 | Ref |
| >60 | 29 | 5.17 ± 4.11 | ns |
| Pathological grade | |||
| I | 13 | 6.83 ± 1.27 | 0.0214 |
| II | 28 | 5.92 ± 0.71 | 0.0291 |
| III | 34 | 3.88 ± 0.59 | Ref |
| TNM stage | |||
| I | 21 | 7.14 ± 0.81 | Ref |
| II | 19 | 4.74 ± 0.84 | 0.0465 |
| III | 10 | 4.90 ± 1.27 | ns |
| IV | 21 | 3.57 ± 0.70 | 0.0018 |
| T stage | |||
| T1 | 54 | 5.63 ± 0.54 | Ref |
| T2 | 11 | 3.36 ± 1.01 | ns |
| T3 | 4 | 3.75 ± 1.65 | ns |
| T4 | 2 | 3.50 ± 0.50 | ns |
| N stage | |||
| N0 | 30 | 6.17 ± 0.73 | Ref |
| N1 | 24 | 5.08 ± 0.69 | ns |
| N2 | 13 | 3.54 ± 0.73 | 0.0362 |
| N3 | 4 | 5.75 ± 2.66 | ns |
| M stage | |||
| M0 | 50 | 5.86 ± 0.53 | Ref |
| M1 | 21 | 3.57 ± 0.79 | 0.0176 |
| EGFR mutation | |||
| Yes | 35 | 4.86 ± 0.69 | Ref |
| No | 36 | 5.06 ± 0.58 | ns |
- Abbreviations: CDK10, cyclin-dependent kinase 10; EGFR, epidermal growth factor receptor; IRS, immunoreactive score; LUAD, lung adenocarcinoma.
3.2 CDK10 inhibits the migration and invasion abilities of LUAD cells
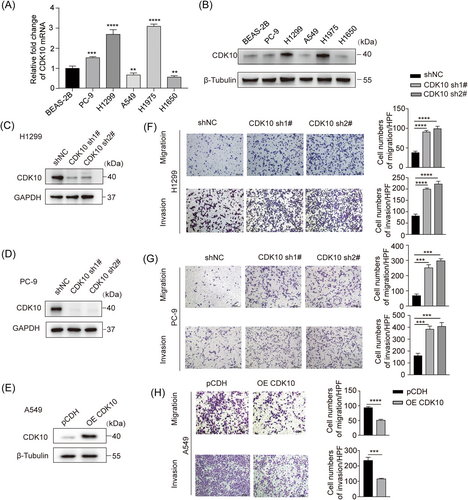
The expression of CDK10 was examined in lung cancer cell lines and the normal lung epithelial cell line, BEAS-2B. As shown in Figure 2A,B, the mRNA and protein levels of CDK10 were significantly higher than those in the LUAD cell lines H1299 and H1975 than in the normal lung epithelial cell line, BEAS-2B. The KD and OE of CDK10 in LUAD cells were achieved by lentivirus-delivered shRNAs or CDK10 cDNA (Figure 2C–E). CCK8 and EdU assays indicated no significant effect of CDK10 on the viability of LUAD cells (Supporting Information Figure S1A,B), which was consistent with the finding that CDK10 KD failed to induce cell cycle arrest in LUAD cells (Supporting Information Figure S1C). Transwell migration and invasion assays showed that CDK10 KD enhanced the migration and invasion capabilities of H1299 and PC-9 cells (Figure 2F,G), and CDK10 OE impaired the migration and invasion capabilities of A549 cells (Figure 2H). Consistently, wound healing assay indicated that CDK10 KD promoted the migration of H1299 cells, and CDK10 OE inhibited the migration of A549 cells (Supporting Information Figure S2). These data suggest that CDK10 plays a suppressive role in the motility and invasiveness of LUAD cells.
3.3 CDK10 represses the formation of in vivo metastatic foci by LUAD cells
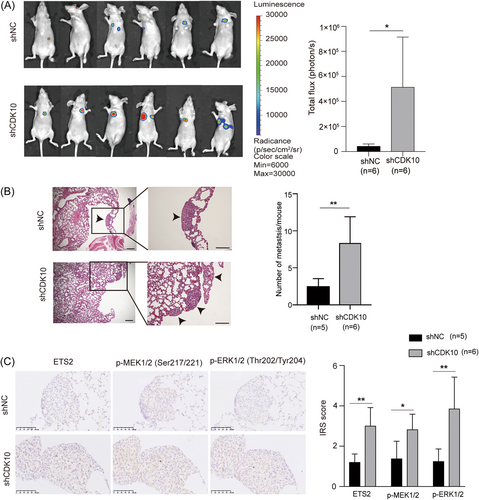
A xenograft metastatic model of LUAD was established in BALB/c nude mice by injection of H1299 cells stably expressing firefly-luciferase via the caudal vein. The results of bioluminescence imaging showed that CDK10 KD significantly enhanced the formation of metastatic lesions (Figure 3A). Consistently, HE staining detected more metastatic lesions in the lungs in mice injected with CDK10-KD H1299 cells than those in control cells (Figure 3B). Therefore, CDK10 counteracts the development of metastases of LUAD cells in vivo.
3.4 CDK10 interacts with ETS2 and inhibits the degradation of ETS2 protein
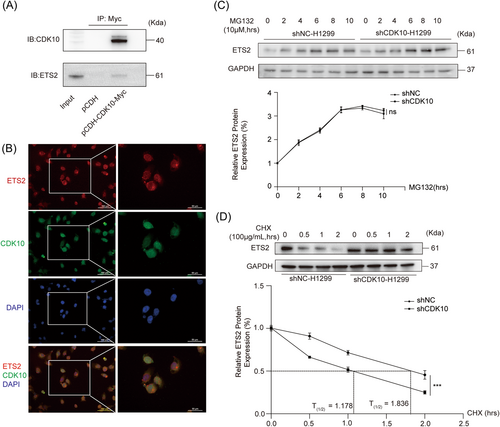
A previous study has reported the interaction between CDK10 and ETS2 proteins in STAR syndrome.15 ETS2 is considered as an oncoprotein, which has been reported to enhance the tumorigenesis and progression of various types of malignancies.26 ETS2 could act as a transcription factor through binding with the promoters of target genes including C-RAF and matrix metalloproteinases (MMPs), both of which are involved in the multistep processes of metastasis.15, 27 Thus, we speculated that the interaction of CDK10 with ETS2 might be involved in the regulation of LUAD metastasis. Immunoprecipitation verified the interaction of CDK10 protein with ETS2 protein in H1299 cells (Figure 4A). Then, dual immunofluorescence staining showed the colocalization of CDK10 and ETS2 proteins in H1299 cells, especially abundant in the nucleus (Figure 4B). No obvious effect of CDK10 KD on the synthesis of ETS2 protein has been found (Figure 4C). However, the silencing of CDK10 in H1299 cells inhibited the degradation and thereby prolonged the half-life time of ETS2 protein (Figure 4D), with the half-life time of 1.178 h and 1.836 h in control and CDK10-KD H1299 cells when treated with CHX (100 μg/mL), respectively. Accordingly, CDK10 might suppress the metastasis of LUAD cells by promoting the degradation of ETS2 protein.
3.5 CDK10 inhibits ETS2/c-Raf/p-MEK/p-ERK signaling, MMP2/9 and impedes EMT of LUAD
The target genes of ETS2, including C-RAF, MMP2, and MMP9, were increased in CDK10-KD H1299 cells (Figure 5A). The c-Raf/p-MEK/p-ERK pathway is widely activated in malignant tumors and could enhance the metastasis of malignant tumors through EMT.28 We found that CDK10 KD increased the protein level of ETS2, and induced the increased expression of MMP2/9 and activation of the c-Raf/p-MEK/p-ERK/EMT signaling cascade in H1299 cells (Figure 5B). Consistently, the immunohistochemical staining in the in vivo model showed increased protein levels of ETS2, p-MEK, and p-ERK in metastatic LUAD lesions in the CDK10-KD group (Figure 3C).
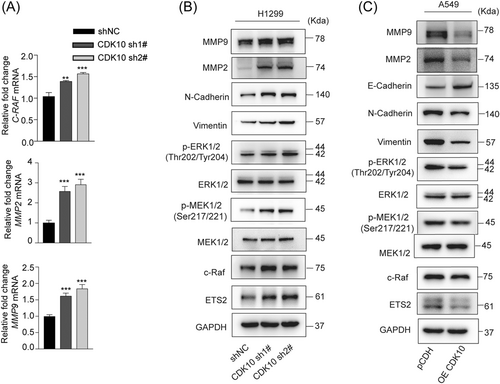
Conversely, the downregulation of ETS2 and inhibitions of MMP2/9 and the c-Raf/p-MEK/p-ERK/EMT pathway were found in CDK10-overexpressed A549 cells compared with control cells (Figure 5C). To test whether ETS2 was a functional target of CDK10, we further knocked down ETS2 in H1299 cells (Figure 6A,B). Transwell migration assay indicated that the effect of CDK10 silencing on cell motility could be blocked by ETS2 KD (Figure 6C). Conversely, while CDK10 OE in A549 cells impaired the capability of invasion, this was rescued by further OE of ETS2 (Figure 6D,E). Moreover, ETS2 deficiency prevented the activation of c-Raf/p-MEK/p-ERK signaling and impaired EMT induced by CDK10 KD, as evidenced by the downregulation of mesenchymal marker N-cadherin and vimentin (Figure 6F). These results showed that ETS2 was a key downstream effector for the inhibitory role of CDK10 on LUAD metastasis. Taken together, these data suggested CDK10 suppressed metastasis of LUAD through ablating ETS2 transactivation of c-Raf and subsequently repressing the p-MEK/p-ERK pathway and EMT of malignant cells.
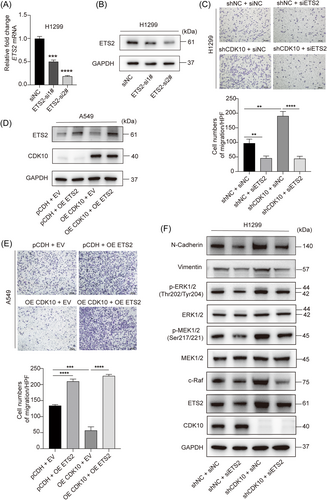
3.6 ETS2 and the c-Raf/p-MEK/p-ERK pathway constitute a positive feedback loop in CDK10-deficient LUAD cells
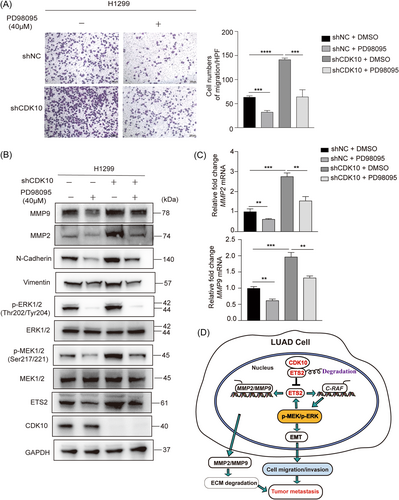
C-Raf/p-MEK/p-ERK signaling represents a canonical intracellular pathway responsible for the regulation of several genes. In line with the aforementioned findings that it plays a key role downstream of CDK10 to regulate the malignant phenotypes of lung cancer cells, we observed that PD98095, a specific inhibitor of MEK,29 reversed the effect of CDK10 KD on the migration of cells (Figure 7A), and inhibited EMT induced by CDK10 ablation (Figure 7B). In addition, decreased expression of ETS2 and MMP2/9 was found in H1299 cells after treatment with PD98095 (40 μM). Consistently, the mRNA levels of MMP2 and MMP9 were also downregulated, upon treatment of cells with PD9809527, 30 (Figure 7C). These findings revealed a positive feedback regulation of ETS2 and MMP2/9 by the p-MEK/p-ERK pathway, which probably contributed to the metastasis of CDK10-deficient LUAD.
4 DISCUSSION
LUAD is the most common histological subtype of lung cancer in Asia. The 5-year survival rate of patients with lung cancer is less than 20%.31 Metastasis accounts for the most deaths in patients with malignant tumors; thus, deep exploration of mechanisms underlying distant metastasis is warranted. In this study, we sought to investigate the role of CDK10, a gene often mutated in tumor specimens, in the metastasis of LUAD. It was determined that downregulation of CDK10 in LUAD was associated with metastasis of tumor and poor prognosis of patients. CDK10 has been documented to play dual roles in tumorigenesis, both as a candidate tumor suppressor in hepatocellular carcinoma, biliary tract cancers and gastric cancer, and as a potential oncogene in colorectal cancer.32 In spite of the previous findings on breast cancer and biliary tract cancer, which disclosed that the downregulation of CDK10 correlated with lymph node metastasis and poor outcome, this was the first report that CDK10 functioned as an essential suppressor of metastasis in LUAD.21, 33 The distinct findings in various types of malignancies suggest that CDK10 plays multifaceted roles in carcinogenesis dependent on the context of the intracellular signal network and the interacting partners available for CDK10. Although varied CDK10 mutations were identified in LUAD specimens, further studies are needed to explore whether CDK10 deficit alone can drive carcinogenesis or clarify how it synergizes with other driving mutations to promote malignant transformation.
Previous studies have identified ETS2 as a regulatory target of CDK10,15, 17 which has also been verified in our study. ETS2 belongs to the ETS transcription factor family, which could modulate the transcription of target genes, thereby regulating multiple processes of tumor progression.34 We found here that the hyperactivity of ETS2 in CDK10-deregulated cells might contribute to metastasis via two processes: activation of c-Raf/p-MEK/p-ERK signaling that elicits EMT, and upregulation of MMP-2/-9 leading to matrix degradation. Since ETS2 is also a target of the p-MEK/p-ERK pathway, CDK10 defect is likely to potentiate metastasis by reinforcing the ETS2/c-Raf/p-MEK/p-ERK signaling loop. However, the detailed mechanisms concerning ETS2 and CDK10 interaction remain elusive. Consistent with the suppressive effect of CDK10 on the transitivity of ETS2, we have identified that CDK10 binds and promotes the degradation of ETS2. The degradation of proteins through the ubiquitin–proteasome pathway could often be regulated by the levels of phosphorylation and dephosphorylation of specific residuals of the proteins. It has been reported that the Ser220/225 on ETS2 could be phosphorylated by the CDK10/cyclin M complex.15 However, another study showed that the degradation of ETS2 was not influenced by the phosphorylation at Ser220/225 by CDK10.35 In addition, a kinase activity-dead mutant and a wild-type CDK10 exert a comparable effect on the activity of ETS2,14 suggesting that CDK10 negatively regulates ETS2 independent of its kinase activity. Nonetheless, further research is needed to clarify whether an intact kinase domain of CDK10 is necessary for the suppression of metastasis of LUAD.
Metastasis is a complex process leading to the spread of cancer to surrounding or distant parts. Its initial steps necessarily involve the acquisition of high motility/invasiveness of cells and the degradation of the extracellular matrix (ECM), which enables local neoplastic cells to invade adjacent tissues. MMPs are a family of zinc-dependent endopeptidases capable of degrading various components of the ECM and thus facilitating metastasis of diverse malignancies.36 In addition to the upregulation of c-Raf and activation of the downstream p-MEK/p-ERK signaling as reported by others and our study, elevated ETS2 in CDK10-ablated lung cancer cells also transcriptionally activated MMP2 and MMP9, which have been identified as target genes of ETS2 in previous studies.15, 37 Although it is still unclear how the classic c-Raf/p-MEK/p-ERK pathway specifically expedites metastasis in LUAD, it has been reported to promote the EMT through upregulation of transcription factors, such as NF-κB, Slug, Zeb1, and Ttwits1.38 MEK inhibitors, combined with B-RAF inhibitor, have been recommended as the first-line therapy for patients with BRAFV600 mutation-positive advanced melanoma.39 In line with those reports and our aforementioned findings, we validated that PD98095, a specific inhibitor of MEK, counteracted the effect of CDK10 silencing on the migration of LUAD cells.
5 CONCLUSION
Our study demonstrates that CDK10 could bind to ETS2 to inhibit its transactivation activity, thereby leading to the downregulation of the ETS2 target genes, C-RAF and MMP-2/-9, in LUAD cells. While c-Raf/p-MEK/p-ERK signaling elicits EMT, a hallmark of neoplastic cells in cancer progression, MMPs also fuel metastasis via the removal of physical barriers for invading cells and decreasing the adhesion of tumor cells. Since ETS2 is also a regulatory target of p-MEK/p-ERK signaling, the dysfunction of CDK10 activates a feedback loop between ETS2 and the c-Raf/p-MEK/p-ERK pathway and thereby contributes to the metastasis of LUAD. The present study unraveled a critical suppressive role of CDK10 in the metastasis of LUAD. This suggests the applicability of targeting the downstream pathways including p-MEK/p-ERK signaling in the treatment of CDK10-deficient lung cancer.
AUTHOR CONTRIBUTIONS
Xiujuan Zhang, Yu Zhao, Yiminniyaze Ruzetuoheti, and Ning Zhu designed the study, performed experiments, analyzed data, interpreted the results, and drafted the original manuscript. Yuanyuan Zhang and Wumaie Gulinuer participated in animal experiments and prepared the figures. Jingwen Xia, Liang Dong, and Daibing Zhu collected tissue samples and clinical data. Jing Wang and Chengwei Li performed the immunohistochemical assays. Youzhi Zhang reviewed and edited the original manuscript. Shengqing Li supervised the project and acquired the funding. All authors contributed to the article and approved the final manuscript.
ACKNOWLEDGMENTS
We acknowledge Liping Zou, a pathologist, for her help in the interpretation of pathology and immunohistochemical results. We also thank Huijun Zhang, Gang Chen, and Yongjun Zhu for their help in collecting the tissue specimens. National Natural Science Foundation of China (No. 81970048) and National Major Science and Technology Projects of China (No. 2018ZX09711002).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The UALCAN database (http://ualcan.path.uab.edu) and Kaplan–Meier Plotter database (https://kmplot.com/analysis/) were used to analyze the relationship between CKD10 mRNA expression and the clinical characteristics of lung adenocarcinoma patients and its relationship with overall survival in lung adenocarcinoma. The data supporting the findings of this study are available from the corresponding author upon reasonable request.




